Abstract
Background
Evidence suggests that increases in synaptic serotonin (5-HT) can reduce the stimulant properties of amphetamine-type drugs. Here we tested the hypothesis that administration of the 5-HT precursor 5-hydroxy-L-tryptophan (5-HTP), along with the peripheral decarboxylase inhibitor benserazide, would decrease locomotor effects of (+)-amphetamine.
Methods
Drug treatments were administered to conscious male rats undergoing in vivo microdialysis in nucleus accumbens. During dialysis sampling, rats were housed in chambers equipped with photobeams to detect forward locomotion (i.e., ambulation) and repetitive movements (i.e., stereotypy). Extracellular concentrations of dopamine (DA) and 5-HT were measured by high-pressure liquid chromatography with electrochemical detection.
Results
5-HTP (10 & 30 mg/kg, i.p.) plus benserazide (30 mg/kg, i.p.) caused dose-related increases in 5-HT but failed to alter other parameters. (+)-Amphetamine (0.3 & 1.0 mg/kg, i.p.) produced dose-related increases in DA, ambulation and stereotypy. Combined administration of 5-HTP and (+)-amphetamine evoked large elevations in extracellular DA and 5-HT, but caused significantly less ambulation than (+)-amphetamine alone (~50% reduction).
Conclusions
Our results confirm that 5-HTP can decrease hyperactivity produced by (+)-amphetamine, even in the presence of elevations in dialysate DA. The data suggest that 5-HTP and (+)-amphetamine may be useful to broadly enhance monoamine function in the clinical setting, while reducing undesirable effects of (+)-amphetamine.
Keywords: amphetamine, 5-hydroxy-L-tryptophan, cocaine, drug addiction, dopamine, serotonin
1. Introduction
Various stimulant medications are established treatments for attention-deficit hyperactivity disorder (Brown et al., 2005), and emerging evidence indicates that (+)-amphetamine may be an effective agonist medication for cocaine dependence (reviewed in (Herin et al., 2010)). In a representative clinical study, cocaine-dependent research participants given sustained-release (+)-amphetamine displayed less cocaine misuse and better retention in treatment when compared to placebo-treated participants (Grabowski et al., 2001). Consistent with the human data, experiments in rhesus monkeys demonstrate that chronic administration of (+)-amphetamine can produce persistent reductions in cocaine self-administration without substantially altering food-reinforced behavior (Negus and Mello, 2003). Taken together, these findings suggest that more studies are warranted to test the efficacy of (+)-amphetamine and other stimulants in treating cocaine dependence.
The high abuse liability of (+)-amphetamine represents a significant concern when prescribing this medication for cocaine-dependent patients (Grabowski et al., 2004). At the molecular level, (+)-amphetamine serves as a substrate for monoamine transporters, especially those expressed on dopamine (DA) and norepinephrine (NE) neurons, thereby causing non-exocytotic release of monoamine transmitters into the synaptic cleft (Fleckenstein et al., 2007; Rothman and Baumann, 2003). In vivo microdialysis studies in rats confirm that administration of (+)-amphetamine causes large elevations in extracellular DA and NE in various brain regions (Berridge and Stalnaker, 2002; Kuczenski et al., 1995). Importantly, the reinforcing properties of amphetamine in animals and humans are thought to involve the release of DA from mesolimbic nerve terminals in the brain (Ikemoto, 2007; Koob and Volkow, 2010).
We have advocated the use of dual DA/serotonin (5-HT) releasing agents as treatments for cocaine dependence (Rothman et al., 2008; Rothman et al., 2005). From a therapeutic standpoint, the addition of a serotonergic component to the catecholamine-releasing properties of (+)-amphetamine would serve two purposes. First, increases in extracellular 5-HT would help to alleviate deficits in central serotonergic function found in cocaine-dependent patients (Ghitza et al., 2007; Haney et al., 2001). Second, increases in extracellular 5-HT would be predicted to reduce stimulant side-effects of (+)-amphetamine (reviewed in (Rothman et al., 2008)). It is well known that 5-HT releasers like fenfluramine can antagonize locomotor actions of amphetamine-type drugs in rodents (Baumann et al., 2000; Bendotti et al., 1980). Wee and Woolverton (Wee and Woolverton, 2006) examined the self-administration of fenfluramine/(+)-amphetamine mixtures in rhesus monkeys and found that increasing the relative amount of fenfluramine (i.e., increasing 5-HT release) markedly decreased the reinforcing potency of the mixture. Such studies support the idea that elevations in synaptic 5-HT can reduce the locomotor and reinforcing effects of (+)-amphetamine.
