Abstract
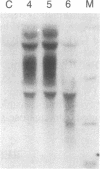
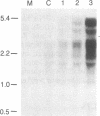
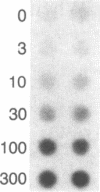
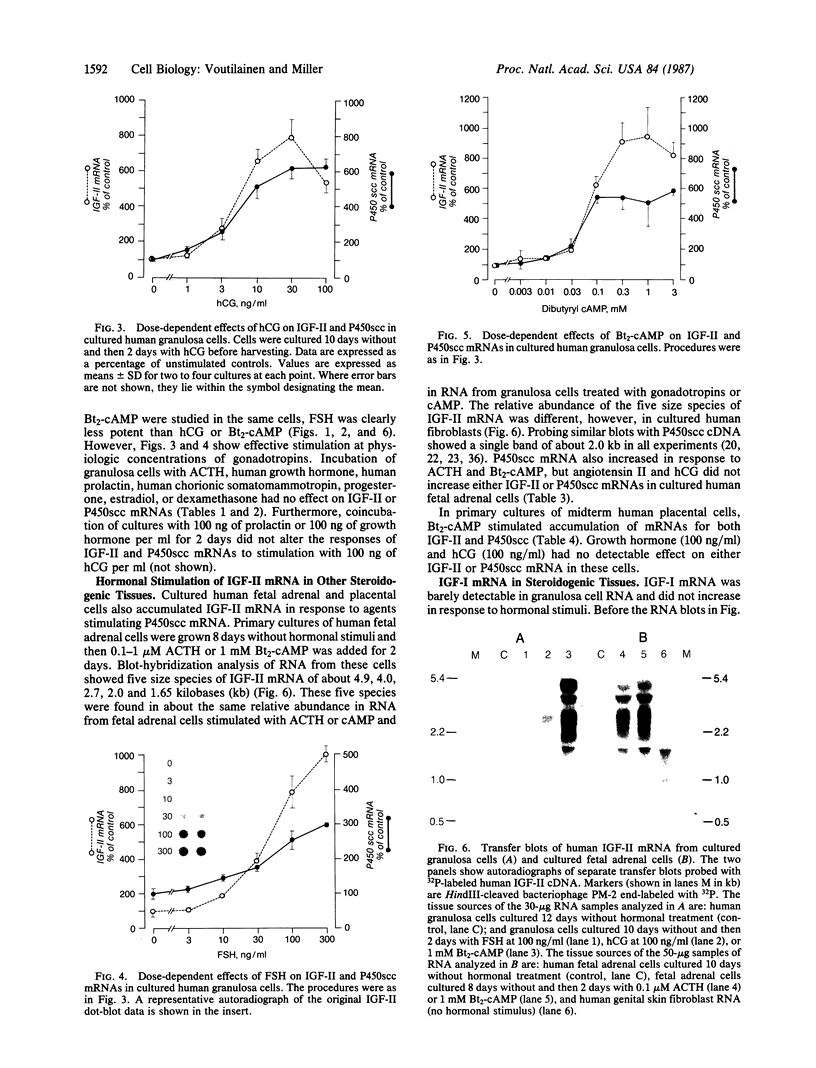
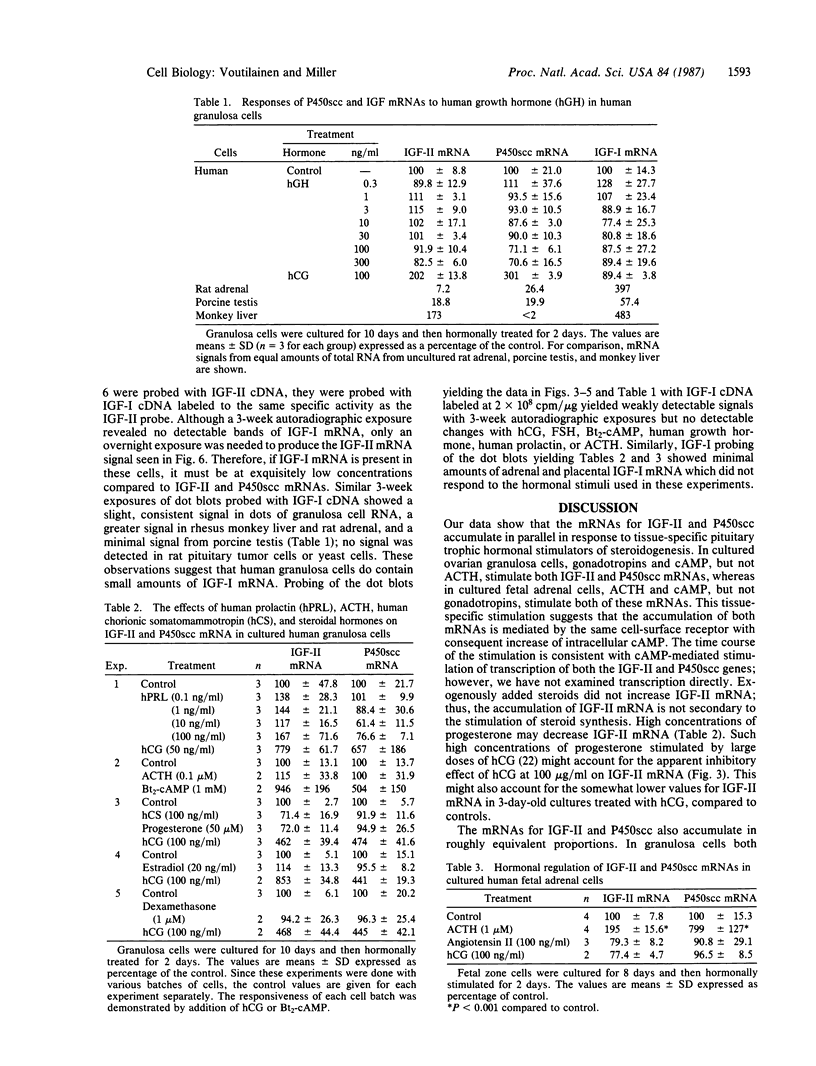
Insulin-like growth factors (IGFs) are single-chain polypeptides important for cell proliferation and growth. IGFs are produced in several tissues, suggesting that they function in a paracrine or autocrine fashion as well as functioning as endocrine hormones. We studied the hormonal regulation of IGF-I and IGF-II mRNA in human steroidogenic tissues. In cultured human ovarian granulosa cells, follicle-stimulating hormone, human chorionic gonadotropin, and dibutyryl cAMP increased IGF-II mRNA, but corticotropin [adrenocorticotropic hormone (ACTH)], chorionic somatomammotropin, growth hormone, prolactin, dexamethasone, estradiol, and progesterone had no effect. In cultured human fetal adrenal cells, ACTH and dibutyryl cAMP increased IGF-II mRNA accumulation, but human chorionic gonadotropin and angiotensin II did not. The same five size species of IGF-II mRNA were detected in transfer blots of RNA from granulosa cells and fetal adrenal cells, and all of these increased after hormonal stimuli. Dibutyryl cAMP also increased IGF-II mRNA accumulation in cultured human placental cells. Accumulation of mRNA for the cholesterol side-chain-cleavage monooxygenase [P450scc [corrected]; cholesterol, reduced-adrenal-ferredoxin:oxygen oxidoreductase (side-chain-cleaving), EC 1.14.15.6] was regulated in parallel with IGF-II mRNA in all these steroidogenic tissues. IGF-I mRNA was not detected in transfer blots of these RNAs, and the minimal amounts detected in dot blots showed no detectable change after any of the hormonal stimuli studied. The data indicate that the IGF-II gene is expressed in human steroidogenic tissues and is regulated by cAMP. These data suggest that IGF-II may act in an autocrine or paracrine fashion to stimulate the adrenal and gonadal growth stimulated by ACTH and gonadotropins, respectively.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adashi E. Y., Resnick C. E., Brodie A. M., Svoboda M. E., Van Wyk J. J. Somatomedin-C-mediated potentiation of follicle-stimulating hormone-induced aromatase activity of cultured rat granulosa cells. Endocrinology. 1985 Dec;117(6):2313–2320. doi: 10.1210/endo-117-6-2313. [DOI] [PubMed] [Google Scholar]
