Abstract
The United States is currently experiencing an entangled epidemic of HIV infection and use of different drugs of abuse, especially of methamphetamine (Meth). Blood monocyte-derived dendritic cells (DC) are the first line of defense against HIV-1 infection, and are the initial target of HIV-1 infection in injection drug users. DC-SIGN present on dendritic cells is the first molecule that facilitates HIV-1 infection independent of CD4 or HIV coreceptors. Chemokines are known to be HIV-1 suppressing molecules, and chemokine receptors also function as HIV-1 coreceptors. We hypothesize that Meth is a cofactor in the pathogenesis of HIV-1 infection, leading to dysregulation of costimulatory molecules and chemokines, with a reciprocal upregulation of DC-SIGN and HIV-1 coreceptors. Our results show that Meth significantly enhances HIV infection, and downregulates the gene expression of chemokines and costimulatory molecules with reciprocal upregulation of HIV coreceptors and DC-SIGN by dendritic cells. Therefore, better understanding of the role of Meth in HIV-1 disease susceptibility and the mechanism through which Meth mediates its effects on HIV-1 infection may help to devise novel therapeutic strategies against HIV-1 infection in Meth using HIV-1 infected population.
Keywords: Methamphetamine, Dendritic cells, HIV-1 coreceptors, CC-chemokines
Introduction
Methamphetamine (Meth) use is associated with a high risk of contracting human immunodeficiency virus (HIV)-1 infection as a result of sharing contaminated needles and increased risky sexual behavior (National Drug Threat Assessment Report 2007; Boddiger 2005). Limited information is available about Meth's effects on the host immune response and the immunopathogenesis of HIV-1 infections. Dendritic cells (DCs) are potent antigen presenting cells that are the initial line of defense against HIV-1 infection (Langhoff et al. 1991; Pope et al. 1994; Weissman et al. 1995). Further, DCs also serve as reservoirs for HIV-1 and play an important role at the interface between the adaptive and the innate immune response. Previously, we have shown that meth act as cofactor in HIV-1 pathogenesis by increasing dendritic cell specific intercellular adhesion molecule-3 (ICAM-3) grabbing non-integrin (DC-SIGN) expression on DCs (Nair et al. 2006). Further we reported that meth enhances HIV-1 infectivity of DCs by upregulating the expression of HIV-1 coreceptors, CXCR4 and CCR5 (Nair et al., 2009). These effects of meth were mediated via dopamine receptors and via downregulation of ERK2 with a reciprocal upregulation of p38MAPK. This review summarizes result from our laboratory supporting the hypothesis that Meth enhances HIV-1 infectivity in DCs and the mechanistic aspects of Meth-mediated effect on HIV-1 disease.
HIV-1 and Methamphetamine
The risk for HIV-1 infection attributable to Meth use continues to increase and the United States is currently experiencing a grave epidemic of Meth use as a recreational drug (National Drug Threat Assessment [NDTA] Report 2007; National Institute of Drug Abuse [NIDA] INFO FACTS 2005). Meth use has surpassed cocaine use as of July, 2005 (National Survey on Drug Use and Health [NSDUH] 2003; http://www.jointtogether.org/news/headlines/inthenews/2006/global-meth-use-exceeds.html). Meth may be smoked, taken orally or injected intravenously. In recent years, the use of meth has spread from the West coast to the East coast. In 2006, a national survey results estimated 5.77% of the US population aged 12 or older used meth at least once in their life time (Office of National Drug Control Policy [NDCP] 2007). Studies indicates that Meth use not only affect the nervous system but also causes heart disease and has immunosuppressive effects (Chang et al. 2005; Nath et al. 2001; Yu et al. 2003).
Meth use increases sex-related HIV-1 risky behavior and meth users are more likely than heroin users to be HIV-1 positive (Boddiger 2005; Frosch et al. 1996; Hirshfield et al. 2004;). Meth effects on the nervous system are mediated via regulation of dopamine (DA) levels, activation of dopamine receptors, and stimulation of glutamate receptors and oxygen-based free radicals (Volkow et al. 2001a-c; Gifford et al. 2000). Five dopamine receptor subtypes (D1R–D5R) have been identified (Sibley et al. 1993); however, the precise roles that these receptors play in the meth-mediated deleterious effects on the host's immune response and the immunopathogenesis of HIV-1 infection remain to be determined. We have shown that meth significantly up-regulates DC-SIGN via dopamine receptors to facilitate virus dissemination (Nair et al. 2006). More recently, we reported that meth exacerbates HIV-1 infectivity in monocyte derived DC by increasing the expression of chemokine receptors CCR5 and CXCR4 and that the activation of the MAPK signal transduction pathways are involved in regulation of HIV-1 infectivity (Nair et al. 2009). The findings, together with previous studies demonstrate that mitogen and other extracellular stimuli that activate MAPK enhance HIV-1 replication by increasing the infectivity of HIV-1 virions (Yang and Gabuzda, 1999; Jacque et al. 1998; Popik et al. 1998). Further, Wilflingseder and coworkers (2004) have also reported that p38 MAPK activation is a prerequisite for HIV-1-induced, CCR7-driven migration of DCs and inhibition of p38 MAPK and simultaneous induction of ERK1/2 is associated with suppression of HIV-1 induced up-regulation of activation markers on DCs. Similarly, Liang and colleagues have also reported that meth promotes HIV infection of macrophages and this is mediated by upregulation of CCR5 expression and the suppression of intracellular interferon-α and signal transducer and activator of transcription-1 in macrophages (Liang et al. 2008).
Dendritic cells and HIV infection
DCs are antigen-presenting cells and are stimulatory for both primary and secondary T-cell responses (Macatonia et al. 1989). Immature DCs (IDC) specialize in capturing and processing antigens. Interaction of IDC with an antigen results in cellular activation or maturation and migration to regional lymphoid tissues where the processed antigens are presented to naïve CD4+ T cells, subsequently enabling T cell activation (Hart 1997; Steinman and Germain, 1998). After further maturation, DC expresses a number of costimulatory molecules CD40, CD80, CD86 and chemokine receptor, CCR7 that are required for efficient antigen presentation by DC (Nair et al. 2007).