At the present time, no 5-HT releasers are clinically available since fenfluramine and (+)-fenfluramine have been removed from the market due to adverse side-effects (Sachdev et al., 2002). A safe alternative strategy for producing elevations in synaptic 5-HT and DA might be to administer a 5-HT precursor, like 5-hydroxy-L-tryptophan (5-HTP), in combination with (+)-amphetamine (Rothman and Baumann, 2009). 5-HTP is a commercially-available dietary supplement, and its administration to rats causes increases in brain extracellular 5-HT (Gartside et al., 1992; Perry and Fuller, 1993). In the present study, we tested the hypothesis that 5-HTP would reduce motor stimulant properties of (+)-amphetamine. Drug treatments were administered to conscious rats undergoing in vivo microdialysis in nucleus accumbens. The peripheral decarboxylase inhibitor, benserazide, was administered prior to 5-HTP to facilitate synthesis of 5-HT in the brain (Halladay et al., 2006). Rats were housed in chambers equipped with photobeams to measure motor activity, which allowed concurrent determination of neurochemistry and behavior during the experiments (Zolkowska et al., 2009).
2. Methods
2.1. Animals and surgery
Male Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA, USA) weighing 300–350 g were double-housed with food and water freely available. Rats were maintained in facilities accredited by the Association for Assessment and Accreditation of Laboratory Animal Care, and procedures were carried out in accordance with the Animal Care and Use Committee of the National Institute on Drug Abuse (NIDA), Intramural Research Program (IRP). After two weeks of acclimation to the vivarium conditions, rats were brought to the surgical facility and anesthetized with sodium pentobarbital (60 mg/kg, i.p.). Indwelling jugular catheters and intracerebral guide cannulae (CMA/12, CMA/Microdialysis, Acton, MA, USA) were surgically implanted as described previously (Zolkowska et al., 2009). Guide cannulae were aimed at the nucleus accumbens according to the coordinates: 1.7 mm lateral and 1.6 mm anterior to bregma, and 6.0 mm ventral to dura. Rats were single-housed postoperatively and allowed at least one week to recover before being used in microdialysis experiments.
2.2. Microdialysis and motor activity
The assessment of in vivo neurochemistry and motor activity was carried out using published methods (Baumann et al., 2008). Briefly, on the evening before microdialysis testing, rats were brought from the vivarium area into the laboratory. Extension tubes were connected to catheters, and microdialysis probes (2 × 0.5 mm, CMA/12, CMA/Microdialysis) were carefully inserted into guide cannulae. Each rat was attached to a tether and placed into a Plexiglas arena equipped with photobeams that allowed movements to be quantified (TruScan; Coulborn Instruments, Allentown, PA, USA). Probes were perfused with Ringers’ solution at 0.6 µl/min overnight. On the next morning, dialysate samples were collected at 20-min intervals then assayed for DA and 5-HT by microbore high-pressure liquid chromatography with electrochemical detection (HPLC-ECD). After collection of three baseline samples, drug or vehicle treatments were administered as described below. Motor activity was monitored throughout the dialysis sampling; ambulation (i.e., forward locomotion) and stereotypy (i.e., repetitive movements) were quantified separately in 20-min bins.
2.3. Reagents and drug treatments
Chemicals and reagents for microdialysis were purchased from Sigma-Aldrich Chemical Company (St. Louis, MO). (+)-Amphetamine ((+)-amphetamine sulfate, FW 368.5) was provided by the NIDA Drug Supply Program (Rockville, MD), whereas 5-HTP (5-hydroxy-L-tryptophan, FW 220.2) and benserazide (DL-Serine 2-(2,3,4-trihydroxybenzyl)hydrazine HCl, FW 293.7) were purchased from Sigma-Aldrich. Drug solutions were prepared in 0.9% NaCl (saline) just before use. Doses are expressed as the salt, and drugs were administered in a volume of 1 ml/kg. Two types of experiments were performed: dose-response and drug combination experiments. For dose-response studies, one group of rats received i.p. injections of 5-HTP (10 & 30 mg/kg) or saline at time zero; these rats were pretreated with i.p. injections of 30 mg/kg benserazide 30 min prior to 5-HTP or saline (Halladay et al., 2006). Another group of rats received single i.p. injections of (+)-amphetamine (0.3 & 1.0 mg/kg.) or saline at time zero. For the drug combination studies, rats received i.p. 5-HTP (30 mg/kg) or saline at time zero, followed by i.p. (+)-amphetamine (1 mg/kg) or saline 40 min later. As in the dose-response studies, rats given 5-HTP were pretreated with i.p. benserazide (30 mg/kg) beforehand. Dialysate samples and behavioral assessments were collected for 2 to 3 h after time zero injections, depending upon the experiment.
