Abstract
Background:
Nutritional supplements are commonly used for a variety of musculoskeletal conditions, including knee and hip degenerative joint disease. Although these supplements are occasionally recommended for patients with degenerative disc disease and spinal degenerative joint disease, the evidence supporting this use is unknown.
Objective:
To systematically search and assess the quality of the literature on the use of glucosamine, chondroitin sulfate, and methylsulfonylmethane for the treatment of spinal osteoarthritis / degenerative joint disease, and degenerative disc disease.
Data Sources:
The Index of Chiropractic Literature, AMED, Medline, and CINAHL were searched for randomized controlled trials in English from 1984 to July 2009.
Data Extraction and Synthesis:
Data from studies meeting the inclusion criteria was extracted and reviewed by three reviewers. The Jadad scale was used to assess study quality. No attempts were made at meta-analysis due to variation in study design.
Results:
Two articles met the inclusion criteria. One study was found to have good quality but reported negative results for the supplemented group compared with placebo, the other study had low quality but reported significant positive results for the supplemented group when compared with a no intervention control group.
Conclusion:
There was little literature found to support the use of common nutritional supplements for spinal degeneration, making it difficult to determine whether clinicians should recommend them.
Keywords: systematic review, glucosamine, chondroitin, methylsulfonylmethane, osteoarthritis, degenerative joint disease, spine
Abstract
Contexte :
Les suppléments alimentaires sont couramment utilisés dans une variété de troubles musculosquelettiques, y compris les maladies dégénératives des articulations des genoux et des hanches. Bien que ces suppléments soient occasionnellement recommandés pour les patients atteints de discarthrose et d’arthrose cervicale, aucune preuve ne vient à l’appui de cette utilisation.
Objectif :
Rechercher systématiquement et évaluer la qualité de la littérature concernant l’utilisation de la glucosamine, du sulfate de chondroïtine et du méthylsulfonylméthane pour le traitement de l’ostéo-arthrite et l’ostéoarthrose cervicale ainsi que de la discarthrose.
Sources :
Des essais cliniques aléatoires en anglais menés entre 1984 et juillet 2009 ont été recherchés dans l’Index of Chiropractic Literature, AMED, Medline, et CINAHL.
Extraction et synthèse des données :
Les données des études respectant les critères d’inclusion ont été extraites et examinées par trois réviseurs. L’échelle de Jadad a été utilisée pour évaluer la qualité des études. Il n’a pas été tenté de procéder à une méta-analyse en raison de la diversité des modèles d’étude.
Résultats :
Deux articles ont respecté les critères d’inclusion. Une étude était de bonne qualité, mais indiquait des résultats négatifs du groupe supplémenté par rapport au groupe placebo. L’autre étude était de moindre qualité mais indiquait des résultats positifs du groupe supplémenté par rapport à un groupe de contrôle.
Conclusion :
Peu de littérature était disponible à l’appui de l’utilisation de suppléments alimentaires communs dans le traitement de la dégénérescence cervicale. Il est par conséquent difficile de déterminer si les cliniciens doivent les recommander.
Keywords: examen systématique, glucosamine, chondroïtine, méthylsulfonylméthane, ostéo-arthrite, ostéoarthrose cervicale, discarthrose, colonne vertébrale
Introduction
Osteoarthritis is a pathology that affects approximately 15% of the world’s population.1 It is a chronic condition that is most prevalent in the elderly and three times more common in women than in men.2 Its characterizing feature is the progressive destruction of the articular cartilage of joint surfaces which can result in impaired joint biomechanics, swelling, pain, and disability.