Dendritic cell subsets that include myeloid DCs, plasmacytoid DCs (pDCs) and Langerhans cells are all susceptible to infection with HIV (Wu and KewalRamani, 2006). Previous studies clearly demonstrate expression of HIV receptor CD4, CCR5, CXCR4, CCR3, CCR8, CCR9 and CXCR6 on DC subsets (Granelli-Piperno et al. 1996; Rubbert et al. 1998). It is also reported that both myeloid DCs and pDCs are susceptible to laboratory adapted R5 HIV strains and X4 HIV strains. Additionally, R5 HIV strain is known to infect myeloid DCs more efficiently than do X4 HIV strains, which is attributed to higher expression of CCR5 on myeloid DCs (Smed-Sorensen et al. 2005).
Several host proteins play a major role in the attachment of HIV-1 to susceptible target cells. DC-SIGN, also called CD209, is the first molecule that facilitates HIV-1 infection independent of CD4 or the HIV-1 coreceptors (Geijtenbeek and van Kooyk 2003). After binding of HIV-1 gp120 to DC-SIGN on DCs, the virus is not internalized and can promote infection of neighboring T cells. Thus, DC-SIGN-bound HIV-1 remains infectious during its transport from the periphery to lymphoid organs (Geijtenbeek et al. 2002). Previous studies have shown that blood monocyte derived DCs are an effective model to study the HIV-1 infection in injection-drug users (Blauvelt et al. 1997).
Effect of Meth on HIV infection in dendritic cells
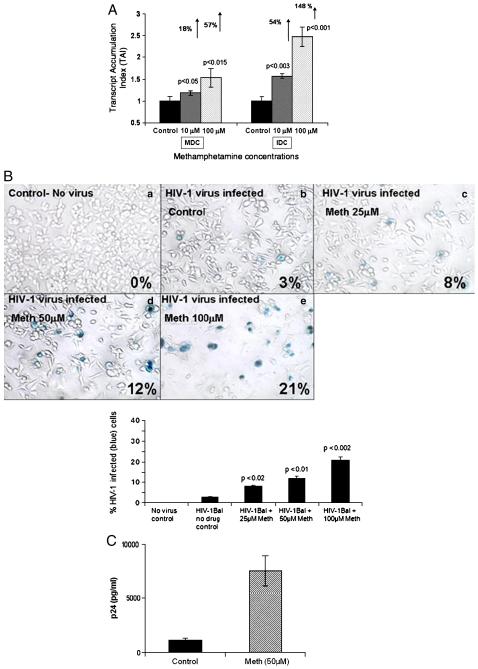
Abuse of meth causes frequent co-morbidity among individuals infected with HIV-1. In an in vitro HIV infection model system, we examined if meth treatment could affect HIV-1 infectivity in monocyte derived dendritic cells. The HIV-1 infectivity was measured by quantitating the expression of the LTR-R/U5 region which represents early stages of reverse transcription of HIV-1. Results (Figure 1A) demonstrated that meth significantly upregulated HIV LTR-R/U5 gene expression in HIV-1 treated immature dendritic cells (IDCs) and mature dendritic cells (MDCs).
Figure 1.
Figure 1A: Meth enhances HIV-1 replication. MDC and IDC (5×105 cells/ml) were infected with native HIV-1 IIIB (X4) (NIH AIDS Research and Reference Reagent Program Cat# 398) at a concentration of 10 3.0 TCID 50/ml cells overnight and washed 3 times with Hank's balanced salt solution (GIBCO-BRL, Grand Island, NY) before being returned to culture with and without Meth (10 & 100 μM) for 24 hr. The RNA was extracted, reverse transcribed and followed by quantitative real time PCR against the LTR-RU5 and the housekeeping gene, β-actin and the 18S RNA primers as internal controls. The data represent mean ± SD of 3 independent experiments. Statistical analysis was done using Student's ‘t’-test.
Figure 1B: Meth enhances HIV-1 infectivity as measured by MAGI assay. MAGI cells (4×104 cells/well) were plated in a 24 well plate, and were treated in duplicate with a 100 μl supertanants from MDC that have been infected with HIV-1 IIIB virus and treated with or without Meth. A total of 200μl of DMEM supplemented with 10% FCS, 100 U/ml penicillin, 100μg/ml streptomycin, 0.25μg/ml fungizone and 300μg/ml glutamine containing DEAE-DEXTRAN at a concentration of 15mg/ml was added to the MAGI cells and infected cells were incubated for 3 days at 37°C, 5%CO2. Cells were fixed and stained with 5-bromo-4-chloro-3-indolyl-D-galactopyranoside (X-Gal) and blue cells were counted as infected cells. The graph represents the percentage of infected cells in each treatment group as observed using a 20X inverted Nikon microscope.
Figure 1C: Meth enhances p24 production. MDC (5×105 cells/ml) from normal subjects were infected with native HIV-1 IIIB (NIH AIDS Research and Reference Reagent Program Cat# 398) at a concentration of 10 3.0 TCID 50/ml cells overnight and washed 3 times with Hank's balanced salt solution (GIBCO-BRL, Grand Island, NY) before being returned to culture with and without Meth (50μM) for 14 days. The culture supernatants were quantitated for p24 antigen using a p24 ELISA kit (ZeptoMetrix Corporation, Buffalo, NY). The data represents the mean ± SD of 3 independent experiments and is expressed as pg/ml. Statistical analysis was done using Students' ‘t’-test.