2.4. Data analysis
Data were analyzed using commercially available software (Graph-Pad Prism, San Diego, CA). All data are presented as mean ± SEM for N=6–7 rats/group. Neurotransmitter data are expressed as pg amounts per 5 µl sample. Ambulation data are expressed as cm traveled per 20 min, while stereotypy data are expressed as number of repetitive events per 20 min. Raw neurochemical and locomotor data were evaluated using two-factor (treatment × time) analysis of variance (ANOVA). When significant main effects were noted, one-factor ANOVAs were run at each time point and Newman-Keuls test was used to identify differences between group means.
3. Results
3.1 Dose-response experiments
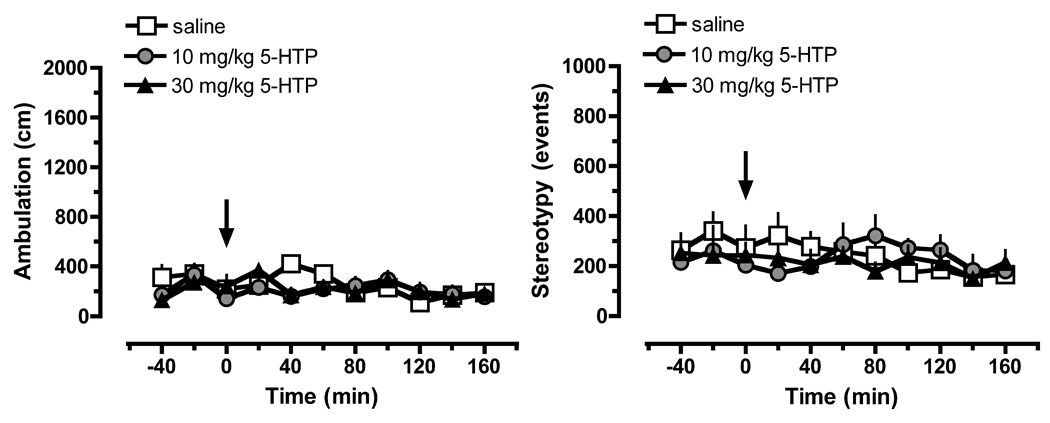
The data in Figure 1 show that administration of 5-HTP/benserazide had no effect on dialysate DA concentrations (F[2,20]=0.1061; NS) but significantly increased dialysate 5-HT (F[2,20]=62.83]; P<0.0001). Post hoc analysis revealed that 30 mg/kg 5-HTP evoked a delayed rise in 5-HT which reached significance from 60 to 160 min after injection, with a maximal effect of 11-fold above saline control values at 100 min. 5-HTP did not alter motor parameters, as depicted in Figure 2.
Figure 1.
Dose-response effects of 5-HTP on extracellular concentrations of DA (left panel) and 5-HT (right panel) in rat nucleus accumbens. Rats undergoing in vivo microdialysis received benserazide pretreatment (30 mg/kg, i.p.) 30 min prior to i.p. injection of 5-HTP or saline at time zero. Data are mean ± SEM expressed as pg/ 5 µl sample for N=7 rats/group. * P<0.05 with respect to saline-treated control at the corresponding time point.
Figure 2.
Dose-response effects of 5-HTP on ambulation (left panel) and stereotypy (right panel) in rats undergoing in vivo microdialysis. Rats received benserazide pretreatment (30 mg/kg, i.p.) 30 min prior to i.p. injection of 5-HTP or saline at time zero. Data are mean ± SEM expressed as cm traveled for ambulation, and number of repetitive events for stereotypy; N=7 rats/group.
The data in Figure 3 show that (+)-amphetamine caused significant dose-related elevations in dialysate concentrations of DA (F[2,20]=48.14; P<0.0001) and 5-HT (F[2,20]=28.24; P<0.0001), but effects on DA were predominant. Specifically, (+)-amphetamine was more potent at releasing DA when compared to 5-HT, since the 0.3 mg/kg dose increased DA only. Moreover, the 1 mg/kg dose produced a much greater magnitude of elevation in dialysate DA (i.e., 22-fold) versus 5-HT (i.e., 3-fold). As illustrated in Figure 4, administration of (+)-amphetamine significantly stimulated ambulation (F[2,20]=61.35; P<0.0001) and stereotypy (F[2,20]=33.88; P<0.0001), with the 1 mg/kg dose increasing both motor parameters from 40 to 120 min post-injection. In general, peak behavioral effects of (+)-amphetamine occurred at about the same time as the peak neurochemical effects.
Figure 3.
