Abstract
Background
The relationship between weight and dementia risk has not been investigated in populations with relatively low body mass index (BMI) such as the Yoruba. This study set out to achieve this objective using a prospective observational design.
Methods
The setting was Idikan Ward in Ibadan City, Nigeria. The participants were all aged 65 years or older and were enrolled in the Indianapolis-Ibadan Dementia Project. Repeated cognitive assessments and clinical evaluations were conducted to identify participants with dementia or MCI during 10 years of follow-up (mean duration: 5.97 years). BMI measures, information on alcohol, smoking history, cancer, hypertension, diabetes, heart attack, stroke and depression were collected at each follow-up evaluation. Mixed effect models adjusted for covariates were used to examine the differences in BMI among participants who developed dementia or MCI and those who remained cognitively normal during the follow-up.
Results
This analysis included 1559 participants who had no dementia at their first BMI measurements. There were 136 subjects with incident dementia, 255 with MCI and 1168 with normal cognition by the end of the study. The mean BMI at baseline was higher for female participants (22.31; SD = 4.39) than for male (21.09; SD = 3.61, p < 0.001). A significantly greater decline in BMI was found in those with either incident dementia (p < 0.001) or incident MCI (p < 0.001) compared to normal subjects.
Conclusion
Decline in BMI is associated with incident MCI and dementia in elderly Yoruba. This observation calls for close monitoring of weight loss in elderly individuals which may indicate future cognitive impairment for timely detection and tailored interventions.
Keywords: body mass index, dementia, mild cognitive impairment, normal cognition
Introduction
The dementing disorders represent a major burden for societies and the magnitude of this burden is likely to increase with the rising tide of dementia worldwide (Larson and Langa, 2008) Vascular factors like hypertension, ischemic heart disease, diabetes mellitus and obesity are associated with increased risk of dementia and can be targeted for preventive strategies (Skoog et al., 1999). Anthropometric measurements have featured prominently in many studies for the diagnosis of obesity. The relationship between weight and dementia risk, however, appears inconsistent with gender variations also reported.
Kivipeto et al. (2005) and Whitner et al. (2005) reported that high body mass index (BMI) increased dementia risk in middle age while other workers reported high BMI to be protective in later life (Fitzpatrick et al., 2009). Another study reported that high BMI in later life increased the risk of Alzheimer’s disease (AD) only in women (Gustafson et al., 2003). Sturman and others (2008) found no association between high BMI and cognitive decline. Low BMI occurring in later life is associated with increased the risk of incident AD and may precede disease onset by many years or even decades (Johnson et al., 2006; Knopman et al., 2007). These contrasting findings have come from studies in Western countries where obesity is more of a problem.
People living in developing countries generally have a lower BMI than those in Western countries. A comparative study of older persons in geographically diverse groups revealed much lower BMI for participants from Nigeria, China and Guatemala when compared with those from developed countries (Launer and Harris, 1996). Also, in the Indianapolis-Ibadan Dementia study, we found significantly lower BMI among the Yoruba participants compared with African Americans (Deeg et al., 2008). Ochayi and Thacher (2006) reported low BMI (<18.5) to be a risk factor for dementia in their cohort in north-central Nigeria. There is paucity of data from prospective studies of dementia from developing countries specifically addressing the issue of weight gain or loss in individuals with cognitive disorders. Extrapolation of results from studies in other parts of the world is fraught with errors.
Therefore, it was anticipated that a prospective study in a unique and relatively understudied population with low BMI, such as the Yoruba, would provide more useful information on markers of dementia as well as relevant strategies for disease prevention. In this paper, we report on the association between repeated BMI measures, incident dementia and mild cognitive impairment (MCI) in a cohort of elderly Yoruba Nigerians, which we believe is the first study of its kind from Africa.
Methods
Study participants
The participants were elderly Yoruba, resident in Idikan Ward of Ibadan City, Nigeria who had been enrolled into the Indianapolis-Ibadan Dementia Project, a longitudinal study examining risk factors for dementia and AD in African Americans and Yoruba, which commenced in 1992. Recruitment into the study was conducted at two time points: 1992 and 2001. The cohort enrolled in 1992 included subjects aged 65 years or older, and those in the 2001 cohort included subjects 70 years and older. Details on the study methodology have been described elsewhere by Hendrie et al. (2001). The study was approved by the Ethics Committee of the University of Ibadan/University College Hospital, Ibadan, Nigeria as well as by the Indiana University-Purdue University of Indianapolis Institutional Review Board. All enrolled participants provided informed consent.