Typically, the literature surrounding osteoarthritis is categorized according to the affected body region. Spinal osteoarthritis is one area that has garnered attention due to its relatively high prevalence and the impact that it can have on those affected. As individuals age, spinal osseous degeneration and age-related changes occur in the macroscopic, histologic and biochemical composition and structure of the nucleus pulposus and the annulus fibrosus. It has been suggested that these changes occur more frequently in the lumbar spine than the thoracic region due to the “splinting” by the costovertebral joints, and again less frequently in the cervical spine due to the relatively low need for weight-bearing.3
A common medicinal treatment for individuals suffering from spinal osteoarthritis is nonsteroidal anti-inflammatory drugs (NSAIDs), but with the associated serious gastrointestinal side effects many patients look towards complementary and alternative medicine to gain symptomatic relief and avoid iatrogenic illness.4
Glucosamine and chondroitin sulfate have been utilized medicinally in Europe for over 40 years and have gained in popularity in North America since the late 1990’s.5 Glucosamine and chondroitin sulfate, studied alone or in combination, appear to be somewhat effective for osteoarthritis of the knee6–8 or hip7,8 but there is no consensus with respect to a specific biochemical rationale or reasoning behind the results. It has been suggested that osteoarthritis is associated with a local deficiency in some key natural substances and that glucosamine acts as a substrate for cartilage repair by stimulating proteoglycan synthesis by chondrocytes.9 In the case of chondroitin, it has been contended that since it constitutes the majority of the glycosaminoglycans (GAGs) in articular cartilage, it helps to maintain the viscosity in joints, stimulates cartilage repair and inhibits enzymes that lead to degeneration of cartilage.10
More recently, methylsulfonylmethane or MSM has been promoted as a possible supplement for osteoarthritis due to its suggested anti-inflammatory and analgesic effects.11 Similar to glucosamine and chondroitin sulfate, most MSM research has evaluated the effects of MSM supplementation on knee osteoarthritis, as Usha and Naidu12 and Kim et al13 both looked at the effects of 12-weeks of supplementation with methylsulfonyl-methane on knee osteoarthritis. In both studies, there was a significant difference between the supplementation and placebo group with the supplementation group showing decreased pain levels. In the study by Usha and Naidu12 specifically, when methylsulfonylmethane and glucosamine were combined there was a significant difference in swelling index, joint function, walking time, joint mobility index, and overall function ability when compared to the placebo and the supplements when taken individually.
The objective of this paper was to systematically search and assess the quality of the literature on the use of glucosamine, chondroitin sulfate, and methylsulfonyl-methane, alone or in combination, for the treatment of spinal osteoarthritis / degenerative joint disease, and degenerative disc disease.
Methods
An electronic search for relevant literature was conducted on the Index of Chiropractic Literature, AMED, Medline, and CINAHL up to and including July 2009. Search terms consisted of combinations of glucosamine sulfate (GS), chondrotin sulfate (CS), or methylsulfonylmethane (MSM) with terms for spinal arthritis or osteoarthritis, spinal degenerative joint disease, or degenerative disc disease (the exact search terms and strategies employed are available from the authors). Relevant MeSH terms were employed whenever possible. The Cochrane Library was also searched for relevant reviews or articles using similar search terms. The authors also hand searched their personal libraries. Two of the authors (KS and SS) scrutinized the electronic search results, titles and abstracts in particular, to determine which full manuscripts should be obtained and evaluated. Each of these authors composed a list of studies from the electronic search results that they felt may be clinical studies using GS, CS, or MSM, these lists were compared and any differences were resolved by discussion to obtain a final list of manuscripts to obtain. The full manuscripts that were obtained were for any clinical studies on spinal arthritis, osteoarthritis, degenerative joint disease, or degenerative disc disease using GS, CS or MSM.
The inclusion criteria used for this review are indicated in Table 1, but consisted of studies that were randomized controlled trials conducted on patients with spinal degenerative joint disease, spinal osteoarthritis/osteoarthrosis, and/or degenerative disc disease. Interventions could include glucosamine (sulfate or HCl), chondroitin sulfate, and methylsulfonylmethane (MSM) in any combination or dosage with co-interventions being allowed; these could be compared to a do nothing control group, placebo, or another active intervention. Outcome measures of interest had to include at least one validated and reliable assessment of pain (such as a visual analog scale or numerical pain rating scale) or disability due to pain (such as the Oswestry Low Back Disability Index). Only articles published in a peer-reviewed journal in English within the past 25 years (1984–2009) were considered. These criteria were applied to all of the obtained full manuscripts. Reference searching was conducted from the reference lists of all retrieved studies.
Table 1.