Dose-response effects of (+)-amphetamine (AMPH) on extracellular concentrations of DA (left panel) and 5-HT (right panel) in rat nucleus accumbens. Rats undergoing in vivo microdialysis received i.p. injection of AMPH or saline at time zero. Data are mean ± SEM expressed as pg/5 µl sample for N=7 rats/group. * P<0.05 with respect to saline-treated control at the corresponding time point.
Figure 4.
Dose-response effects of (+)-amphetamine (AMPH) on ambulation (left panel) and stereotypy (right panel) in rats undergoing in vivo microdialysis. Rats received AMPH or saline at time zero. Data are mean ± SEM expressed as cm traveled for ambulation, and number of repetitive events for stereotypy; N=7 rats/group. * P<0.05 with respect to saline-treated control at the corresponding time point.
3.2 Drug combination experiments
In the drug combination study, 5-HTP (30 mg/kg) was administered 40 min prior to (+)-amphetamine (1 mg/kg) so that peak neurochemical effects of both treatments would occur simultaneously (see Results 3.1 above). For the time course data depicted in Figures 5 and 6, the injection of 5-HTP or saline pretreatment is considered time zero. Figure 5 shows that treatment condition significantly influenced extracellular DA (F[3,33]=79.30, P<0.0001) and 5-HT (F[3,33]=64.63, P<0.0001). With regard to dopaminergic effects, (+)-amphetamine caused significant increases in dialysate DA from 80 to 160 min, with a maximal rise of 21-fold above corresponding saline control values. Rats receiving 5-HTP and (+)-amphetamine displayed a similar profile of DA elevations, but the amount of dialysate DA was significantly greater than (+)-amphetamine alone at 140 and 160 min. For serotonergic effects, 5-HTP increased dialysate 5-HT concentrations up to 7-fold higher than saline control values, while rats receiving the combination of 5-HTP and (+)-amphetamine displayed a markedly enhanced 5-HT response that reached 32-fold greater than saline control. Post hoc analysis revealed that the enhancement of dialysate 5-HT levels produced by the combination treatment was significantly greater than 5-HTP alone from 60 to 160 min.
Figure 5.
Effects of 5-HTP and (+)-amphetamine (AMPH), administered alone or together, on extracellular concentrations of DA (left panel) and 5-HT (right panel) in rat nucleus accumbens. Rats received 5-HTP (30 mg/kg, i.p.) or saline pretreatment at time zero, followed by AMPH (1 mg/kg, i.p.) or saline 40 min later. All rats pretreated with 5-HTP were given benserazide (30 mg/kg, i.p.) at −30 min. Data are mean ± SEM for N=7 rats/group. * P<0.05 with respect to saline/AMPH group at the corresponding time point; # P<0.05 with respect to 5-HTP/saline group at the corresponding time point.
Figure 6.
Effects of 5-HTP and (+)-amphetamine (AMPH), administered alone or together, on ambulation (left panel) and stereotypy (right panel) in rats undergoing in vivo microdialysis. Rats received 5-HTP (30 mg/kg, i.p.) or saline pretreatment at time zero, followed by AMPH (1 mg/kg, i.p.) or saline 40 min later. All rats pretreated with 5-HTP were given benserazide (30 mg/kg, i.p.) at −30 min. Data are mean ± SEM for N=7 rats/group. * P<0.05 with respect to saline/AMPH group at the corresponding time point.
The data in Figure 6 show that drug treatment significantly affected ambulation (F[3,33]=38.81, P<0.0001) and stereotypy (F[3,33]=97.85, P<0.0001). In the case of ambulation, (+)-amphetamine increased activity up to 18-fold greater than corresponding saline control values. Rats receiving 5-HTP and (+)-amphetamine exhibited increases in ambulation, but post hoc analysis demonstrated that the degree of motor stimulation was significantly less than the corresponding (+)-amphetamine group, reaching a maximum of only 8-fold above saline control. With regard to effects on stereotypy, (+)-amphetamine caused sustained elevations in repetitive movements that reached 4-fold above saline control values. Rats receiving the drug combination displayed similar increases in stereotypy, where the magnitude and time course of effects was not significantly different from that elicited by (+)-amphetamine. It is noteworthy that rats receiving 5-HTP/amphetamine did not display signs of serotonin behavioral syndrome, despite large elevations in extracellular 5-HT.