- Adashi E. Y., Resnick C. E., D'Ercole A. J., Svoboda M. E., Van Wyk J. J. Insulin-like growth factors as intraovarian regulators of granulosa cell growth and function. Endocr Rev. 1985 Summer;6(3):400–420. doi: 10.1210/edrv-6-3-400. [DOI] [PubMed] [Google Scholar]
- Adashi E. Y., Resnick C. E., Svoboda M. E., Van Wyk J. J. Somatomedin-C enhances induction of luteinizing hormone receptors by follicle-stimulating hormone in cultured rat granulosa cells. Endocrinology. 1985 Jun;116(6):2369–2375. doi: 10.1210/endo-116-6-2369. [DOI] [PubMed] [Google Scholar]
- Adashi E. Y., Resnick C. E., Svoboda M. E., Van Wyk J. J. Somatomedin-C synergizes with follicle-stimulating hormone in the acquisition of progestin biosynthetic capacity by cultured rat granulosa cells. Endocrinology. 1985 Jun;116(6):2135–2142. doi: 10.1210/endo-116-6-2135. [DOI] [PubMed] [Google Scholar]
- Baranao J. L., Hammond J. M. Comparative effects of insulin and insulin-like growth factors on DNA synthesis and differentiation of porcine granulosa cells. Biochem Biophys Res Commun. 1984 Oct 30;124(2):484–490. doi: 10.1016/0006-291x(84)91579-1. [DOI] [PubMed] [Google Scholar]
- Bell G. I., Gerhard D. S., Fong N. M., Sanchez-Pescador R., Rall L. B. Isolation of the human insulin-like growth factor genes: insulin-like growth factor II and insulin genes are contiguous. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6450–6454. doi: 10.1073/pnas.82.19.6450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell G. I., Merryweather J. P., Sanchez-Pescador R., Stempien M. M., Priestley L., Scott J., Rall L. B. Sequence of a cDNA clone encoding human preproinsulin-like growth factor II. 1984 Aug 30-Sep 5Nature. 310(5980):775–777. doi: 10.1038/310775a0. [DOI] [PubMed] [Google Scholar]
- Brissenden J. E., Ullrich A., Francke U. Human chromosomal mapping of genes for insulin-like growth factors I and II and epidermal growth factor. 1984 Aug 30-Sep 5Nature. 310(5980):781–784. doi: 10.1038/310781a0. [DOI] [PubMed] [Google Scholar]
- Chung B. C., Matteson K. J., Voutilainen R., Mohandas T. K., Miller W. L. Human cholesterol side-chain cleavage enzyme, P450scc: cDNA cloning, assignment of the gene to chromosome 15, and expression in the placenta. Proc Natl Acad Sci U S A. 1986 Dec;83(23):8962–8966. doi: 10.1073/pnas.83.23.8962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Ercole A. J., Hill D. J., Strain A. J., Underwood L. E. Tissue and plasma somatomedin-C/insulin-like growth factor I concentrations in the human fetus during the first half of gestation. Pediatr Res. 1986 Mar;20(3):253–255. doi: 10.1203/00006450-198603000-00011. [DOI] [PubMed] [Google Scholar]