Review Inclusion Criteria
|
One of the authors (KS) initially extracted data (such as sample details, interventions, outcome measures, results, adverse events, withdrawals/dropouts) from the studies meeting the inclusion criteria into a data extraction sheet that was checked and edited by the other authors (SS, KK). The Jadad scale (or Oxford quality scoring system) was used to assess study quality.14 The Jadad scale is among the most referenced and widely used of all quality scoring systems and considered valid and reliable.14,15 The Jadad scale asks questions about three different aspects of study design: double blinding, randomization, and the handling of withdrawals and dropouts.14 There are seven questions which lead to a score that out of five, with zero being the lowest score and five being the highest.14 We applied the classification developed by Abraham et al to determine whether included trials were of good or poor quality, where they defined a good quality trial as receiving four or higher on the Jadad scale and a poor quality trial as one earning three or less.16 All three authors independently reviewed the included studies and resolved any differences by discussion. No attempts were made at meta-analysis as it was felt there would be too much variation in study parameters to allow for suitable synthesis.
Results
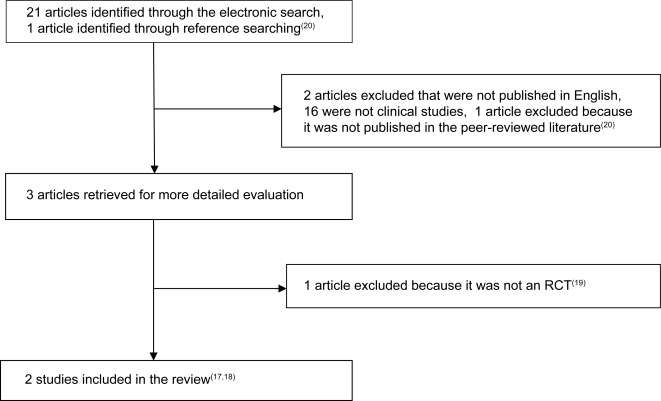
Figure 1 depicts the flow of trials through the review. The electronic database search initially yielded 17 articles from Medline, 2 from CINAHL, 2 from the Index to Chiropractic Literature, 1 from AMED, and none from the Cochrane Library, for a total or 21 articles excluding overlap. These search results were scrutinized and only three articles were obtained for full manuscript review. Upon review of these three manuscripts, two were found to be RCTs17,18 and one was a case report19 and thus excluded. One article was identified by reference searching from a previous systematic review of glucosamine for osteoarthritis8 but was excluded as it was not published in the peer-reviewed literature.20 Thus only two articles were accepted for analysis.17,18 Table 2 depicts the quality rating of the two articles included in the review using the Jadad scale. There was complete (100%) agreement between the authors on the rating of these articles.
Figure 1.
Literature Search Flow
Table 2.
Jadad Scale Scoring Results
| Jadad Scale Item | Leffler CT, et al. Military Med 1999; 164(2): 85–91. | Fujita T, et al. J Bone Miner Metab 2002; 20: 298–302. |
|---|---|---|
| Study described as randomized? | 1 | 1 |
| Randomization method described and appropriate? | 0 | 0 |
| Study described as double blind? | 1 | 0 |
| Double blinding method described and appropriate? | 1 | 0 |
| Description of withdrawals and dropouts? | 1 | 0 |
| Subtract 1 point if the randomization method was described but was inappropriate. | 0 | 0 |
| Subtract 1 point if the double blinding method was described but was inappropriate. | 0 | 0 |
| Total Score (/5) | 4 | 1 |
The paper by Leffler et al17 received a score of 4/5 which corresponds with a good quality article16, with the only point missing being for the description of randomization method. Leffler et al compared the use of a combination of glucosamine, chondroitin, and manganese ascorbate with placebo for patients with degenerative joint disease of the knee or low back. The subjects were 34 males in the United States Navy with x-ray proven degenerative changes in the knees or low back. The 23 subjects with low back DJD were 43.6 years old on average. The subjects received either oral Cosamin (at a dosage of 1500 mg/day of glucosamine HCl, 1200 mg/day of chondroitin sulphate, and 228 mg/day of manganese ascorbate) or a matching placebo, each taken three times daily. Subjects spent three weeks in a baseline period, then received either 8 weeks of Cosamin or placebo, then crossed over to the other group for a final 8 weeks. Subjects were not permitted to take NSAIDs during the trial but they could take acetaminophen as necessary.