4. Discussion
The aim of the present study was to determine whether 5-HT precursor loading with 5-HTP could alter neurochemical and behavioral effects of (+)-amphetamine. We found that administration of 5-HTP, after benserazide pretreatment, produced increases in extracellular 5-HT but no change in DA, in agreement with previous findings (Halladay et al., 2006). 5-HTP failed to alter ambulation or stereotypy, suggesting that elevation of synaptic 5-HT alone is not sufficient to stimulate forward locomotion (Baumann et al., 2000; Bendotti et al., 1980). Administration of (+)-amphetamine produced increases in extracellular DA and motor activity, with a much smaller rise in 5-HT. Our data with (+)-amphetamine are consistent with many microdialysis reports demonstrating a role for extracellular DA in mediating stimulant-induced motor stimulation (Sharp et al., 1987; Kuczenski et al., 1995; Rothman et al., 2005). Combined administration of 5-HTP and (+)-amphetamine caused marked elevations in extracellular concentrations of 5-HT and DA, but engendered significantly less forward locomotion than (+)-amphetamine alone. Thus, we provide support for the hypothesis that elevations in brain extracellular 5-HT are associated with reductions in DA-mediated properties of drugs like amphetamine (reviewed in (Rothman et al., 2008)).
Neurochemical effects of the 5-HTP/(+)-amphetamine combination deserve comment. In particular, the drug combination produced greater effects on extracellular 5-HT and DA than the additive actions of each drug alone, and the apparent synergism was most pronounced for 5-HT. For example, 5-HTP evoked a 7-fold rise in dialysate 5-HT whereas the drug combination evoked elevations that reached 30-fold greater than baseline. It is known that 5-HTP administration can lead to the synthesis of 5-HT in dopaminergic cells in rat brain (Arai et al., 1995; Lynn-Bullock et al., 2004). Thus, 5-HTP which is converted to 5-HT in DA nerve terminals may be subsequently released into the extracellular space by (+)-amphetamine via a transporter-dependent mechanism. Consistent with this idea, we have observed similar, albeit less pronounced, synergistic increases in extracellular 5-HT with 5-HTP/phentermine combinations in previous studies (Halladay et al., 2006). It is likely that the elevations of 5-HT produced by 5-HTP and (+)-amphetamine in this study are larger than what is necessary to reduce the stimulant properties of (+)-amphetamine. Future studies should be carried out examine the effects of 5-HTP/ (+)-amphetamine combinations at lower doses in rats.
The mechanisms responsible for the ability of 5-HT to decrease motor effects of (+)-amphetamine are not known, but previous research has shown that the magnitude of drug-induced transmitter response (e.g., % increase above baseline) for DA vs. 5-HT is critical (reviewed in (Rothman et al., 2008)). More specifically, the % increase in extracellular 5-HT must exceed that of extracellular DA to observe antagonism of DA-mediated behavioral effects. The data presented in Figures 5 and 6 serve to illustrate this point. (+)-Amphetamine increased DA 20-fold above baseline while 5-HT rose only 2-fold. The combination of 5-HTP/(+)-amphetamine, on the other hand, increased DA 20-fold and 5-HT 30-fold above baseline; when the extent of 5-HT elevation exceeded that of DA, forward locomotion was decreased. The 5-HT receptor subtypes which might be involved with antagonism of amphetamine-induced motor actions are not known, but 5-HT2C receptors are likely candidates (Alex and Pehek, 2007; Bubar and Cunningham, 2006). It is well known that 5-HT2C sites exert an inhibitory effect on DA transmission and reduce motor activity produced by stimulant drugs (Di Matteo et al., 2001; Di Matteo et al., 2000; Fletcher et al., 2004). Moreover, the data from our study indicate that at least some inhibitory 5-HT receptor mechanisms must be downstream from DA neurons (i.e., the receptors involved are not located on DA neurons) because 5-HT antagonized forward locomotion even in the presence of large elevations in extracellular DA. Careful investigations with receptor-selective antagonists will be required to determine the role of specific 5-HT subtypes in this phenomenon.
Although it is difficult to extrapolate our data to the clinical situation, it seems possible that combined administration of 5-HTP and (+)-amphetamine might be a safe approach to broadly enhance monoamine function in humans, while limiting stimulant effects of amphetamine. We have previously suggested using (+)-amphetamine and 5-HTP, in conjunction with the aromatic L-amino acid decarboxylase inhibitor carbidopa, as a treatment for patients with stimulant dependence (Rothman et al., 2005). The available human data indicate that this combination should be safe, including recent reports that the combination of phentermine plus 5-HTP/carbidopa is being used as an efficacious treatment for obesity (Hendricks et al., 2009; Rothman, 2009). 5-HTP has not been associated with dangerous side effects or toxicity including the eosinophilic myalgia syndrome and the serotonin syndrome (Das et al., 2004; Turner et al., 2006). Although these are all clinically prescribed medications, the combination is novel and further studies in animals and appropriate safety studies are needed. As noted in the Introduction, (+)-amphetamine has shown promise as a medication for treating stimulant addiction and has been administered safely in this patient population (Grabowski et al., 2004). 5-HTP is available for human use as an over-the-counter supplement and has been administered with and without carbidopa for the treatment of depression, obesity and insomnia (Amer et al., 2004; Cangiano et al., 1992; Cangiano et al., 1998; Turner et al., 2006; Zmilacher et al., 1988). Carbidopa, like benserazide, selectively blocks the conversion of 5-HTP to 5-HT in the periphery, but not in the brain. It is noteworthy that carbidopa has been used safely in stimulant addicts, when administered as a component in L-DOPA/carbidopa (Mooney et al., 2007).