- D'Ercole A. J., Stiles A. D., Underwood L. E. Tissue concentrations of somatomedin C: further evidence for multiple sites of synthesis and paracrine or autocrine mechanisms of action. Proc Natl Acad Sci U S A. 1984 Feb;81(3):935–939. doi: 10.1073/pnas.81.3.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daughaday W. H., Yanow C. E., Kapadia M. Insulin-like growth factors I and II in maternal and fetal guinea pig serum. Endocrinology. 1986 Aug;119(2):490–494. doi: 10.1210/endo-119-2-490. [DOI] [PubMed] [Google Scholar]
- Davoren J. B., Hsueh A. J. Growth hormone increases ovarian levels of immunoreactive somatomedin C/insulin-like growth factor I in vivo. Endocrinology. 1986 Feb;118(2):888–890. doi: 10.1210/endo-118-2-888. [DOI] [PubMed] [Google Scholar]
- Dull T. J., Gray A., Hayflick J. S., Ullrich A. Insulin-like growth factor II precursor gene organization in relation to insulin gene family. 1984 Aug 30-Sep 5Nature. 310(5980):777–781. doi: 10.1038/310777a0. [DOI] [PubMed] [Google Scholar]
- Fant M., Munro H., Moses A. C. An autocrine/paracrine role for insulin-like growth factors in the regulation of human placental growth. J Clin Endocrinol Metab. 1986 Aug;63(2):499–505. doi: 10.1210/jcem-63-2-499. [DOI] [PubMed] [Google Scholar]
- Gunning P., Ponte P., Okayama H., Engel J., Blau H., Kedes L. Isolation and characterization of full-length cDNA clones for human alpha-, beta-, and gamma-actin mRNAs: skeletal but not cytoplasmic actins have an amino-terminal cysteine that is subsequently removed. Mol Cell Biol. 1983 May;3(5):787–795. doi: 10.1128/mcb.3.5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond J. M., Baranao J. L., Skaleris D., Knight A. B., Romanus J. A., Rechler M. M. Production of insulin-like growth factors by ovarian granulosa cells. Endocrinology. 1985 Dec;117(6):2553–2555. doi: 10.1210/endo-117-6-2553. [DOI] [PubMed] [Google Scholar]