Evaluation of participants
Participants enrolled in 1992 had additional cognitive assessment and clinical evaluations done in 1995, 1997, 2001, 2004 and 2007 while those recruited in 2001 were further evaluated in 2004 and 2007. At each wave of the study, a two-stage assessment was utilized. The first stage consisted of cognitive assessment and collection of information on potential risk factors including anthropometric measurements. The subjects were screened in their homes using the Community Screening Interview for Dementia (CSI-D), which consists of cognitive assessment that tests memory, orientation, language, attention, calculation and reasoning. The results are combined to produce a Cognitive Score. A concurrent structured interview with an informant (usually a close relative) provided information on the onset and progression of any cognitive symptoms and the adequacy of the subject’s daily functioning, which are combined to produce an Informant Score. Based on the Cognitive and Informant scores, the subjects were classified into three performance groups – good, intermediate and poor. They were then sampled for the clinical assessment stage from prior analysis for high likelihood of dementia as follows: all subjects in the poor performance group, 75% of those in the intermediate performance category, and 5% of those identified as good performers.
The second stage included an in-home interview using the Clinician Home-based Interview to assess Function (Hendrie et al., 2006); a neuropsychological battery adapted from the Consortium to Establish a Registry of Alzheimer’s Disease (Morris et al., 1988); a standardized neurologic and physical examination and a structured interview with a close relative adapted from the Cambridge Examination for Mental Disorders of the Elderly informant interview (Hendrie et al., 1988). Following the second stage evaluation, participants were diagnosed and placed into one of three mutually exclusive cognitive diagnostic categories: normal cognitive function, dementia, or MCI. Diagnoses were made during consensus diagnostic conference of clinicians after reviewing the CERAD neuropsychological test battery, the physician’s assessment and the informant interview. The clinicians were blinded to CSI-D scores and the screening performance groups. Dementia was diagnosed using criteria from both the International Classification of Diseases, 10th Revision (World health Organization, 1992) and the Diagnostic and Statistical Manual of Mental Disorders, Revised Third Edition (American Psychiatric Association, 1987). MCI was diagnosed if the following criteria were fulfilled: informant reported a clinically significant decline in cognitive function; evidence of significant cognitive decline on physician examination, or impaired CERAD test performance (test scores 1.5 SD below the mean of the normative reference sample) and impaired normal daily functioning (based on informant interview).
BMI measures
Anthropometric measurements were consistently recorded starting from the 1997 evaluation wave during each in-home interview. Four sets of values were obtained for the original cohort and three measures for the enrichment cohort of 2001. BMI was calculated as weight in kilograms divided by height in meters squared.
Other covariates
Demographic information including age, sex and whether they attended school or not were available on all study participants as well as life style information on alcohol consumption and smoking history. At each wave of the study, we also documented prior diagnosis of the following medical conditions: cancer, hypertension, diabetes, heart attack, stroke and depression, which could affect cognitive performance. Subjects with other psychiatric diagnoses and brain trauma were excluded as well as those with acute confusional state.
Statistical analyses
This analysis included subjects without dementia from the 1997 evaluation for those in the original cohort, and subjects without dementia from the 2001 evaluation for those enrolled in 2001. Repeated BMI measures were used as the outcome variable. The changes in BMI mainly reflected variations in weight since height was held constant for all BMI computations. Three groups of subjects were defined in the analysis. The first group consisted of subjects with incident dementia diagnosed after the 1997 evaluation for those in the original cohort and after the 2001 evaluation for the enrichment cohort. A second group consisted of subjects diagnosed with MCI at their last evaluation. The third group consisted of participants who were evaluated in 2007 during the in-home cognitive assessment and were found to have good cognitive function. Participants with only one BMI measure were excluded from the analysis.
