Abstract
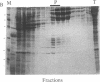
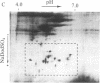
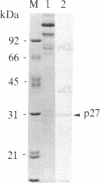
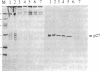
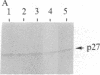
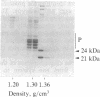
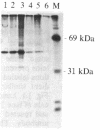
A cytoplasmic particle displaying properties in common with a structure present in duck erythroblasts, termed the prosome, has been isolated from eggs and embryos of two species of sea urchin. This particle was partially purified by sedimentation in sucrose gradients containing 0.5 M KCl, and some of its physical properties and its behavior during early development were determined. The prosome sediments between 16 and 19 S and has a buoyant density of 1.30 g/cm3 in Cs2SO4 gradients. Biochemically, the particle is characterized as 20-25 polypeptides of molecular size 24-35 kDa with about 10 small RNAs. A monoclonal antibody directed against the 27-kDa protein of duck erythroblast prosome recognizes a 27-kDa protein of the sea urchin prosome. We have used this protein, as representative of the prosome, to immunologically determine the level and the subcellular localization of the particle during development. Immunoblotting and cellular fractionation studies show that the 27-kDa prosome polypeptide is present almost entirely in the postribosomal supernatant of unfertilized egg lysates. After fertilization and during early development, the total amount of 27-kDa protein per embryo remains constant, but the amount in the postribosomal supernatant decreases; there is a concomitant increase in the level of the 27-kDa protein in a rapidly sedimenting, particulate fraction containing nuclei. Immunofluorescence studies further show that the 27-kDa protein is located mainly in the cytoplasm of eggs and two-cell embryos. The subcellular location of the prosome, therefore, appears to change during development. In vivo labeling experiments have failed to detect the synthesis of either the prosome proteins or RNAs in eggs and embryos up to 48 hr of development, suggesting that this cytoplasmic particle is not synthesized de novo in early embryogenesis and thus is metabolically stable. The prosome is thus a normal cellular constituent of the sea urchin and is most likely synthesized during oogenesis and stored in the unfertilized egg.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arrigo A. P., Darlix J. L., Khandjian E. W., Simon M., Spahr P. F. Characterization of the prosome from Drosophila and its similarity to the cytoplasmic structures formed by the low molecular weight heat-shock proteins. EMBO J. 1985 Feb;4(2):399–406. doi: 10.1002/j.1460-2075.1985.tb03642.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibring T., Baxandall J. Tubulin synthesis in sea urchin embryos. II. Ciliary A tubulin derives from the unfertilized egg. Dev Biol. 1981 Apr 15;83(1):122–126. doi: 10.1016/s0012-1606(81)80014-0. [DOI] [PubMed] [Google Scholar]
- Busch H., Reddy R., Rothblum L., Choi Y. C. SnRNAs, SnRNPs, and RNA processing. Annu Rev Biochem. 1982;51:617–654. doi: 10.1146/annurev.bi.51.070182.003153. [DOI] [PubMed] [Google Scholar]
- Castaño J. G., Ornberg R., Koster J. G., Tobian J. A., Zasloff M. Eukaryotic pre-tRNA 5' processing nuclease: copurification with a complex cylindrical particle. Cell. 1986 Aug 1;46(3):377–385. doi: 10.1016/0092-8674(86)90658-6. [DOI] [PubMed] [Google Scholar]
- De Robertis E. M., Lienhard S., Parisot R. F. Intracellular transport of microinjected 5S and small nuclear RNAs. Nature. 1982 Feb 18;295(5850):572–577. doi: 10.1038/295572a0. [DOI] [PubMed] [Google Scholar]
- Dworkin M. B., Infante A. A. Relationship between the mRNA of polysomes and free ribonucleoprotein particles in the early sea urchin embryo. Dev Biol. 1976 Oct 1;53(1):73–90. doi: 10.1016/0012-1606(76)90210-4. [DOI] [PubMed] [Google Scholar]