In summary, this is the first report to demonstrate that precursor loading with 5-HTP can reduce motor stimulant properties of (+)-amphetamine, without altering the DA-releasing effect of the drug. Further studies are required to determine the precise molecular underpinnings of this phenomenon. Our data from rats support the evaluation of (+)-amphetamine and 5-HTP/carbidopa in preclinical models of addiction, such as conditioned place preference assays, as well in clinical trials after appropriate safety studies are carried out.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alex KD, Pehek EA. Pharmacologic mechanisms of serotonergic regulation of dopamine neurotransmission. Pharmacol Ther. 2007;113:296–320. doi: 10.1016/j.pharmthera.2006.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amer A, Breu J, McDermott J, Wurtman RJ, Maher TJ. 5-Hydroxy-L-tryptophan suppresses food intake in food-deprived and stressed rats. Pharmacol Biochem Behav. 2004;77:137–143. doi: 10.1016/j.pbb.2003.10.011. [DOI] [PubMed] [Google Scholar]
- Arai R, Karasawa N, Nagatsu T, Nagatsu I. Exogenous L-5-hydroxytryptophan is decarboxylated in neurons of the substantia nigra pars compacta and locus coeruleus of the rat. Brain Res. 1995;669:145–149. doi: 10.1016/0006-8993(94)01259-k. [DOI] [PubMed] [Google Scholar]
- Baumann MH, Ayestas MA, Dersch CM, Brockington A, Rice KC, Rothman RB. Effects of phentermine and fenfluramine on extracellular dopamine and serotonin in rat nucleus accumbens: therapeutic implications. Synapse. 2000;36:102–113. doi: 10.1002/(SICI)1098-2396(200005)36:2<102::AID-SYN3>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Baumann MH, Clark RD, Rothman RB. Locomotor stimulation produced by 3,4-methylenedioxymethamphetamine (MDMA) is correlated with dialysate levels of serotonin and dopamine in rat brain. Pharmacol Biochem Behav. 2008;90:208–217. doi: 10.1016/j.pbb.2008.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendotti C, Borsini F, Zanini MG, Samanin R, Garattini S. Effect of fenfluramine and norfenfluramine stereoisomers on stimulant effects of d-amphetamine and apomorphine in the rat. Pharmacol Res Commun. 1980;12:567–574. doi: 10.1016/s0031-6989(80)80142-1. [DOI] [PubMed] [Google Scholar]
- Berridge CW, Stalnaker TA. Relationship between low-dose amphetamine-induced arousal and extracellular norepinephrine and dopamine levels within prefrontal cortex. Synapse. 2002;46:140–149. doi: 10.1002/syn.10131. [DOI] [PubMed] [Google Scholar]
- Brown RT, Amler RW, Freeman WS, Perrin JM, Stein MT, Feldman HM, Pierce K, Wolraich ML. Treatment of attention-deficit/hyperactivity disorder: overview of the evidence. Pediatrics. 2005;115:e749–e757. doi: 10.1542/peds.2004-2560. [DOI] [PubMed] [Google Scholar]
- Bubar MJ, Cunningham KA. Serotonin 5-HT2A and 5-HT2C receptors as potential targets for modulation of psychostimulant use and dependence. Curr Top Med Chem. 2006;6:1971–1985. doi: 10.2174/156802606778522131. [DOI] [PubMed] [Google Scholar]
- Cangiano C, Ceci F, Cascino A, Del Ben M, Laviano A, Muscaritoli M, Antonucci F, Rossi-Fanelli F. Eating behavior and adherence to dietary prescriptions in obese adult subjects treated with 5-hydroxytryptophan. AmJClinNutr. 1992;56:863–867. doi: 10.1093/ajcn/56.5.863. [DOI] [PubMed] [Google Scholar]
- Cangiano C, Laviano A, Del Ben M, Preziosa I, Angelico F, Cascino A, Rossi-Fanelli F. Effects of oral 5-hydroxy-tryptophan on energy intake and macronutrient selection in non-insulin dependent diabetic patients. IntJObesRelatMetabDisord. 1998;22:648–654. doi: 10.1038/sj.ijo.0800642. [DOI] [PubMed] [Google Scholar]
- Das YT, Bagchi M, Bagchi D, Preuss HG. Safety of 5-hydroxy-L-tryptophan. Toxicol Lett. 2004;150:111–122. doi: 10.1016/j.toxlet.2003.12.070. [DOI] [PubMed] [Google Scholar]