To compare changes in BMI prior to the clinical diagnosis of dementia and MCI to normal subjects, we aligned the timing of the BMI measurements to an index time point defined as the time of diagnosis for participants in the incident dementia group or the MCI group, and the time at the 2007 evaluation for the normal subjects. Therefore, all BMI measurements taken during the course of the study were aligned by the number of years BMI was measured prior to the index time. In all the statistical models, index time was coded as time zero. For example, a subject with BMI measured at 1997 and diagnosed with dementia in 2007 would have time coded as −10 indicating that the BMI measure was taken 10 years prior to the dementia diagnosis. Comparisons of demographic characteristics and medical history among the three groups of participants were conducted using χ2 tests for categorical variables and analysis of variance for continuous variables. To control for potential confounding effects of chronic medical conditions on BMI change, we compared the frequencies of medical conditions collected at index time as potential covariates.
Mixed effect models with random intercept and random slope for time were used to analyze the effect of each variable on BMI and BMI changes. We explored separate single covariate mixed effect models, main effect of time and time squared, and also checked for interaction between the covariates and time. Variables that were significantly different among the three groups and significant in the single covariate BMI models were considered in a multivariate model. The final multivariate model included variables that either had significant main effects or significant interaction with time. The level of statistical significance was set at p < 0.05. The statistical software SAS version 9.2 was used for the analyses.
Results
A total of 1559 study participants with baseline BMI data were studied. During follow-up, 136 cases of dementia were diagnosed (26 in 2001, 53 in 2004 and 57 in 2007) and 255 cases of MCI (21 in 2001, 44 in 2004, and 190 in 2007). The remaining 1168 subjects were found to have good cognitive function. At baseline, the mean BMI for the total sample was 21.91 (SD = 4.19) and by gender, it was 22.31 (SD = 4.39) for women and 21.09 (SD = 3.61) for men. Eighty subjects (5.13%) were obese, i.e. BMI > 30; 235 (15.07%) were overweight; 1000 (64.14%) had a BMI between 18 and 25; and 244 (15.65%) had a mean BMI below 18. Mean numbers of BMI measurements were 3.14 (SD = 0.53) for the normal group, 2.51 (SD = 0.66) for the dementia group, and 2.89 (SD = 0.68) for the MCI group.
As shown in Table 1, the mean BMI values of female participants, irrespective of final diagnosis, were significantly higher than the corresponding values for males (p < 0.001). The summary statistics and participant characteristics in the three diagnostic groups are also presented in Table 1. It shows significant differences between the three groups of subjects in age, gender, schooling, BMI and smoking status. The normal group has the longest mean follow-up time of 5.97 years which was significantly higher than for the dementia group (4.95 years). The MCI group was followed-up for an average of 5.69 years. At index time (diagnosis time for dementia and MCI subjects, last follow-up evaluation for normal subjects), the dementia and MCI subjects were significantly older. The normal cognition group had the highest proportion of males, included more participants who went to school and had the lowest frequency of current smokers. The three groups did not differ significantly with respect to alcohol consumption, history of cancer, hypertension, diabetes, heart attack or depression.
Table 1.
Subjects’ characteristics by group (incident dementia, incident MCI and cognitively normal subjects). Information was collected at the time of diagnosis for the dementia and MCI subjects and at the 2007 evaluation for the normal subjects
| SUBJECTS’ CHARACTERISTICS | INCIDENT DEMENTIA (N = 136) | INCIDENT MCI (N = 255) | COGNITIVELY NORMAL (N = 1168) | P -VALUE |