We also measured HIV-1 infectivity using the MAGI (multinuclear activation of a galactosidase indicator) assay, which allows detection of HIV-1 after a single viral replication cycle in supernatant of infected cultures. The MAGI cells are HeLa derived cells stabily transfected with CD4 and a reporter construct consisting of the β-galactosidase gene (which is modified to localize to the nucleus) driven by a truncated HIV-1 LTR. Expression of the β-galactosidase gene is Tat-dependent such that an incoming virus must produce active Tat protein to drive expression of the reporter. Meth treated MDC cultures showed an increase in number of HIV-1 infected cells as represented by the increased number of blue cells with increasing concentrations of meth. Percentage of HIV-1 infected cells at 25, 50, and 100 μM concentrations of meth were 8% (p=0.02), 12% (p=0.01), and 21% (p=0.002), respectively as compared to the 3% in the HIV-1 infected meth untreated control (Figure 1B).
HIV-1 p24 antigen levels in the culture supernatants were quantitated using a p24 ELISA kit (ZeptoMetrix Corporation, Buffalo, NY) on day 15 post infection. The p24 antigen level in 50 μM meth treated HIV-1 infected MDC was 7600±3005 (p=0.01) compared to the untreated control (1100±520 pg/ml) (Figure 1C).
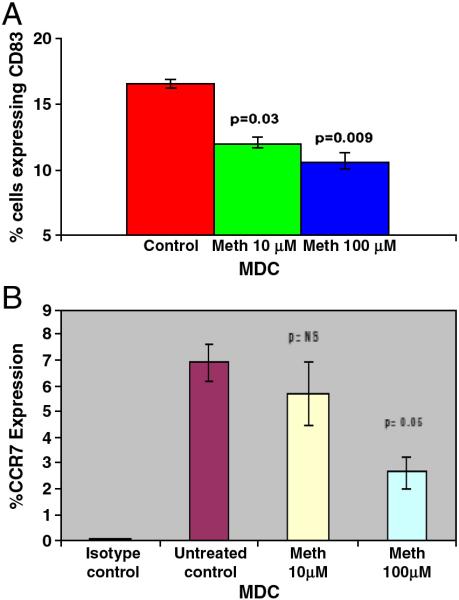
Meth decreases costimulatory molecule expression by dendritic cells
We hypothesized that meth increases HIV-1 infection, leading to downregulation of costimulatory molecules and chemokines, with a reciprocal upregulation of DC-SIGN and HIV coreceptors. Costimulatory molecule CD83 is a hallmark of MDC and plays an important role in antigen presentation, stimulation of T cells and the anti-HIV-1 immune response (Nair et al., 2007). The effect of meth on expression of costimulatory molecule CD83 by MDC was evaluated by flow cytometry analysis. Figure 2A shows that meth significantly downregulated the expression of CD83 on MDC. The percentage CD83 positive cells at meth concentration of 10 and 100μM, respectively were 11.3 (p<0.03) and 9.8% (p<0.009) compared to 16.9% in the untreated control culture. Since DC change their phenotypes and functional properties during maturation (Caux 1998; Yanagihara et al. 1998), we further investigated the expression of maturation marker CCR7 on MDC treated in vitro with meth. Our results indicate that meth at a concentration of 100μM significantly reduced the numbers of CCR7 positive MDC compared to untreated control culture (Figure 2B). These results are in agreement with our previous report showing that the number of MDC expressing costimulatory molecules is significantly lower in polydrug using HIV-1 infected patients compared to non-drug using HIV-1 infected subjects (Nair et al. 2005).
Figure 2.
Figure 2A: Meth decreases the expression of costimulatory molecule, CD83 on MDC. Normal MDC cultures (~ 5×105 cell/ml) were treated with Meth for 24 hr following which FACS analysis was done to determine the percentage of MDC expressing costimulatory molecule, CD83. Statistical significance was calculated by Students' ‘t’-test (n=3).
Figure 2B: Meth inhibits MDC maturation by decreasing the expression of CCR7. MDC (~ 5×105 cell/ml) were treated with Meth for 24 hr following which FACS analysis was done to determine the percentage of cells expressing CCR7. Statistical significance was calculated by Students' ‘t’-test (n=3).
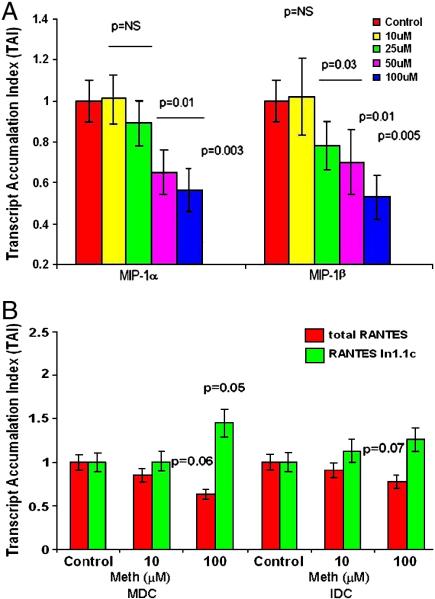
Meth decreases CCchemokine expression by dendritic cells
Chemokines have gained major attention because of their specific inhibitory effects on HIV-1 infection (Lusso 2002; Verani and Lusso 2002). CC-chemokines such as MIP-1α (CCL3), MIP-1β (CCL4) and RANTES (CCL5) are natural ligands for the HIV-1 coreceptor CCR5 and are known to block HIV-CCR5 interactions (Alkhatib et al. 1996). Decreased production of these chemokines may reverse their anti-HIV effects thereby facilitating HIV-1 infection. Further, drugs of abuse especially meth is known to affect the immune response thereby enhancing the HIV-1 infectivity of the DCs. Therefore, we evaluated the effects of Meth on gene expression of HIV-1 protective CC-chemokines, MIP-1α, and MIP-1β. Figure 3A shows a dose dependent decrease in MIP-1α and MIP-1β gene expression by DCs on treatment with meth (25-100 μM).
Figure 3.