Outcome measure assessment occurred after weeks 2 and 3 of the baseline period and after weeks 7 and 8 of each treatment period (thus 6 times in total); there was no long term follow-up. Outcome measures for the low back degenerative joint disease subjects included the Roland-Morris questionnaire for back disability, patient subjective assessment of handicap (from 0 to 5), physician assessment of severity (from 0 to 3), an 11 point visual analog scale, tenderness with movement of the low back (from 0 to 3), a sprint and stair run, Pavelka physical examination maneuvers, the Modified Schober technique for assessing lumbar flexion, and patient’s subjective assessment of results of treatment (from −3 to +3) (Leffler). All of these assessments were totalled to provide an overall summary score.
By the end of the trial, four patients withdrew from the low back degenerative joint disease group. There were no statistically significant changes in the low back group when considering the overall summary score or individual outcome measures. However, the summary score and patient assessment of treatment effect did show wide 95% Confidence Interval’s indicating that clinically meaningful results may have been obtained. No statistically significant differences were identified between groups with respect to reported adverse effects.
Fujita et al’s paper18 scored 1/5 which corresponds with a low quality article,16 with the only point allocated for the study being described as randomized. This study compared the use of a combination of glucosamine (1800 mg/day), active absorbable algal calcium (900 mg/day), porcine skin collagen (10,500 mg/day), composite muco-polysaccharide (600 mg/day), and vitamin C (600 mg/day) with control for 80 patients with knee or low back pain. The number of subjects in this trial with low back degenerative joint disease was not indicated. The subjects were randomly divided into two groups: one that would receive three daily doses of the glucosamine combination treatment over a four month time period (after a suitable washout period) and a second group that did not receive supplements. Subjects were not permitted analgesics during the trial. The average age of the subjects was approximately 65 years old and 75 out of 80 were female. The supplement group had an average of 1.47/3 in terms of radiographic degree of spondylosis deformans, compared with 1.65 in the control group (not a significant difference).
Outcome measures were assessed at baseline and after the 4 month trial period and included a subjective pain rating (from 0–3). Pain levels were also measured by skin impedance when quietly sitting (the basal value), when standing up, walking, squatting, climbing up and down stairs (which were all expressed in terms of percentage change from the basal value). Lumbar spinal radiographs and bone mineral densities were performed prior to and following the trial. The supplement group had a significant decrease in skin impedance from the beginning to conclusion of the trial; this change was not seen in the control group. Significant decreases in skin impedance during various tasks (standing up, walking, squatting, climbing up and down stairs) when compared with rest were again noted in the supplement group. Subjective pain values decreased significantly in the supplement group, but not the control group. The lumbar bone mineral density increased significantly in the supplement group; however the degree of vertebral deformity did not change in either group.
Discussion
Only two papers met the inclusion criteria for this review and these articles by Leffler et al17 and Fujita et al18 are contradictory in their findings. Leffler et al17 examined the effects of glucosamine, chrondroitin sulfate, and manganese ascorbate on degenerative joint disease of the low back or knee. These authors found no discernable improvements or change in the supplemented group when compared to the control group. The article was rated as having good quality.16 Fujita et al18 looked at the effect of glucosamine, active absorbable algal calcium, porcine skin collage, composite mucopolysaccharide, and vitamin C on back or knee pain. The results indicated that the group assigned to supplements had significant decreases in skin impedance and pain over the duration of the study when compared to the control group. This article was rated as having poor quality.16
The supplements used by the active treatment (i.e. non-control) groups in both Leffler et al’s17 and Fujita et al’s18 studies each had several components, thus it cannot be ascertained which component(s) of the supplements produced the improvements (if any) in those subjects. For the purposes of this review, it cannot be discerned whether the glucosamine or chondroitin sulfate produced any beneficial effects in the study by Leffler et al17 or if glucosamine was responsible for any improvements noted in the study by Fujita et al.18 The methods of randomization used in both of the included studies were not revealed. Both studies indicated that randomization of subjects did take place, but the exact methods were not disclosed and thus we cannot discern if the methods were appropriate. The follow-up periods of 7 to 8 weeks employed by Leffler et al17 and 4 months by Fujita et al18 were likely inadequate, given that spinal osteoarthritis is a long-term condition. It would seem more suitable for researchers to utilize longer follow-up periods of at least one year and preferably to five years or more. Both of the involved studies had mixed populations with Leffler et al including patients with low back and/or knee degenerative joint disease17 and Fujita et al including patients with back or knee pain.18 In the case of Fujita et al18 the average degree of spondylosis deformans on a scale of zero to three was noted at baseline indicating that there was some amount of radiograph-proven spinal osteoarthritis present on average, however the number of subjects diagnosed with spinal degeneration was not indicated.18
Thus, for the clinician there is contradictory evidence to support the use of glucosamine in the treatment of spinal osteoarthritis or disc degeneration based on the results of one positive study with low quality and one negative study with good quality. We identified no articles to support the use of chondroitin sulfate based on one negative study with good quality, or MSM based on no identified studies. Regardless, use of these supplements and their recommendation in practice is widespread.