|---|---|---|---|---|
| Years of follow-up (mean, SD) | 4.95 (2.12) | 5.69 (2.29) | 5.97 (1.91) | <0.001 |
| Mean age, (SD) | 83.1 (7.8) | 82.1 (5.8) | 80.3 (4.4) | <0.001 |
| Female, N (%) | 103 (75.7%) | 203 (79.6%) | 748 (64.0%) | <0.001 |
| Have education, N, (%) | 16 (11.8%) | 17 (6.7%) | 193 (16.5%) | <0.001 |
| Mean BMI, (SD), Female | 21.1 (3.7) | 21.0 (4.2) | 22.6 (4.6) | <0.001 |
| Mean BMI, (SD), Male | 19.5 (4.4) | 20.2 (3.1) | 20.9 (3.9) | |
| Drink alcohol, N, (%) | ||||
| Current | 12 (8.8%) | 22 (8.6%) | 124 (10.6%) | 0.554 |
| Past | 28 (20.6%) | 51 (20.0%) | 268 (23.0%) | |
| Never | 96 (70.6%) | 182 (71.4%) | 776 (66.4%) | |
| Smoking, N, (%) | ||||
| Current | 19 (14.0%) | 37 (14.5%) | 134 (11.5%) | 0.014 |
| Past | 62 (45.6%) | 82 (32.2%) | 391 (33.5%) | |
| Never | 55 (40.4%) | 136 (53.3%) | 643 (55.0%) | |
| History of: | ||||
| Cancer, N, (%) | 8 (5.88%) | 10 (3.92%) | 35 (3.00%) | 0.188 |
| Hypertension, N, (%) | 124 (91.2%) | 223 (87.5%) | 1039 (89.0%) | 0.532 |
| Diabetes, N, (%) | 2 (1.47%) | 16 (6.27%) | 44 (3.77%) | 0.052 |
| Heart attack, N, (%) | 24 (17.7%) | 58 (22.8%) | 200 (17.1%) | 0.106 |
| Stroke, N, (%) | 9 (6.62%) | 8 (3.14%) | 33 (2.83%) | 0.059 |
| Depression, N, (%) | 33 (24.3%) | 53 (20.8%) | 250 (21.4%) | 0.706 |
The results of the mixed effect models containing a single covariate in each model with repeated BMI measures as outcomes are displayed in Table 2. It shows significant effects with age, gender and smoking status. A significant main effect would indicate differences in BMI measures at index time among the levels defined by the covariate. Significant interaction between a covariate and time indicates differences in BMI changes over time among the levels defined by the covariate. We included significant factors from both Table 1 and Table 2 in multivariate mixed effect models to determine their respective contribution to BMI and BMI changes. The final multivariate model, including all significant factors associated with BMI change, is shown in Table 3. Male subjects, current smokers, dementia subjects and MCI subjects all had lower BMI at index time. There is also a significant quadratic time effect in BMI in all subjects. Most importantly, subjects with incident dementia and MCI showed a greater decline in BMI over time than the normal subjects (P < 0.001). Subjects with incident dementia declined 0.19 kg/m2 per year more than the normal cognition group (p < 0.001) and the MCI subjects declined 0.16 kg/m2 per year more than the normal group (p < 0.001). Both the incident dementia and the MCI subjects had significantly lower BMI than the normal cognition group at index time (p < 0.001).
Table 2.
Results of mixed effect single covariate models on repeated BMI measures. Each model includes one covariate, main effect of time and time squared, and an interaction term between the covariate and time
| VARIABLES | MAIN EFFECT |
INTERACTION WITH TIME (SLOPE) |
||||
|---|---|---|---|---|---|---|
| PARAMETER ESTIMAT E | SE ESTIMATE | P -VALUE | PARAMETER ESTIMATE | SE ESTIMATE | P -VALUE | |
| Age | −0.089 | 0.022 | <0.001 | −0.011 | 0.002 | <0.001 |
| Female | 1.414 | 0.236 | <0.001 | 0.016 | 0.026 | 0.546 |
| Educated | 0.520 | 0.317 | 0.101 | 0.037 | 0.033 | 0.266 |
| Alcohol | ||||||
| current | 0.033 | 0.212 | 0.878 | −0.007 | 0.036 | 0.854 |
| past | −0.120 | 0.205 | 0.559 | −0.053 | 0.033 | 0.106 |
| never | 0 | – | – | 0 | – | – |
| Smoking | ||||||
| current | −1.046 | 0.235 | <0.001 | −0.032 | 0.036 | 0.383 |
| past | −0.762 | 0.209 | <0.001 | −0.027 | 0.027 | 0.326 |
| never | 0 | – | – | 0 | – | – |
| History of | ||||||
| cancer | −0.029 | 0.394 | 0.942 | 0.084 | 0.090 | 0.351 |
| hypertension | 0.340 | 0.205 | 0.097 | −0.017 | 0.034 | 0.607 |
| diabetes | −0.733 | 0.364 | 0.044 | −0.083 | 0.068 | 0.226 |
| heart attack | 0.450 | 0.232 | 0.052 | 0.022 | 0.030 | 0.466 |
| stroke | −0.100 | 0.406 | 0.805 | −0.104 | 0.086 | 0.225 |
| depression | −0.058 | 0.193 | 0.765 | 0.018 | 0.030 | 0.553 |
SE = standard error.