Figure 3A: Meth down regulates MIP-1α and β gene expression in MDC. MDC (~ 5×105 cell/ml) were cultured with and without Meth (10-100 μM) for 24 hr, RNA was extracted and reverse transcribed followed by quantitative real time PCR for MIP-1α and MIP-1β and housekeeping genes (β-actin) primers. The data represents the mean ± SD of 3 independent experiments. Statistical significances between controls and treated samples was calculated by Students' ‘t’-test (n=3).
Figure 3B: Meth downregulates total RANTES and upregulates RANTES variant In1.1c gene expression in IDC and MDC. MDC and IDC from normal subjects (~ 5×105cell/ml) were cultured with Meth (10 & 100μM) for 24 hr, RNA was extracted and reverse transcribed followed by quantitative real time PCR for total RANTES and RANTES variant In1.1c and house keeping gene β-actin. The data represents the mean ± SD of 3 independent experiments. Statistical significance was calculated by Students' ‘t’-test (n=3).
Previous studies show that polymorphism in RANTES chemokines (RANTES variant In1.1c) has been associated with accelerated HIV-1 disease progression (Duggal et al. 2005). In our in vitro model using both IDCs and MDCs we examined the effect of meth on total RANTES and its variant, In1.1c gene expression. Data presented in Figure 3B indicates that meth significantly decreased total RANTES gene expression in a dose dependent manner, whereas RANTES variant In1.1c expression was significantly upregulated in a dose dependent manner by both IDC and MDC. This finding support our notion that increased HIV-disease progression in meth using subjects may be mediated through down regulation of these protective chemokines.
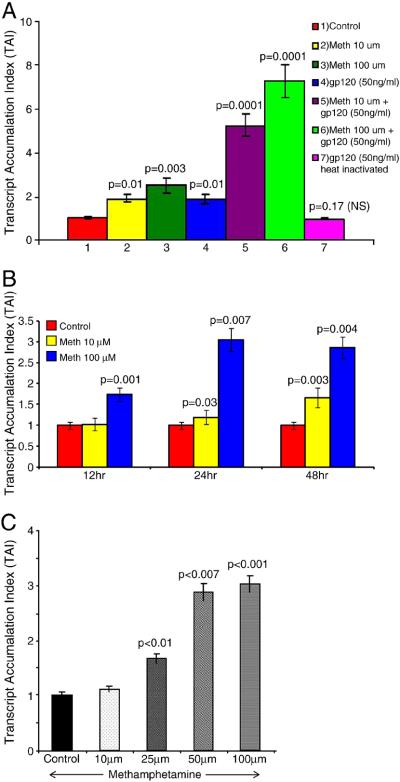
Effect of Meth and gp120 on DC-SIGN gene expression
Previously, we have shown that cocaine up-regulates DC-SIGN expression in MDCs (Nair et al. 2005). Since DC-SIGN facilitates HIV-1 infection independent of CD4 or viral coreceptors, an up-regulation in this receptor induced by cocaine or meth may facilitate virus dissemination.
Further, cocaine and morphine in synergy with HIV-1-derived proteins up-regulate various biological functions (Nair et al. 2000, 2005; Mahajan et al. 2005), we investigated whether meth in synergy with gp120 can up-regulate DC-SIGN gene expression. MDCs were treated with meth alone, gp120 alone, or in combination and DC-SIGN gene expression was determined by real-time Q-PCR. As shown in Figure 4A, MDCs cultured with meth at 10 (TAI = 1.9, p = 0.01) and 100 μM (TAI = 2.5, p = 0.003) produced significant up-regulation of DC-SIGN gene expression compared to the control culture (TAI = 1.0). MDCs cultured with 50 ng/ml of gp120 also produced a significant up-regulation of DC-SIGN gene expression (TAI = 1.9, p = 0.01) compared to the control culture (TAI = 1.0). However, when MDCs were cultured with 10 μM of meth and 50 ng/ml of gp120 in combination, DC-SIGN expression was significantly up-regulated (TAI = 5.2, p < 0.0001) compared to either meth alone (TAI = 1.9), or gp120 (TAI = 1.9) alone, or the numerical sum of meth- and gp120-treated cultures (TAI = 3.8). Similarly when meth at increased concentration of 100 μM and 50 ng/ml gp120 were used in combination, DC-SIGN expression was further significantly up-regulated (TAI = 7.2, p < 0.0001) compared to either meth alone (TAI = 2.5), or gp120 alone (TAI = 1.9) or the numerical sum of meth- and gp120-treated cultures (TAI = 4.4). Heat-inactivated gp120 (50 ng/ml; TAI = 0.9, p = 0.17) was not significantly different from control cultures. These data suggest that meth in synergy with gp120 up-regulates DC-SIGN expression. These further support the premise that HIV-1-infected individuals who are injecting drug users may undergo an accelerated rate of HIV-1 disease progression by up-regulating the CD4-independent virus attachment factor, DC-SIGN.
Figure 4.
Figure 4A: Meth in synergy with gp120 up-regulates DC-SIGN gene expression. MDCs were cultured for 24 hr with either meth (10 and 100 μM) or gp120 (50 ng/ml) or in combination and with heat inactivated (HI) gp120 (50 ng/ml). RNA was extracted, reverse transcribed, and QPCR amplified using DC-SIGN-specific primers. Data represent the mean ± SD of three independent experiments. Statistical analysis was performed using one-way ANOVA with Bonferroni multiple comparison posttests.
Figure 4B: Meth upregulates CXCR4 gene expression. MDC (5×105 cells/ml) were cultured with Meth (10 & 100μM) for 12-48 hr, RNA extracted, reverse transcribed and QPCR amplified using a CXCR4 specific primer. The data represent mean ± SD of 3 independent experiments. Statistical analysis was done using Student's ‘t’-test.
Figure 4C: Meth upregulates CCR5 gene expression. MDC (5×105 cells/ml) were cultured with Meth (10-100μM) for 24 hr, RNA extracted, reverse transcribed and QPCR amplified using a CCR5 specific primer. The data represent mean ± SD of 3 independent experiments. Statistical analysis was done using Student's ‘t’-test.