In a recent randomized clinical trial analyzing the use of alternative therapies by individuals with osteoarthritis, the authors found that 47% of their participants reported utilizing at least one type of alternative care with the most common types being massage therapy (57% of alternative care users), chiropractic (20.7%) and non-prescribed alternative medications (17.2%).21 A survey of over 2,500 full-time chiropractors in the United States revealed that on average they treat patients with osteoarthritis/degenerative joint disease “often” which was equivalent to one to two times per week.22 The specific anatomic locations of the osteoarthritis/degenerative joint disease were not indicated in that survey. The specific methods of treatment for a patient with osteoarthritis/degenerative joint disease was not assessed, however 89% of the chiropractors surveyed utilized nutritional counselling, therapy, or supplementation in their practices over the previous year and on average they indicated that 34.6% of their patients would receive this type of passive adjunctive procedure.22
A prospective cohort study of a random sample of 9423 Canadians found that 11.5% of their participants were taking glucosamine five years into the trial compared with 1.6% at baseline.23 This increased usage was associated with several factors including age, presence of arthritis and or back pain, calcium intake, regular physical activity, and use of glucosamine previously.23 The authors felt that some participants use glucosamine to manage the symptoms of arthritis and/or back pain, while others use it on a preventive basis.23 In 2007 glucosamine was the second most commonly used natural health product, used by 19.9% of participants over the previous thirty days in a survey of adults in the general population of the United States who used nonvitamin, nonmineral health products.24 In the same survey chondroitin was used by 11.9% of the participants, ranking eighth, while MSM was used by 4.1% of the participants, ranking eighteenth.24
For clinicians who do choose to recommend these supplements, it is important to bear in mind that there are some potential side-effects or contraindications to their use. It has been proposed that glucosamine sulfate could potentially alter glucose control, specifically interfering with the hexosamine biosynthesis pathway,25 and as such down-regulating cellular glucose uptake and leading to hypergylcemia and insulin resistance. To date, no effects on glucose concentrations were documented in studies evaluating the use of long-term oral glucosamine for osteoarthritis.9,26 Although no specific glucosamine sulfate induced changes in glycemic control are found in the literature, it should be noted that the subjects in these studies had well-controlled type II diabetes and it is unclear how glucosamine sulfate would affect individuals with type I diabetes who are unable to secrete additional endogenous insulin to compensate for the potential glucosamine-induced insulin resistance.27 Recently, a case report by Knudsen and Sokol addressed the effects of glucosamine sulfate on an individual utilizing warfarin.28 The authors suggested that the supplementation of glucosamine or glucosamine combined with chondroitin sulfate in individuals consuming warfarin could potentiate the anticoagulant effects of war-farin and thereby increase the risk of bleeding.28 Although this was only a case report, chiropractors should be cognizant of this potential glucosamine-warfarin interaction as some of the patients for whom they may consider a recommendation for glucosamine may be currently taking war-farin as an anti-coagulant.