- Gander E. S., Stewart A. G., Morel C. M., Scherrer K. Isolation and characterization of ribosome-free cytoplasmic messenger-ribonucleoprotein complexes from avian erythroblasts. Eur J Biochem. 1973 Oct 18;38(3):443–452. doi: 10.1111/j.1432-1033.1973.tb03078.x. [DOI] [PubMed] [Google Scholar]
- Gordon K., Infante A. A. Utilization of maternal and embryonic histone RNA in early sea urchin development. Dev Biol. 1983 Feb;95(2):414–420. doi: 10.1016/0012-1606(83)90042-8. [DOI] [PubMed] [Google Scholar]
- Infante A. A., Heilmann L. J. Distribution of messenger ribonucleic acid in polysomes and nonpolysomal particles of sea urchin embryos: translational control of actin synthesis. Biochemistry. 1981 Jan 6;20(1):1–8. doi: 10.1021/bi00504a001. [DOI] [PubMed] [Google Scholar]
- Infante A. A., Nemer M. Heterogeneous ribonucleoprotein particles in the cytoplasm of sea urchin embryos. J Mol Biol. 1968 Mar 28;32(3):543–565. doi: 10.1016/0022-2836(68)90342-2. [DOI] [PubMed] [Google Scholar]
- Kleinschmidt J. A., Hügle B., Grund C., Franke W. W. The 22 S cylinder particles of Xenopus laevis. I. Biochemical and electron microscopic characterization. Eur J Cell Biol. 1983 Nov;32(1):143–156. [PubMed] [Google Scholar]
- Kuhn O., Wilt F. H. Chromatin proteins of sea urchin embryos: dual origin from an oogenetic reservoir and new synthesis. Dev Biol. 1981 Jul 30;85(2):416–424. doi: 10.1016/0012-1606(81)90273-6. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lerner M. R., Steitz J. A. Snurps and scyrps. Cell. 1981 Aug;25(2):298–300. doi: 10.1016/0092-8674(81)90047-7. [DOI] [PubMed] [Google Scholar]
- Martins de Sa C., Grossi de Sa M. F., Akhayat O., Broders F., Scherrer K., Horsch A., Schmid H. P. Prosomes. Ubiquity and inter-species structural variation. J Mol Biol. 1986 Feb 20;187(4):479–493. doi: 10.1016/0022-2836(86)90328-1. [DOI] [PubMed] [Google Scholar]
- Mazia D., Schatten G., Sale W. Adhesion of cells to surfaces coated with polylysine. Applications to electron microscopy. J Cell Biol. 1975 Jul;66(1):198–200. doi: 10.1083/jcb.66.1.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy T. L., Siegel E., Mroczkowski B., Heywood S. M. Characterization of translational-control ribonucleic acid isolated from embryonic chick muscle. Biochemistry. 1983 Feb 15;22(4):935–941. doi: 10.1021/bi00273a035. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. Z., Goodman H. M., O'Farrell P. H. High resolution two-dimensional electrophoresis of basic as well as acidic proteins. Cell. 1977 Dec;12(4):1133–1141. doi: 10.1016/0092-8674(77)90176-3. [DOI] [PubMed] [Google Scholar]
- Preobrazhensky A. A., Spirin A. S. Informosomes and their protein components: the present state of knowledge. Prog Nucleic Acid Res Mol Biol. 1978;21:1–38. doi: 10.1016/s0079-6603(08)60265-2. [DOI] [PubMed] [Google Scholar]
- Schmid H. P., Akhayat O., Martins De Sa C., Puvion F., Koehler K., Scherrer K. The prosome: an ubiquitous morphologically distinct RNP particle associated with repressed mRNPs and containing specific ScRNA and a characteristic set of proteins. EMBO J. 1984 Jan;3(1):29–34. doi: 10.1002/j.1460-2075.1984.tb01757.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuldt C., Kloetzel P. M. Analysis of cytoplasmic 19 S ring-type particles in Drosophila which contain hsp 23 at normal growth temperature. Dev Biol. 1985 Jul;110(1):65–74. doi: 10.1016/0012-1606(85)90064-8. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent A., Goldenberg S., Scherrer K. Comparisons of proteins associated with duck-globin mRNA and its polyadenylated segment in polyribosomal and repressed free messenger ribonucleoprotein complexes. Eur J Biochem. 1981 Feb;114(2):179–193. doi: 10.1111/j.1432-1033.1981.tb05135.x. [DOI] [PubMed] [Google Scholar]
- Weber K., Bibring T., Osborn M. Specific visualization of tubulin-containing structures in tissue culture cells by immunofluorescence. Cytoplasmic microtubules, vinblastine-induced paracrystals, and mitotic figures. Exp Cell Res. 1975 Oct 1;95(1):111–120. doi: 10.1016/0014-4827(75)90615-1. [DOI] [PubMed] [Google Scholar]