- Haselbacher G. K., Schwab M. E., Pasi A., Humbel R. E. Insulin-like growth factor II (IGF II) in human brain: regional distribution of IGF II and of higher molecular mass forms. Proc Natl Acad Sci U S A. 1985 Apr;82(7):2153–2157. doi: 10.1073/pnas.82.7.2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John M. E., John M. C., Ashley P., MacDonald R. J., Simpson E. R., Waterman M. R. Identification and characterization of cDNA clones specific for cholesterol side-chain cleavage cytochrome P-450. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5628–5632. doi: 10.1073/pnas.81.18.5628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura T. ACTH stimulation on cholesterol side chain cleavage activity of adrenocortical mitochondria. Transfer of the stimulus from plasma membrane to mitochondria. Mol Cell Biochem. 1981 Apr 27;36(2):105–122. doi: 10.1007/BF02354909. [DOI] [PubMed] [Google Scholar]
- Matteson K. J., Chung B. C., Urdea M. S., Miller W. L. Study of cholesterol side-chain cleavage (20,22 desmolase) deficiency causing congenital lipoid adrenal hyperplasia using bovine-sequence P450scc oligodeoxyribonucleotide probes. Endocrinology. 1986 Apr;118(4):1296–1305. doi: 10.1210/endo-118-4-1296. [DOI] [PubMed] [Google Scholar]
- Milner R. D., Hill D. J. Fetal growth control: the role of insulin and related peptides. Clin Endocrinol (Oxf) 1984 Oct;21(4):415–433. doi: 10.1111/j.1365-2265.1984.tb03229.x. [DOI] [PubMed] [Google Scholar]
- Moses A. C., Nissley S. P., Short P. A., Rechler M. M., White R. M., Knight A. B., Higa O. Z. Increased levels of multiplication-stimulating activity, an insulin-like growth factor, in fetal rat serum. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3649–3653. doi: 10.1073/pnas.77.6.3649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeve A. E., Eccles M. R., Wilkins R. J., Bell G. I., Millow L. J. Expression of insulin-like growth factor-II transcripts in Wilms' tumour. Nature. 1985 Sep 19;317(6034):258–260. doi: 10.1038/317258a0. [DOI] [PubMed] [Google Scholar]
- Rotwein P. Two insulin-like growth factor I messenger RNAs are expressed in human liver. Proc Natl Acad Sci U S A. 1986 Jan;83(1):77–81. doi: 10.1073/pnas.83.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SALMON W. D., Jr, DAUGHADAY W. H. A hormonally controlled serum factor which stimulates sulfate incorporation by cartilage in vitro. J Lab Clin Med. 1957 Jun;49(6):825–836. [PubMed] [Google Scholar]
- Savion N., Lui G. M., Laherty R., Gospodarowicz D. Factors controlling proliferation and progesterone production by bovine granulosa cells in serum-free medium. Endocrinology. 1981 Aug;109(2):409–420. doi: 10.1210/endo-109-2-409. [DOI] [PubMed] [Google Scholar]