The literature regarding possible contraindications for MSM is limited, as no formal safety data or long-term assessment was available. Animal toxicity studies have shown only minor adverse effects in levels that are 5 to 7 times the proposed maximum recommended human dose of 6 grams per day.29 The only proposed adverse effects regarding human supplementation with MSM include allergic gastrointestinal disruptions and skin rashes.30
This review has some possible limitations including the limitations of the literature itself as there were only two articles that met our inclusion criteria. This calls into question whether there may be some publication bias in this area. It is striking that there have only been two RCTs published on these supplements for spinal osteoarthritis when compared with the number of available studies for these supplements on hip and knee osteoarthritis. Reference searching of the Cochrane systematic review by Towheed et al8 yielded an additional short term pilot RCT article pertaining to the use of glucosamine for osteoarthritis of the spine.20 However, this article was excluded from the review by Towheed et al8 and did not meet the inclusion criteria for this review as it was not published in the peer-reviewed literature; it was an unpublished technical report by a pharmaceutical company.20
From this limited evidence base firm conclusions cannot be drawn with respect to the effectiveness of glucosamine, chondroitin, or MSM for spinal osteoarthritis, because their effectiveness is still largely untested. It is difficult to generalize the findings from systematic reviews pertaining to topics that are as poorly studied as this one and if more studies had been eligible for this review it would have led to a stronger conclusion. However it is still important to present the results of such systematic reviews so that clinicians can make evidence-based decisions; there have even been systematic reviews published that had zero articles meet their inclusion criteria.31 From this overt lack of studies it can be easily stated that there is a definite need for more research in this area.
Another possible limitation of this review is by way of a language bias as we only permitted articles published in English. Furthermore we did not search the “grey” literature or additional electronic databases such as EMBASE. However, we conducted a multi-modal search strategy using several electronic databases with hand reference searching of obtained articles, thus numerous steps were taken to thoroughly evaluate the literature.
It could also be argued that a weakness in the methods of this systematic review was only including RCTs according to the inclusion criteria employed. However, in looking at the results of the literature search as seen in Figure 1, the only other clinical study identified in the electronic literature search, which was not limited by study type, was a case report by van Blitterswijkk et al19 that would have been excluded from the review regardless as it did not employ suitable outcome measures of pain and/or disability due to pain, along with the single study found through reference searching, the aforementioned pilot RCT20 which was excluded as it was not published in a peer-reviewed journal. As such, use of this particular inclusion criterion did not affect the outcome of the review.” The use of the Jadad scale may be questioned as it is a relatively simple tool for rating the quality of RCTs.14 The Jadad scale only evaluates randomization, blinding, and withdrawals and dropouts, and does not look at other areas to assess study quality.15 However the Jadad scale is the most frequently used health care literature quality assessment tool and it has been tested extensively and found to be valid and reliable.15
Future research in this area should take place to determine the rates at which health care professionals recommend or prescribe nutritional supplements such as glucosamine sulfate, chondroitin sulfate, and MSM to patients with spinal arthritis and disc degeneration, as well as the consumer usage rates of these supplements. Further clinical research by way of randomized controlled trials on the effects of these supplements is also suggested as there have been only two RCTs in this area using vastly different supplements and without solely examining patients with spinal osteoarthritis and/or disc degeneration.
Conclusions
Given the paucity of evidence surrounding the use of glucosamine, chondroitin, and MSM for spinal arthritis and disc degeneration and the conflicting results in the two studies that were identified, it would be difficult for evidence-based practitioners to justify the recommendation of these supplements for the pain and resultant disability from spinal degenerative conditions. There is an inadequate amount of literature examining the use of these supplements for lumbar spinal degenerative conditions in comparison with the volume available pertaining to their use for knee or hip osteoarthritis. Further research is necessary to clarify if these supplements are of any potential benefit for patients with spinal degenerative conditions.
Acknowledgments
The authors would like to thank and acknowledge Ms. Anne Taylor-Vaisey, reference librarian at the Canadian Memorial Chiropractic College for her assistance with the literature search.
Footnotes
No funding or support was received in conducting this study or preparing the manuscript.