- Di Matteo V, De Blasi A, Di Giulio C, Esposito E. Role of 5-HT(2C) receptors in the control of central dopamine function. Trends Pharmacol Sci. 2001;22:229–232. doi: 10.1016/s0165-6147(00)01688-6. [DOI] [PubMed] [Google Scholar]
- Di Matteo V, Di Giovanni G, Di Mascio M, Esposito E. Biochemical and electrophysiological evidence that RO 60-0175 inhibits mesolimbic dopaminergic function through serotonin(2C) receptors. Brain Res. 2000;865:85–90. doi: 10.1016/s0006-8993(00)02246-0. [DOI] [PubMed] [Google Scholar]
- Fleckenstein AE, Volz TJ, Riddle EL, Gibb JW, Hanson GR. New insights into the mechanism of action of amphetamines. Annu Rev Pharmacol Toxicol. 2007;47:681–698. doi: 10.1146/annurev.pharmtox.47.120505.105140. [DOI] [PubMed] [Google Scholar]
- Fletcher PJ, Chintoh AF, Sinyard J, Higgins GA. Injection of the 5-HT2C receptor agonist Ro60-0175 into the ventral tegmental area reduces cocaine-induced locomotor activity and cocaine self-administration. Neuropsychopharmacology. 2004;29:308–318. doi: 10.1038/sj.npp.1300319. [DOI] [PubMed] [Google Scholar]
- Gartside SE, Cowen PJ, Sharp T. Effect of 5-hydroxy-L-tryptophan on the release of 5-HT in rat hypothalamus in vivo as measured by microdialysis. Neuropharmacology. 1992;31:9–14. doi: 10.1016/0028-3908(92)90154-h. [DOI] [PubMed] [Google Scholar]
- Ghitza UE, Rothman RB, Gorelick DA, Henningfield JE, Baumann MH. Serotonergic responsiveness in human cocaine users. Drug Alcohol Depend. 2007;86:207–213. doi: 10.1016/j.drugalcdep.2006.06.007. [DOI] [PubMed] [Google Scholar]
- Grabowski J, Rhoades H, Schmitz J, Stotts A, Daruzska LA, Creson D, Moeller FG. Dextroamphetamine for cocaine-dependence treatment: a double-blind randomized clinical trial. J Clin Psychopharmacol. 2001;21:522–526. doi: 10.1097/00004714-200110000-00010. [DOI] [PubMed] [Google Scholar]
- Grabowski J, Shearer J, Merrill J, Negus SS. Agonist-like, replacement pharmacotherapy for stimulant abuse and dependence. Addict Behav. 2004;29:1439–1464. doi: 10.1016/j.addbeh.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Halladay AK, Wagner GC, Sekowski A, Rothman RB, Baumann MH, Fisher H. Alterations in alcohol consumption, withdrawal seizures, and monoamine transmission in rats treated with phentermine and 5-hydroxy-L-tryptophan. Synapse. 2006;59:277–289. doi: 10.1002/syn.20239. [DOI] [PubMed] [Google Scholar]
- Haney M, Ward AS, Gerra G, Foltin RW. Neuroendocrine effects of d-fenfluramine and bromocriptine following repeated smoked cocaine in humans. Drug Alcohol Depend. 2001;64:63–73. doi: 10.1016/s0376-8716(00)00232-5. [DOI] [PubMed] [Google Scholar]
- Hendricks EJ, Rothman RB, Greenway FL. How physician obesity specialists use drugs to treat obesity. Obesity (Silver Spring, Md. 2009;17:1730–1735. doi: 10.1038/oby.2009.69. [DOI] [PubMed] [Google Scholar]
- Herin DV, Rush CR, Grabowski J. Agonist-like pharmacotherapy for stimulant dependence: preclinical, human laboratory, and clinical studies. Ann N Y Acad Sci. 2010;1187:76–100. doi: 10.1111/j.1749-6632.2009.05145.x. [DOI] [PubMed] [Google Scholar]
- Ikemoto S. Dopamine reward circuitry: two projection systems from the ventral midbrain to the nucleus accumbens-olfactory tubercle complex. Brain Res Rev. 2007;56:27–78. doi: 10.1016/j.brainresrev.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuczenski R, Segal DS, Cho AK, Melega W. Hippocampus norepinephrine, caudate dopamine and serotonin, and behavioral responses to the stereoisomers of amphetamine and methamphetamine. J Neurosci. 1995;15:1308–1317. doi: 10.1523/JNEUROSCI.15-02-01308.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynn-Bullock CP, Welshhans K, Pallas SL, Katz PS. The effect of oral 5-HTP administration on 5-HTP and 5-HT immunoreactivity in monoaminergic brain regions of rats. J Chem Neuroanat. 2004;27:129–138. doi: 10.1016/j.jchemneu.2004.02.003. [DOI] [PubMed] [Google Scholar]