Effect of Meth on CXCR4 and CCR5 gene expression in MDC
Since meth facilitates HIV-1 infection of DCs, we further examined whether meth could modulate the expression of HIV-1 entry coreceptors. The kinetics and dose response effect of meth on CXCR4 and CCR5 expression was evaluated using a quantitative real time PCR. Granelli-Piperno et al. (1996) have shown that in purified dendritic cells, HIV-1 infectivity occurs via interaction with multiple chemokine co-receptors. Data presented in Figure 4B show a dose and time kinetics in MDC treated with meth (10, and 100 μM) at 12, 24 and 48 hr. At 12 hr, 24 and 48 hr after Meth treatment, the CXCR4 gene expression at 100μM was significantly increased as compared to the untreated control. Time kinetics demonstrate, that at 24 and 48 hrs the CXCR4 gene expression was higher compared to 12 hr. Data in Figure 4C show a dose dependent effect of Meth on CCR5 gene expression. Our results show that MDC treated with meth for 24 hr showed a significant dose dependent increase in the CCR5 gene expression at 10μM (TAI = 1.11, 11% increase), 25μM (TAI = 1.68, 68% increase), 50μM (TAI = 2.88, 188% increase), and 100μM (TAI = 3.03, 203% increase) compared to the untreated control (TAI=1.0).
Signal transduction mechanisms that mediate Meth induced effects on HIV-1 entry co-receptor expression
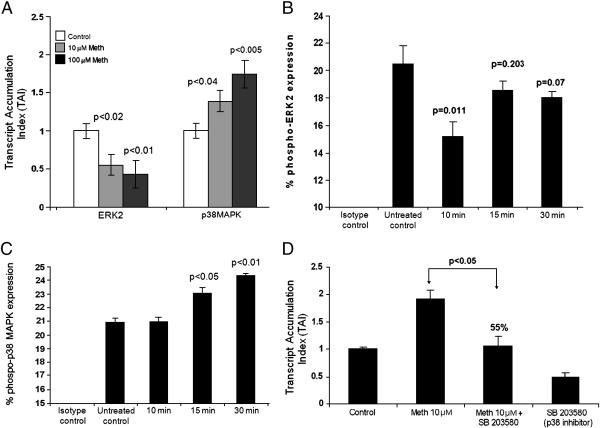
Previous studies implicate ERK/MAPK as the virion associated kinases which regulate HIV-1 infectivity (Yang and Gabuzda 1999; Jacque et al. 1998; Popik et al. 1998). Thus, we examined whether meth induced modulation of HIV-1 infectivity via coreceptor modulation are mediated via virion associated MAPK by real time quantitative PCR. Our results show that meth significantly decreased ERK2 gene expression at 10 (TAI=0.55, 45 % decrease, P=0.02) and 100μM (TAI=0.43, 57 % decrease, P=0.01) concentrations. However Meth significantly increased p38 gene expression at both 10 (TAI=1.38, 38 % increase, P=0.04) and 100μM (TAI=1.74, 74 % increase, P=0.005) (Figure 5A).
Figure 5.
Figure 5A: Meth differentially regulates signal transduction molecules in MDC. MDC (5×105 cells/ml) were cultured for 24 hr with and without Meth (10 & 100μM), RNA was extracted, reverse transcribed and QPCR amplified against various signal transduction molecules ERK2 and p38 MAPK. The data represent mean ± SD of 3 independent experiments. Statistical significance was determined by Student's ‘t’- test.
Figure 5B: Effect of Meth on the phosphorylation of ERK2. MDC (~ 5×105 cell/ml) were treated with Meth (100μM) for 10, 15, and 30 min following which FACS analysis was done to determine the percentage of cells expressing phosphorylated ERK2. Statistical significance was calculated by Student's ‘t’-test. The histogram is a graphical representation of 3 independent FACS experiments.
Figure 5C: Effect of Meth on the phosphorylation of p38 MAPK. MDC (~ 5×105 cell/ml) were treated with Meth (100μM) for 10, 15, and 30 min following which FACS analysis was done to determine the percentage of cells expressing phosphorylated p38 MAPK. Statistical significance was calculated by Student's ‘t’-test. The histogram is a graphical representation of 3 independent FACS experiments.
Figure 5D: p38 MAPK inhibitor reverses Meth induced upregulation of CCR5 gene expression. MDC (5×105cells/ml) were cultured alone or with Meth (10μM) or the p38 MAPK inhibitor (SB203580) (10μM) alone and in combination with Meth (10μM) plus inhibitor (SB203580) (10μM) for 24 hr and CCR5 specific mRNA expression was quantitated using real time PCR. The data represent mean ± SD of 2 independent experiments. Statistical analysis was done using ANOVA.
Further we examined the effect of Meth on phosphorylated form of ERK2 and p38 MAPK on MDC by flow cytometry analysis (Figure 5B and 5C). Results indicate that Meth significantly downregulated (15.16%, p<0.01) the phenotypic expression of ERK2 positive MDC at 10 min of meth treatment compared to untreated control culture (20.4%). Meth also significantly upregulated the percentage of MDC expressing phosphorylated form of p38 MAPK, (Figure 5C) at 15 (23.4%; p<0.05) and 30 mins (24.34 % p<0.01) of treatment as compared to the untreated control (20.8%). Thus FACS analysis confirms our gene expression results.
In order to confirm the role of p38 MAPK pathways in the modulation of meth induced effects, we used a p38 MAPK inhibitor SB203580 for its potential ability to reverse the meth induced upregulation of CCR5 co-receptor gene expression by MDC. In these experiments (Figure 5D) treatment of MDC with SB203580 significantly reversed meth induced upregulation of CCR5 gene expression (55%, p<0.05) compared to meth alone treated cultures. These results suggest that meth induced upregulation of CCR5 co-receptor may be mediated through the activation of p38 MAPK.