Table 3.
Multivariate mixed effect models on repeated BMI measures prior to dementia/MCI diagnosis
| PARAMETER ESTIMATE | SE ESTIMATE | P - VALUE | |
|---|---|---|---|
| Main effects | |||
| Female | 1.342 | 0.232 | <0.001 |
| Smoking | |||
| current | −0.589 | 0.201 | 0.004 |
| past | −0.214 | 0.199 | 0.282 |
| never | 0 | – | – |
| Group | |||
| Dementia | −1.162 | 0.399 | 0.004 |
| MCI | −1.236 | 0.302 | <0.004 |
| Normal | 0 | – | – |
| Time | −0.114 | 0.026 | <0.001 |
| Time2 | −0.013 | 0.003 | <0.001 |
| Interaction with time | |||
| Group*time | |||
| Dementia | −0.194 | 0.049 | <0.001 |
| MCI | −0.164 | 0.033 | <0.001 |
| Normal | 0 | – | – |
SE = standard error; MCI = mild cognitive impairment.
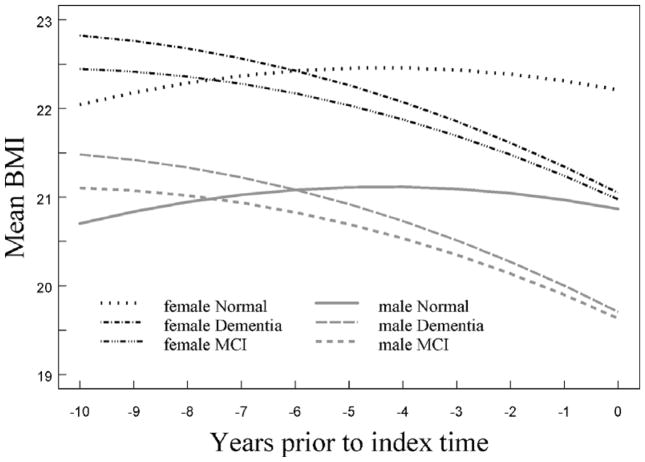
Figure 1 shows plots of the predicted BMI values at different evaluation times leading to the time of diagnosis or the end of follow-up for the three groups according to gender. For both women and men, there was a progressive drop in the plots of mean BMI for the MCI and incident dementia groups, with a steeper decline obvious as the measurement time approached the time of diagnosis.
Figure 1.
Plot of predicted mean BMI over time by gender and diagnostic group from the final multivariate model.
Discussion
We found significantly greater weight loss in this cohort of elderly Yoruba subjects diagnosed with incident dementia and incident MCI when compared with those with normal cognition. This association of weight loss with incident dementia in Nigerians corroborates the finding of Ochayi and Thatcher (2006) in the same environment as well as agreeing with other authors who have examined the relationship between BMI and dementia in different communities (Johnson et al., 2006; Knopman et al., 2007). In conformity with severity of cognitive impairment, our results showed a significant and progressive increase in the magnitude of BMI change from subjects with normal cognition to those diagnosed as incident MCI while incident dementia subjects had the greatest decline. Approximately one-fifth of the study participants were either overweight or obese and it is interesting that the phenomenon of weight loss in association with incident dementia and MCI was also found in the cohort since previous studies were carried out in societies confronting obesity and being overweight. This consistent finding in different environments is in line with the conclusion of Buchman et al. (2006) that loss of BMI may reflect pathologic processes that contribute to the subsequent development of AD.