- Mooney ME, Schmitz JM, Moeller FG, Grabowski J. Safety, tolerability and efficacy of levodopa-carbidopa treatment for cocaine dependence: two double-blind, randomized, clinical trials. Drug Alcohol Depend. 2007;88:214–223. doi: 10.1016/j.drugalcdep.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, Mello NK. Effects of chronic d-amphetamine treatment on cocaine-and food-maintained responding under a second-order schedule in rhesus monkeys. Drug Alcohol Depend. 2003;70:39–52. doi: 10.1016/s0376-8716(02)00339-3. [DOI] [PubMed] [Google Scholar]
- Perry KW, Fuller RW. Extracellular 5-hydroxytryptamine concentration in rat hypothalamus after administration of fluoxetine plus L-5-hydroxytryptophan. J Pharm Pharmacol. 1993;45:759–761. doi: 10.1111/j.2042-7158.1993.tb07105.x. [DOI] [PubMed] [Google Scholar]
- Rothman RB. Treatment of Obesity with “Combination” Pharmacotherapy. Am J Ther. 2009 doi: 10.1097/MJT.0b013e31818e30da. in press. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Baumann MH. Monoamine transporters and psychostimulant drugs. Eur J Pharmacol. 2003;479:23–40. doi: 10.1016/j.ejphar.2003.08.054. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Baumann MH. Appetite Suppressants, Cardiac Valve Disease and Combination Pharmacotherapy. Am J Ther. 2009;16:354–364. doi: 10.1097/MJT.0b013e31817fde95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman RB, Blough BE, Baumann MH. Dopamine/serotonin releasers as medications for stimulant addictions. Prog Brain Res. 2008;172:385–406. doi: 10.1016/S0079-6123(08)00919-9. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Blough BE, Woolverton WL, Anderson KG, Negus SS, Mello NK, Roth BL, Baumann MH. Development of a rationally designed, low abuse potential, biogenic amine releaser that suppresses cocaine self-administration. J Pharmacol Exp Ther. 2005;313:1361–1369. doi: 10.1124/jpet.104.082503. [DOI] [PubMed] [Google Scholar]
- Sachdev M, Miller WC, Ryan T, Jollis JG. Effect of fenfluramine-derivative diet pills on cardiac valves: a meta-analysis of observational studies. Am Heart J. 2002;144:1065–1073. doi: 10.1067/mhj.2002.126733. [DOI] [PubMed] [Google Scholar]
- Sharp T, Zetterstrom T, Ljungberg T, Ungerstedt U. A direct comparison of amphetamine-induced behaviours and regional brain dopamine release in the rat using intracerebral dialysis. Brain Res. 1987;401:322–330. doi: 10.1016/0006-8993(87)91416-8. [DOI] [PubMed] [Google Scholar]
- Turner EH, Loftis JM, Blackwell AD. Serotonin a la carte: supplementation with the serotonin precursor 5-hydroxytryptophan. Pharmacol Ther. 2006;109:325–338. doi: 10.1016/j.pharmthera.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Wee S, Woolverton WL. Self-administration of mixtures of fenfluramine and amphetamine by rhesus monkeys. Pharmacol Biochem Behav. 2006;84:337–343. doi: 10.1016/j.pbb.2006.05.022. [DOI] [PubMed] [Google Scholar]
- Zmilacher K, Battegay R, Gastpar M. L-5-hydroxytryptophan alone and in combination with a peripheral decarboxylase inhibitor in the treatment of depression. Neuropsychobiology. 1988;20:28–35. doi: 10.1159/000118469. [DOI] [PubMed] [Google Scholar]
- Zolkowska D, Jain R, Rothman RB, Partilla JS, Roth BL, Setola V, Prisinzano TE, Baumann MH. Evidence for the involvement of dopamine transporters in behavioral stimulant effects of modafinil. J Pharmacol Exp Ther. 2009;329:738–746. doi: 10.1124/jpet.108.146142. [DOI] [PMC free article] [PubMed] [Google Scholar]