Role of Dopamine receptors in Meth modulation of HIV-1 infectivity
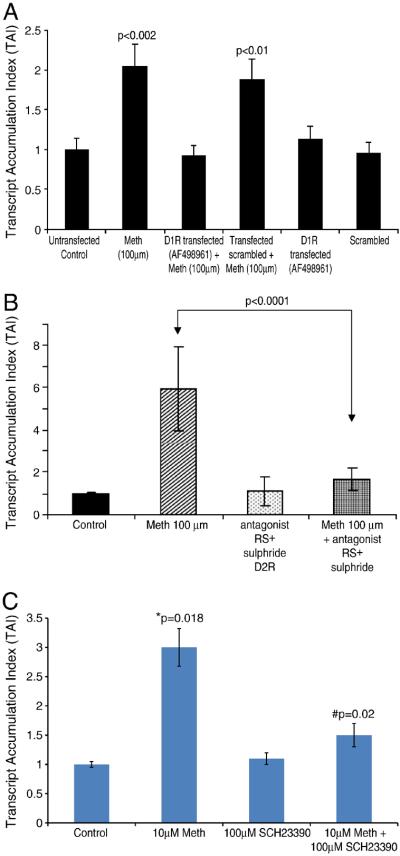
Meth is known to exert its effects through interaction with dopamine receptors on cells, inducing the release of neurotransmitters and by inhibiting their uptake resulting in the increase of extracellular dopamine (DA) concentrations (Granelli-Piperno et al. 1996). In order to examine whether Meth induced effects on HIV-1 infectivity was mediated through the dopamine receptor D1 and D2, we investigated the effect of siRNA directed against D1R and the use of a D2R antagonist for their potential effect to reverse meth induced upregulation of CCR5 gene expression. Data presented in Figure 6A shows that MDC treated with Meth (100μM) significantly upregulated CCR5 gene expression (Lane 2; TAI=2.03, p<0.002) compared to the untransfected control culture (Lane 1; TAI=1.0) or scrambled siRNA transfected culture (Lane 6; TAI=1.05). However MDC transfected with D1R specific siRNA and treated with Meth completely reversed Meth induced CCR5 gene upregulation (Lane 3; TAI=0.85; p<0.03) compared to non transfected Meth treated control (Lane 2, TAI=2.32). Data presented in Figure 6B show the effects of D2 receptor antagonist, RS± Sulphride on meth induced upregulation of CCR5. The results indicate that D2 receptor antagonist reversed meth induced upregulation of CCR5 gene. Thus studies with D1R and D2R antagonists suggest that both D1 and D2 receptors are involved in meth mediated effects on CCR5 gene expression. The reversal of the meth induced upregulation of the HIV-1 co-receptor CCR5 by both the D1 receptor antagonist and the D2 receptor antagonist suggest the specificity of the meth induced effects. The dopamine agonist SKF-82958 has previously shown to produce similar effects in a rat model, suggesting dopaminergic involvement (Munzar and Goldberg, 2000).
Figure 6.
Figure 6A: Effects of D1R specific siRNA on CCR5 gene expression in siRNA transfected MDC. MDCs were transfected with siRNA using lipofectamine reagent. Our results show that Meth treated D1R siRNA transfected cells showed a significant reduction in CCR5 gene expression indicating that the effect of Meth may be mediated via the D1 receptor. Data represents Mean ± SD of 2 separate experiments and statistical analysis was done using ANOVA.
Figure 6B: D2 receptor antagonist reverses Meth induced upregulation of CCR5 gene expression. MDC were cultured with and without the D2 receptor antagonist RS± sulphiride (100μM) alone and in combination with Meth (100μM) for 24 hr and the CCR5 specific mRNA expression was quantitated using real time PCR. The data represent mean ± SD of 3 independent experiments. Statistical analysis was done using Student's ‘t’-test.
Figure 6C: D1 receptor antagonist reverses meth-induced up-regulation of DC-SIGN gene expression. MDCs were cultured alone, with meth (10 μM), with the D1 receptor antagonist SCH23390 (100 μM), and in combination with meth (10 μM) plus antagonist (100 μM) for 24 hr, and DC-SIGN-specific mRNA expression was quantitated using real-time Q-PCR. Data represent the mean ± SD of three independent experiments. Statistical analyses were performed using one-way ANOVA with Bonferroni multiple comparison posttests. *Compared to control cultures, # compared to meth-treated cultures.
Further we evaluated the role of dopamine D1 receptor in meth mediated upregulation of DC-SIGN by MDCs. Data presented in Figure 6C show that MDC cultures treated with 10 μM meth alone significantly upregulated the expression of DC-SIGN-specific mRNA (TAI = 3.0, p = 0.018) as quantitated by real time Q-PCR compared to the control culture (TAI = 1.0). MDCs incubated with the dopamine D1 receptor antagonist, SCH23390 (100 μM) alone showed no effect on DC-SIGN gene expression (TAI = 1.1, p = ns) and was comparable to the control culture. However, in a mixing experiment when MDC were cultured with meth plus SCH23390, meth-induced up-regulation of DC-SIGN gene expression was reversed by SCH23390 (TAI = 1.5, p = 0.02) compared to meth-alone treated cultures (TAI = 3.0). This suggests that meth may mediate its effects on DC-SIGN through the dopamine D1 receptor.
Conclusions
These studies suggest that drugs of abuse, Meth enhances HIV-1 infectivity in human monocyte-derived DC and the mechanisms of these effects may be by upregulating DC-SIGN and HIV-1 coreceptors with reciprocal downregulation of co-stimulatory molecules and CC-chemokine. Additionally, studies suggest that both dopaminergic D1 and D2 are associated with Meth-induced modulation of HIV-1 infectivity and coreceptor modulation. These studies provide important information on molecular aspects of Meth and HIV-1 transmission and pathogenesis and may help to develop novel anti-HIV therapeutic strategies targeting DC-SIGN/HIV-1 coreceptors or costimulatory molecules.