Weight loss in old age could also result from diminished caloric intake that is insufficient to counter-balance the demands of high physical activity especially in developing countries. The other reasons for accelerated weight loss experienced by subjects with dementia, which had been observed to antedate diagnosis by many years in some studies, include forgetfulness to eat, apathy and loss of initiative in the prodromal stage of dementia, impaired olfactory function and loss of taste (Aziz et al., 2008; Luchsinger and Gustafson, 2009). Difficulty with swallowing has also been alluded to in some studies (Easterling and Robbins, 2008). All these tend to create a nutritional deficit ultimately leading to weight loss. Gradual weight loss is believed to be associated with changes in the regulation of food intake during the aging process especially with the increased satiating effect of cholecystokinin, which tends to cause decrease in food intake (MacIntosh et al., 2001). We think the reasons for the weight loss in our patients may be multifactorial rather than being due to a single factor. Further studies on the biological mechanisms of weight balance and disease pathogenesis may provide clues, including analysis of biomarkers.
In a recent study, Burns et al. (2010) reported association between lean body mass, whole brain volume and global cognitive performance. They found accelerated loss of lean mass associated with brain atrophy. This was presumed to be due to either a direct or indirect consequence of AD pathophysiology or through shared mechanisms common to both AD and sarcopenia. Early degenerative changes in AD resulting in cognitive dysfunction involve the hippocampus, trans entorhinal cortex and the entorhinal cortex (Braak and Braak, 1991; Luchsinger and Gustafson 2009). The hypothalamus is also affected by virtue of the fact that it maintains body homeostasis including weight maintenance as well as regulating insulin signaling pathways, and cognitive dysfunction in Alzheimer disease is associated with insulin deficiency within the brain (Stockhorst et al., 2004; Muzumdar et al., 2009). Unintended weight loss is not peculiar to dementia as it has also been observed in other neurodegenerative diseases like Parkinson’s disease and Huntington’s chorea (Buchman et al., 2005; Aziz et al., 2008). Findings from the Religious Orders Study provided further supportive evidence linking Alzheimer’s disease pathology and fall in BMI over time quite unlike the presence of Lewy body pathology and ischemic lesions in the brain (Buchman et al., 2006).
Our results showed that current smokers had low BMI at index time but they did not show significant weight loss over time. We had reported in an earlier study that smoking significantly increased the risk of mortality of elderly Yoruba with dementia (Perkins et al., 2002). Weight loss adds to the frailty syndrome of the older person with dementia and could predispose them to increased susceptibility to infections, falls, poor quality of life and increased mortality. We found no association between weight loss and depression, cancer and cardiovascular disease, which are other important causes of such loss in the elderly. It is possible, however, that individuals with these conditions might have dropped out in earlier time periods due to death or other reasons. However, we had in place a regular follow-up of the study participants in their homes to ensure that important information on outcome was carefully documented and such errors eliminated.
Weight loss in older persons is largely due to a decrease in lean mass and some authors have argued that weight and hip measurements may reflect changes in body adiposity better than BMI (Luchsinger and Gustafson, 2009). The non-availability of the waist-hip ratio measurements on these study participants could be regarded as a limitation. However, Ukoli et al. (1995) reported that BMI correlated better with blood pressure measurements in Nigerian elderly urban populations than weight and hip measurements. Our study confirmed this observation in Nigerians.
To our knowledge, ours is the first study to examine the relationship between BMI changes prior to a diagnosis of MCI and dementia in a population of community-dwelling older persons in a developing country. The methodology employed, in which serial BMI measurements were made in a prospective study design, and the ten-year span of the study are strong points in favor of our findings. The mean duration of six years in our study was similar to that of the Chicago Health and Aging Project of 6.4 years and the Cardiovascular Health Study of 5.4 years (Sturman et al., 2008; Fitzpatrick et al., 2009).
In summary, in this community-based elderly Yoruba cohort with an average low BMI, we found that subjects with incident dementia and/or MCI exhibited significantly greater weight loss prior to diagnosis compared to those who had normal cognition. We conclude that decline in BMI can be used as a marker of cognitive impairment/dementia, and this points to the need for close monitoring of weight loss in the elderly.
Acknowledgments
The research was supported by NIH grant R01 AG09956.
We thank our field interviewers and supervisors of the Ibadan Dementia Research Project for data collection. We acknowledge the support of the following: Drs. A. Akinbiyi, R. O. Akinyemi, L. F. Owolabi, F. T. N. Nuhu, A. K. O. Adebayo and A. A. O. Adesokan for clinical assessment of study participants.