References
- 1.Huskisson EC. Glucosamine and chondroitin for osteoarthritis. J Int Med Res. 2008;36(2):1161–1179. doi: 10.1177/147323000803600602. [DOI] [PubMed] [Google Scholar]
- 2.Hopman WM, Harrison MB, Friedberg E, Buchanan M, VanDenKerkhof EG. Associations between chronic disease, age and physical and mental health status. Chronic Dis Can. 2009;29(3):08–116. [PubMed] [Google Scholar]
- 3.Sarzi-Puttini P, Atzeni F, Fumagalli M, Capsoni F, Carrabba M. Osteoarthritis of the spine. Semin Arthritis Rheum. 2004;6(Suppl 2):38–43. [PubMed] [Google Scholar]
- 4.Lichtenstein DR, Syngal S, Wolfe MM. Nonsteroidal anti-inflammatory drugs and the gastrointestinal tract. The double-edged sword. Arthritis Rheum. 1995;38:5–18. doi: 10.1002/art.1780380103. [DOI] [PubMed] [Google Scholar]
- 5.Vangsness CT, Spiker W, Erickson J. A review of evidence-based medicine for glucosamine and chondroitin sulfate use in knee osteoarthritis. Arthroscopy. 2009;25(1):86–94. doi: 10.1016/j.arthro.2008.07.020. [DOI] [PubMed] [Google Scholar]
- 6.Richy F, Bruyere O, Ethgen O, Cucherat M, Henrotin Y, Reginster JY. Structural and symptomatic efficacy of glucosamine and chondroitin: a comprehensive meta-analysis. Arch Intern Med. 2003;163(13):1514–1522. doi: 10.1001/archinte.163.13.1514. [DOI] [PubMed] [Google Scholar]
- 7.McAlindon TE, LaValley MP, Gullin JP, Felson DT. Glucosamine and chondroitin for treatment of osteoarthritis: a systematic quality assessment and meta-analysis. JAMA. 2000;283(11):1469–1475. doi: 10.1001/jama.283.11.1469. [DOI] [PubMed] [Google Scholar]
- 8.Towheed T, Maxwell L, Anastassiades TP, Shea B, Houpt JB, Welch V, Hochberg MC, Wells GA. Glucosamine therapy for treating osteoarthritis. Cochrane Database of Systematic Reviews. 2005;(2) doi: 10.1002/14651858.CD002946.pub2. Art. No.: CD002946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pavelka K, Gatterova J, Olejarova M, Machacek S, Giacovelli G, Rovati LC. Glucosamine sulfate use and delay of progression of knee osteoarthritis: a 3-year, randomized, placebo-controlled, double-blind study. Arch Intern Med. 2002;162(18):2113–2123. doi: 10.1001/archinte.162.18.2113. [DOI] [PubMed] [Google Scholar]
- 10.Lippiello L, Woodward J, Karpman R, Hammad TA. In vivo chondroprotection and metabolic synergy of glucosamine and chondroitin sulfate. Clin Orthop Relat Res. 2000 Dec;(381):229–240. doi: 10.1097/00003086-200012000-00027. [DOI] [PubMed] [Google Scholar]
- 11.Ameye LG, Chee WS. Osteoarthrithis and nutrition. From nutriceuticals to functional foods: A systematic review of the scientific evidence. Arthritis Res Ther. 2006;8(4):R127. doi: 10.1186/ar2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Usha PR, Naidu MUR. Randomized double blind, parallel, placebo controlled study of oral glucosamine, methylsulfonylmethane and their combination in osteoarthritis. Clin Drug Investig. 2004;24(6):353–363. doi: 10.2165/00044011-200424060-00005. [DOI] [PubMed] [Google Scholar]
- 13.Kim LS, Axelrod LJ, Howard P, Buratovich N, Waters RF. Efficacy of methylsulfonylmethane (MSM) in osteoarthritis pain of the knee: A pilot clinical trial. Osteoarthritis Cartilage. 2006;14(3):286–294. doi: 10.1016/j.joca.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 14.Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJM, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Controlled Clinical Trials. 17(1):1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 15.Olivo SA, Macedo LG, Gadotti IC, Fuentes J, Stanton T, Magee DJ. Scales to assess the quality of randomized controlled trials: a systematic review. Phys Ther. 2008;88(2):156–75. doi: 10.2522/ptj.20070147. [DOI] [PubMed] [Google Scholar]
- 16.Abraham NS, Moayyedi P, Daniels B, Veldhuyzen Van Zanten SJ. Systematic review: the methodological quality of trials affects estimates of treatment efficacy in functional (non-ulcer) dyspepsia. Aliment Pharmacol Ther. 2004 Mar 15;19(6):631–41. doi: 10.1111/j.1365-2036.2004.01878.x. [DOI] [PubMed] [Google Scholar]
- 17.Leffler CT, Philippi AF, Leffler SG, Mosure JC, Kim PD. Glucosamine, chondroitin, and manganese ascorbate for degenerative joint disease of the knee or low back: a randomized, double-blind, placebo-controlled pilot study. Military Med. 1999;164(2):85–91. [PubMed] [Google Scholar]
- 18.Fujita T, Ohue M, Fujii Y, Miyauchi A, Takagi Y. The effect of active absorbable algal calcium (AAA Ca) with collagen and other matrix components on back and joint pain and skin impedance. J Bone Miner Metab. 2002;20:298–302. doi: 10.1007/s007740200043. [DOI] [PubMed] [Google Scholar]
- 19.van Blitterswijkk WJ, van de Nes JCM, Wuisman PIJM. Glucosamine and chondroitin sulphate supplementation to treat symptomatic disc degeneration: biochemical rational and case report. BMC Comp Alt Med. 2003;3(2) doi: 10.1186/1472-6882-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rovati LC, Poma A, Biavati G, et al. A multicenter, randomized, double-blind, parallel-group study to investigate efficacy and safety of oral glucosamine sulfate in osteoarthritis of the spine. Milan, Italy: Rotthapharm; 1993. Unpublished technical report prepared by Rottapharm.