Acknowledgements
This work was supported in part by National Institute on Drug Abuse Grants RO1-DA012366, RO1-DA025576, RO1-DA021537 and RO1-DA027049.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alkhatib G, Combadiere C, et al. CC CKR5: A RANTES, MIP-1α, MIP-1β receptor as a fusion cofactor for macrophage-tropic HIV- 1. Science. 1996;272:1955–1958. doi: 10.1126/science.272.5270.1955. [DOI] [PubMed] [Google Scholar]
- Blauvelt A, Asada HM, et al. Productive Infection of Dendritic Cells by HIV-1 and Their Ability to Capture Virus Are Mediated through Separate Pathways. Journal of Clinical Investigation. 1997;100:2043–2053. doi: 10.1172/JCI119737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boddiger D. Methamphetamine use linked to rising HIV transmission. Lancet. 2005;365:1217–1218. doi: 10.1016/S0140-6736(05)74794-2. [DOI] [PubMed] [Google Scholar]
- Caux C. Pathways of development of human dendritic cells. European Journal of Dermatology. 1998;8:375–384. [PubMed] [Google Scholar]
- Chang L, Ernst T, et al. Additive effects of HIV and chronic methamphetamine use on brain metabolite abnormalities. American Journal of Psychiatry. 2005;162(2):361–369. doi: 10.1176/appi.ajp.162.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duggal P, Winkler CA, et al. The effects of RANTES chemokine genetic variants on Early HIV-1 plasma RNA among African American injection drug users. Journal of Acquired Immune Deficiency Syndrome. 2005;38:584–589. doi: 10.1097/01.qai.0000134741.49208.03. [DOI] [PubMed] [Google Scholar]
- Frosch D, Shoptaw S, et al. Sexual HIV risk among gay and bisexual male methamphetamine abusers. Journal of Substance Abuse Treatment. 1996;13:483–486. doi: 10.1016/s0740-5472(96)00098-0. [DOI] [PubMed] [Google Scholar]
- Geijtenbeek TB, Engering A, van Kooyk Y. DC-SIGN, a C type lectin on DC that unveils many aspects of dendritic cell biology. Leukocyte Biology. 2002;71:921–931. [PubMed] [Google Scholar]
- Geijtenbeek TB, van Kooyk Y. DC-SIGN: a novel HIV receptor on DCs that mediates HIV-1 transmission. Current Topics in Microbiology and Immunology. 2003;276:31–54. doi: 10.1007/978-3-662-06508-2_2. [DOI] [PubMed] [Google Scholar]
- Gifford AN, Park MH, et al. Effect of amphetamine-induced dopamine release on radiotracer binding to D1 and D2 receptors in rat brain striatal slices. Naunyn Schmiedebergs Archives of Pharmacology. 2000;362(4–5):413–418. doi: 10.1007/s002100000293. [DOI] [PubMed] [Google Scholar]
- Granelli-Piperno A, Moser B, et al. Efficient interaction of HIV-1 with purified dendritic cells via multiple chemokine coreceptors. Journal of Experimental Medicine. 1996;184:2433–2438. doi: 10.1084/jem.184.6.2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart DN. Dendritic cells: unique leukocyte populations which controls the primary immune response. Blood. 1997;90:3245–3287. [PubMed] [Google Scholar]
- Hirshfield S, Remien RH, et al. Substance use and high-risk sex among men who have sex with men: a national online study in the USA. AIDS Care. 2004;16:1036–1047. doi: 10.1080/09540120412331292525. [DOI] [PubMed] [Google Scholar]
- Jacque JM, Mann A, et al. Modulation of HIV-1 infectivity by MAPK, a virion-associated kinase. EMBO Journal. 1998;17:2607–2618. doi: 10.1093/emboj/17.9.2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langhoff E, Terwilliger EF, et al. Replication of human immunodeficiency virus type 1 in primary dendritic cell cultures. Proceeding of National Academy of Science USA. 1991;88:7998–8002. doi: 10.1073/pnas.88.18.7998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang H, Wang X, et al. Methamphetamine enhances HIV infection of macrophages. American Journal of Pathology. 2008;172(6):1617–1624. doi: 10.2353/ajpath.2008.070971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lusso P. HIV and chemokines: implications for therapy and vaccine. Vaccine. 2002;20:1964–1967. doi: 10.1016/s0264-410x(02)00079-8. [DOI] [PubMed] [Google Scholar]
- Macatonia SE, Patterson S, Knight SC. Suppression of immune responses by dendritic cells infected with HIV. Immunology. 1989;67:285–289. [PMC free article] [PubMed] [Google Scholar]
- Mahajan SD, Aalinkeel R, et al. Morphine exacerbates HIV-1 viral protein gp120 induced modulation of chemokine gene expression in U373 astrocytoma cells. Current HIV Research. 2005;3(3):277–288. doi: 10.2174/1570162054368048. [DOI] [PubMed] [Google Scholar]
- Munzar P, Goldberg SR. Dopaminergic involvement in the discriminative-stimulus effects of methamphetamine in rats. Psychopharmacology (Berl) 2000;148:209–216. doi: 10.1007/s002130050044. [DOI] [PubMed] [Google Scholar]
- Nair MPN, Mahajan S, Schwartz SA. Immunomodulatory roles of heroin and HIV protein on nitric oxide production by brain microvascular endothelial cells. NIDA Research Monograph. 2000;181:85–86. [Google Scholar]
- Nair MP, Mahajan S, et al. Cocaine modulates dendritic cell-specific C type intercellular adhesion molecule-3-grabbing nonintegrin expression by dendritic cells in HIV-1 patients. Journal of Immunology. 2005;174:6617–6626. doi: 10.4049/jimmunol.174.11.6617. [DOI] [PubMed] [Google Scholar]
- Nair MPN, Mahajan S, et al. Methamphetamine modulates DC-SIGN expression by mature dendritic cells. Journal of Neuroimmune Pharmacology. 2006;1:296–304. doi: 10.1007/s11481-006-9027-1. [DOI] [PubMed] [Google Scholar]
- Nair MPN, Rodriguez JW, et al. Methamphetamine inhibits β-chemokines and co-stimulatory molecule expression by dendritic cells. American Journal of Infectious Diseases. 2007;3(4):217–224. [Google Scholar]
- Nair MPN, Saiyed ZM, et al. Methamphetamine enhances HIV-1 infectivity in monocyte derived dendritic cells. Journal of Neuroimmune Pharmacolgy. 2009;4:129–139. doi: 10.1007/s11481-008-9128-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nath A, Maragos WF, et al. Acceleration of HIV dementia with methamphetamine and cocaine. Journal of Neurovirology. 2001;7(1):66–71. doi: 10.1080/135502801300069737. [DOI] [PubMed] [Google Scholar]
- National Drug Threat Assessment (NDTA) 2008 Methamphetamine. 2007 October; http://www.usdoj.gov/ndic/pubs25/25921/meth.htm#Top.