Footnotes
Description of authors’ roles
H. C. Hendrie designed the study, reviewed data collection, participated in data analysis and helped to write the paper. K. S. Hall designed the study, reviewed data collection, participated in consensus diagnosis and helped to write the paper. S. Gao formulated the research question, supervised statistical analysis and helped to write the paper. O. Baiyewu and O. Gureje participated in data review and consensus diagnosis, and helped to write the paper. F. W. Unverzagt designed the study, participated in consensus diagnosis and data analysis, and helped to write the paper. V. Smith-Gamble participated in consensus diagnosis and reviewed the paper. J. R. Murrell participated in study design and helped to write the paper. A. M. Hake participated in consensus diagnosis and reviewed the paper. J. T. Nguyen was responsible for statistical analysis. A. Ogunniyi participated in consensus diagnosis, data analysis and helped to write the paper.
Conflict of interest
None.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 3. Washington, DC: American Psychiatric Assocation; 1987. revised version. [Google Scholar]
- Aziz NA, van der Marck MA, Pijl H, Olde Rikkert MGM, Bloem BR, Roos RAC. Weight loss in neurodegenerative disorders. Journal of Neurology. 2008;255:1872–1880. doi: 10.1007/s00415-009-0062-8. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathologica (Berlin) 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Buchman AS, Wilson RS, Bienias JL, Shah RC, Evans DA, Bennett DA. Change in body mass index and risk of incident Alzheimer disease. Neurology. 2005;65:892–897. doi: 10.1212/01.wnl.0000176061.33817.90. [DOI] [PubMed] [Google Scholar]
- Buchman AS, Schneider JA, Wilson RS, Bienas JL, Bennett DA. Body mass index in older persons is associated with Alzheimer disease pathology. Neurology. 2006;67:1949–1954. doi: 10.1212/01.wnl.0000247046.90574.0f. [DOI] [PubMed] [Google Scholar]
- Burns JM, Johnson DK, Watts A, Swerdlow RH, Brooks WM. Reduced lean mass in early Alzheimer disease and its association with brain atrophy. Archives of Neurology. 2010;67:428–433. doi: 10.1001/archneurol.2010.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deeg M, et al. A comparison of cardiovascular disease risk factor biomarkers in African Americans and Yoruba Nigerians. Ethnicity and Disease. 2008;18:427–433. [PMC free article] [PubMed] [Google Scholar]
- Easterling CS, Robbins E. Dementia and dysphagia. Geriatric Nursing. 2008;29:275–285. doi: 10.1016/j.gerinurse.2007.10.015. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick AL, et al. Midlife and late-life obesity and the risk of dementia: cardiovascular health study. Archives of Neurology. 2009;66:336–342. doi: 10.1001/archneurol.2008.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafson D, Rothenberg E, Blennow K, Steen B, Skoog I. An 18-year follow-up of overweight and risk of Alzheimer disease. Archives of Internal Medicine. 2003;163:1524–1528. doi: 10.1001/archinte.163.13.1524. [DOI] [PubMed] [Google Scholar]
- Hendrie HC, et al. The CAMDEX: a standardized instrument for the diagnosis of mental disorder in the elderly: a replication with a US sample. Journal of the American Geriatric Society. 1988;36:402–408. doi: 10.1111/j.1532-5415.1988.tb02378.x. [DOI] [PubMed] [Google Scholar]
- Hendrie HC, et al. Incidence of dementia and Alzheimer disease in two communities: Yoruba residing in Ibadan, Nigeria and African Americans residing in Indianapolis, Indiana. JAMA. 2001;285:739–747. doi: 10.1001/jama.285.6.739. [DOI] [PubMed] [Google Scholar]
- Hendrie HC, et al. The development of a semi-structured home interview (CHIF) to directly assess function in cognitively impaired elderly people in two cultures. International Psychogeriatrics. 2006;18:653–666. doi: 10.1017/S104161020500308X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson DK, Wilkins CH, Morris JC. Accelerated weight loss may precede diagnosis in Alzheimer disease. Archives of Neurology. 2006;63:1312–1317. doi: 10.1001/archneur.63.9.1312. [DOI] [PubMed] [Google Scholar]