- Scott J., Cowell J., Robertson M. E., Priestley L. M., Wadey R., Hopkins B., Pritchard J., Bell G. I., Rall L. B., Graham C. F. Insulin-like growth factor-II gene expression in Wilms' tumour and embryonic tissues. Nature. 1985 Sep 19;317(6034):260–262. doi: 10.1038/317260a0. [DOI] [PubMed] [Google Scholar]
- Simpson E. R. Cholesterol side-chain cleavage, cytochrome P450, and the control of steroidogenesis. Mol Cell Endocrinol. 1979 Mar;13(3):213–227. doi: 10.1016/0303-7207(79)90082-0. [DOI] [PubMed] [Google Scholar]
- Tricoli J. V., Rall L. B., Scott J., Bell G. I., Shows T. B. Localization of insulin-like growth factor genes to human chromosomes 11 and 12. 1984 Aug 30-Sep 5Nature. 310(5980):784–786. doi: 10.1038/310784a0. [DOI] [PubMed] [Google Scholar]
- Veldhuis J. D., Furlanetto R. W. Trophic actions of human somatomedin C/insulin-like growth factor I on ovarian cells: in vitro studies with swine granulosa cells. Endocrinology. 1985 Apr;116(4):1235–1242. doi: 10.1210/endo-116-4-1235. [DOI] [PubMed] [Google Scholar]
- Veldhuis J. D., Rodgers R. J., Dee A., Simpson E. R. The insulin-like growth factor, somatomedin C, induces the synthesis of cholesterol side-chain cleavage cytochrome P-450 and adrenodoxin in ovarian cells. J Biol Chem. 1986 Feb 25;261(6):2499–2502. [PubMed] [Google Scholar]
- Voutilainen R., Miller W. L. Developmental expression of genes for the stereoidogenic enzymes P450scc (20,22-desmolase), P450c17 (17 alpha-hydroxylase/17,20-lyase), and P450c21 (21-hydroxylase) in the human fetus. J Clin Endocrinol Metab. 1986 Nov;63(5):1145–1150. doi: 10.1210/jcem-63-5-1145. [DOI] [PubMed] [Google Scholar]
- Voutilainen R., Tapanainen J., Chung B. C., Matteson K. J., Miller W. L. Hormonal regulation of P450scc (20,22-desmolase) and P450c17 (17 alpha-hydroxylase/17,20-lyase) in cultured human granulosa cells. J Clin Endocrinol Metab. 1986 Jul;63(1):202–207. doi: 10.1210/jcem-63-1-202. [DOI] [PubMed] [Google Scholar]
- Zapf J., Walter H., Froesch E. R. Radioimmunological determination of insulinlike growth factors I and II in normal subjects and in patients with growth disorders and extrapancreatic tumor hypoglycemia. J Clin Invest. 1981 Nov;68(5):1321–1330. doi: 10.1172/JCI110379. [DOI] [PMC free article] [PubMed] [Google Scholar]