- National Survey on Drug Use and Health (NSDUH) 2003 https://nsduhweb.rti.org.
- NIDA (National Institute of Drug abuse) INFO FACTS Methamphetamine . National Institute of Drug Abuse; Baltimore: May, 2005. [Google Scholar]
- Office of National Drug Control Policy (ONDCP) Drug facts Methamphetamine. 2007 http://www.whitehousedrugpolicy.gov/drugfact/methamphetamine/index.html#go3.
- Pope M, Betjes MGH, et al. Conjugates of dendritic cells and memory T lymphocytes from skin facilitate productive infection with HIV-1. Cell. 1994;78:389–398. doi: 10.1016/0092-8674(94)90418-9. [DOI] [PubMed] [Google Scholar]
- Popik W, Hesselgesser E, Pitha PM. Binding of human immunodeficiency virus type 1 to CD4 and CXCR4 receptors differentially regulates expression of inflammatory genes and activates the MEK/ERK signaling pathway. Journal of Virology. 1998;72:6406–6413. doi: 10.1128/jvi.72.8.6406-6413.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubbert A, Combadiere C, et al. Dendritic cells express multiple chemokine receptors used as coreceptors for HIV entry. Journal of Immunology. 1998;160:3933–3941. [PubMed] [Google Scholar]
- Sibley DR, Monsma FJ., Jr Shen Y. Molecular neurobiology of dopaminergic receptors. International Review of Neurobiology. 1993;35:391–415. doi: 10.1016/s0074-7742(08)60573-5. [DOI] [PubMed] [Google Scholar]
- Smed-Sorensen A, Lore AK, et al. Differential susceptibility to human immunodeficiency virus type 1 infection of myeloid and plasmacytoid dendritic cells. Journal of Virology. 2005;79:8861–8869. doi: 10.1128/JVI.79.14.8861-8869.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman RM, Germain RN. Antigen presentation and related immunological aspects of HIV-1 vaccines. AIDS 12S A. 1998:S97–112. [PubMed] [Google Scholar]
- Urbina A, Jones K. Crystal methamphetamine, its analogues, and HIV infection: medical and psychiatric aspects of a new epidemic. Clinical Infectious Diseases. 2004;38:890–894. doi: 10.1086/381975. [DOI] [PubMed] [Google Scholar]
- Verani A, Lusso P. Chemokines as natural HIV antagonists. Current Molecular Medicine. 2002;2:691–702. doi: 10.2174/1566524023361862. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Chang L, et al. Association of dopamine transporter reduction with psychomotor impairment in methamphetamine abusers. American Journal of Psychiatry. 2001a;158(3):377–382. doi: 10.1176/appi.ajp.158.3.377. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Chang L, et al. Low level of brain dopamine D2 receptors in methamphetamine abusers: association with metabolism in the orbitofrontal cortex. American Journal of Psychiatry. 2001b;158(12):2015–2021. doi: 10.1176/appi.ajp.158.12.2015. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Chang L, et al. Loss of dopamine transporters in methamphetamine abusers recovers with protracted abstinence. Journal of Neuroscience. 2001c;21(23):9414–9418. doi: 10.1523/JNEUROSCI.21-23-09414.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman D, Li Y, Orenstein JM, Fauci AS. Both a precursor and a mature population of dendritic cells can bind HIV. However, only the mature population that expresses CD80 can pass infection to unstimulated CD4+ T cells. Journal of Immunology. 1995;155:4111–4117. [PubMed] [Google Scholar]
- Wilflingseder D, Müllauer B, et al. HIV-1-induced migration of monocyte-derived dendritic cells is associated with differential activation of MAPK pathways. Journal of Immunology. 2004;173:7497–7505. doi: 10.4049/jimmunol.173.12.7497. [DOI] [PubMed] [Google Scholar]
- Wu L, KewalRamani VN. Dendritic-cell interactions with HIV: infection and viral dissemination. Nature Review. 2006;6:859–867. doi: 10.1038/nri1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagihara S, Komura E, et al. EBI1/CCR7 is a new member of dendritic cell chemokine receptor that is up-regulated upon maturation. Journal of Immunology. 1998;161:3096–3102. [PubMed] [Google Scholar]
- Yang X, Gabuzda D. Regulation of human immunodeficiency virus type 1 infectivity by the ERK mitogen-activated protein kinase signaling pathway. Journal of Virology. 1999;73:3460–3466. doi: 10.1128/jvi.73.4.3460-3466.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Q, Larson DF, Watson RR. Heart disease, methamphetamine and AIDS. Life Sciences. 2003;73(2):129–140. doi: 10.1016/s0024-3205(03)00260-1. [DOI] [PubMed] [Google Scholar]