- Kivipelto M, et al. Obesity and vascular risk factors at midlife and the risk of dementia and Alzheimer disease. Archives of Neurology. 2005;62:1556–1560. doi: 10.1001/archneur.62.10.1556. [DOI] [PubMed] [Google Scholar]
- Knopman DS, Edland SD, Cha RH, Peterson RC, Rocca WA. Incident dementia in women is preceded by weight loss by at least a decade. Neurology. 2007;69:739–746. doi: 10.1212/01.wnl.0000267661.65586.33. [DOI] [PubMed] [Google Scholar]
- Larson EB, Langa KM. The rising tide of dementia worldwide. Lancet. 2008;372:430–432. doi: 10.1016/S0140-6736(08)61003-X. [DOI] [PubMed] [Google Scholar]
- Launer LJ, Harris T. Weight, height and body mass index distributions in geographically and ethnically diverse samples of older persons. Age and Ageing. 1996;25:300–306. doi: 10.1093/ageing/25.4.300. [DOI] [PubMed] [Google Scholar]
- Luchsinger JA, Gustafson DR. Adiposity and Alzheimer’s disease. Current Opinion in Clinical Nutrition and Metabolic Care. 2009;12:15–21. doi: 10.1097/MCO.0b013e32831c8c71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacIntosh CG, et al. Effect of exogenous cholecystokinin (CCK)-8 on food intake and plasma CCK, leptin, and insulin concentrations in older and young adults: evidence for increased CCK activity as a cause of the anorexia of aging. Journal of Clinical Endocrinology and Metabolism. 2001;86:5830–5837. doi: 10.1210/jcem.86.12.8107. [DOI] [PubMed] [Google Scholar]
- Morris JC, Mohs RC, Rogers H, Fillenbaum G, Heyman A. Consortium to Establish a Registry for Alzheimer’s Disease (CERAD): clinical and neuropsychological assessment of Alzheimer’s disease. Psychopharmacology Bulletin. 1988;24:641–652. [PubMed] [Google Scholar]
- Muzumdar RH, et al. Humanin: a novel central regulator of peripheral insulin action. Public Library of Science One. 2009;4:e6334. doi: 10.1371/journal.pone.0006334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochayi B, Thacher TD. Risk factors for dementia in central Nigeria. Aging and Mental Health. 2006;10:616–620. doi: 10.1080/13607860600736182. [DOI] [PubMed] [Google Scholar]
- Perkins AJ, et al. Risk of mortality for dementia in a developing country: the Yoruba in Nigeria. International Journal of Geriatric Psychiatry. 2002;17:566–573. doi: 10.1002/gps.643. [DOI] [PubMed] [Google Scholar]
- Skoog I, Kalaria RN, Breteler MM. Vascular factors and Alzheimer’s disease. Alzheimer Disease and Associated Disorders. 1999;13:S106–S114. doi: 10.1097/00002093-199912003-00016. [DOI] [PubMed] [Google Scholar]
- Stockhorst U, de Fries D, Steingrueber HJ, Scherbaum WA. Insulin and the CNS: effects on food intake, memory, and endocrine parameters and the role of intranasal insulin administration in humans. Physiology and Behavior. 2004;83:47–54. doi: 10.1016/j.physbeh.2004.07.022. [DOI] [PubMed] [Google Scholar]
- Sturman MT, Mendes de Leon CF, Bienias JL, Morris MC, Wilson RS, Evans DA. Body mass index and cognitive decline in a biracial community population. Neurology. 2008;70:360–367. doi: 10.1212/01.wnl.0000285081.04409.bb. [DOI] [PubMed] [Google Scholar]
- Ukoli FA, Bunker CH, Fabio A, Olomu AB, Egbagbe EE, Kuller LH. Body fat distribution and other anthropometric blood pressure correlates in a Nigerian urban elderly population. Central African Journal of Medicine. 1995;41:154–161. [PubMed] [Google Scholar]
- Whitmer RA, Gunderson EP, Barrett-Connor E, Quesenberry CP, Jr, Yaffe K. Obesity in middle age and future risk of dementia: a 27-year longitudinal population-based study. BMJ. 2005;330:1360. doi: 10.1136/bmj.38446.466238.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. The International Statistical Classification of Diseases and Related Health Problems: 1 and 2. Geneva: World Health Organization; 1992. ICD-10. [Google Scholar]