- 21.Ramsey SD, Spencer AC, Topolski TD, Belza B, Patrick DL. Use of alternative therapies by older adults with osteoarthritis. Arthritis Rheum. 2001;45(3):222–227. doi: 10.1002/1529-0131(200106)45:3<222::AID-ART252>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 22.Christensen MG, Kollasch MW, Ward R, Webb KR, Day AA, zumBrunnen J, editors. National Board of Chiropractic Examiners: Job analysis of chiropractic 2005. 2005. pp. 1–205.
- 23.Hopman WM, Towheed TE, Gao Y, Berger C, Joseph L, Vik SA, Hanley D, Carran J, Anastassiades T. Prevalence of and factors associated with glucosamine use in Canada. OsteoArthritis Cartilage. 2006;14(12):1288–1293. doi: 10.1016/j.joca.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 24.Barnes PM, Bloom B, Nahim RL. National Health Statistics Reports. Hyattsville, MD: National Center for Health Statistics; 2008. Complementary and alternative medicine use among adults and children: United States, 2007; p. 12. [PubMed] [Google Scholar]
- 25.McClain DA, Crook ED. Hexosamines and insulin resistance. Diabetes. 1996;45(8):1003–1009. doi: 10.2337/diab.45.8.1003. [DOI] [PubMed] [Google Scholar]
- 26.Reginster JY, Deroisy R, Rovati LC, Lee Rl, Lejeune E, Bruyere O, Giacovelli G, Henrotin Y, Dacre JE, Gossett C. Long-term effects of glucosamine sulphate on osteoarthritis progression: a randomized, placebo-controlled clinical trial. Lancet. 2001;357(9252):251–256. doi: 10.1016/S0140-6736(00)03610-2. [DOI] [PubMed] [Google Scholar]
- 27.Stumpf JL, Lin S-W. Effect of glucosamine on glucose control. Ann Pharmacother. 2006;40(4):694–698. doi: 10.1345/aph.1E658. [DOI] [PubMed] [Google Scholar]
- 28.Knudsen JF, Sokol GH. Potential glucosamine-warfarin interaction resulting in increased international normalized ratio: case report and review of literature and medwatch database. Pharmocotherapy. 2008;28(4):540–548. doi: 10.1592/phco.28.4.540. [DOI] [PubMed] [Google Scholar]
- 29.Horvath K, Noker PE, Somfai-Relle S, Glavits R, Financsek I, Schauss AG. Toxicity of methylsulfonylmethane in rats. Food Chem Toxicol. 2002;40(10):1459–1462. doi: 10.1016/s0278-6915(02)00086-8. [DOI] [PubMed] [Google Scholar]
- 30.Brien S, Prescott P, Bashir N, Lewith H, Lewith G. Systematic review of the nutritional supplements dimethyl sulfoxide (DMSO) and methysulfonlmethane (MSM) in the treatment of osteoarthritis. Osteoarthritis and Cartilage. 2008;16(11):1277–1288. doi: 10.1016/j.joca.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 31.Richards CE, Magin PJ, Callister R. Is your prescription of distance running shoes evidence-based? Br J Sports Med. 2009;43(3):159–162. doi: 10.1136/bjsm.2008.046680. [DOI] [PubMed] [Google Scholar]