Abstract
Background
Robotic-assisted laparoscopic surgery (RALS) is evolving as an important surgical approach in the field of colorectal surgery. We aimed to evaluate the learning curve for RALS procedures involving resections of the rectum and rectosigmoid.
Methods
A series of 50 consecutive RALS procedures were performed between August 2008 and September 2009. Data were entered into a retrospective database and later abstracted for analysis. The surgical procedures included abdominoperineal resection (APR), anterior rectosigmoidectomy (AR), low anterior resection (LAR), and rectopexy (RP). Demographic data and intraoperative parameters including docking time (DT), surgeon console time (SCT), and total operative time (OT) were analyzed. The learning curve was evaluated using the cumulative sum (CUSUM) method.
Results
The procedures performed for 50 patients (54% male) included 25 AR (50%), 15 LAR (30%), 6 APR (12%), and 4 RP (8%). The mean age of the patients was 54.4 years, the mean BMI was 27.8 kg/m2, and the median American Society of Anesthesiologists (ASA) classification was 2. The series had a mean DT of 14 min, a mean SCT of 115.1 min, and a mean OT of 246.1 min. The DT and SCT accounted for 6.3% and 46.8% of the OT, respectively. The SCT learning curve was analyzed. The CUSUMSCT learning curve was best modeled as a parabola, with equation CUSUMSCT in minutes equal to 0.73 × case number2 − 31.54 × case number − 107.72 (R = 0.93). The learning curve consisted of three unique phases: phase 1 (the initial 15 cases), phase 2 (the middle 10 cases), and phase 3 (the subsequent cases). Phase 1 represented the initial learning curve, which spanned 15 cases. The phase 2 plateau represented increased competence with the robotic technology. Phase 3 was achieved after 25 cases and represented the mastery phase in which more challenging cases were managed.
Conclusions
The three phases identified with CUSUM analysis of surgeon console time represented characteristic stages of the learning curve for robotic colorectal procedures. The data suggest that the learning phase was achieved after 15 to 25 cases.
Keywords: Cumulative sum analysis, Learning curve, Rectosigmoid, Robotic-assisted laparoscopic surgery
Surgical proficiency is improved with ongoing innovations in surgical instrumentation and technology. The acquisition of competency in novel surgical techniques represents a “learning curve” for each surgeon. The learning curve, in addition to being a function of the surgeon’s understanding of the new technique, technical modifications to the technique, and improvements in support staff and perioperative care [1], is a function of the surgeon’s evolving ease with the procedure and performance of more challenging cases.
In 1995, a robotic-assisted laparoscopic system prototype was developed for abdominal procedures [2], and in 2001, Weber et al. [3] performed the first robotic-assisted laparoscopic colectomy for benign disease. Soon afterward, Hashizume and Tsugawa [4] reported three colonic resections for malignant disease using a robotic system. Since then, the robotic-assisted laparoscopic system has gained popularity, with more surgeons using it for colorectal procedures of the pelvis, both benign and malignant. With the adoption of new techniques, it is important to assess the effect on the surgeon’s learning curve.
The cumulative sum (CUSUM) technique is a method originally devised for monitoring performance and detecting areas for improvement in the industrial sector. This method was adopted by the medical profession in the 1970s to analyze the learning curve for surgical procedures [5, 6]. Cumulative sum analysis transforms raw data into the running total of data deviations from their group mean, enabling investigators to visualize the data for trends not discernable with other approaches.
Multiple reports on robotic-assisted laparoscopic surgery have been published, but none have evaluated the learning curve in robotic colorectal surgery using CUSUM analysis. We aimed to analyze the learning curve for robotic-assisted laparoscopic rectosigmoid surgery using CUSUM methodology.
Materials and methods
This study was approved by the institutional review board. Data were abstracted into a retrospective database for analysis. Fifty consecutive robotic-assisted laparoscopic surgery (RALS) procedures were performed between August 2008 and September 2009 by an experienced laparoscopic colorectal surgeon (E.M.H.).
Docking time (DT) was defined as the time required to position the robot and secure the robotic arms to the corresponding port sites. The surgeon console time (SCT) was the actual time the surgeon spent at the robotic console during the procedure, which directly corresponded to the robotic portion of the procedure. The total operative time (OT) spanned the time from the first incision to the final closure. The surgical procedures included abdominoperineal resection (APR), anterior rectosigmoidectomy (AR), low anterior resection (LAR), and rectopexy (RP).
All the procedures were performed in a medial-to-lateral approach, with early identification and ligation of the inferior mesenteric artery and vein when indicated. The left ureter and hypogastric plexus were routinely identified and preserved. When required, the splenic flexure was taken down using conventional laparoscopic technique. The anastomoses were performed intracorporeally with the ECS29 circular stapling device (Endopath® ILS; Ethicon Endo-Surgery, Cincinnati, OH, USA).
Patients who underwent pelvic radiation were routinely diverted with loop ileostomy. Demographic data including patient gender, age, body mass index (BMI), and American Society of Anesthesiologists (ASA) score were tabulated. Intraoperative parameters including DT, SCT, OT, and estimated blood loss (EBL) were analyzed as well as the patient’s hospital length of stay (LOS).
Cumulative sum analysis
The CUSUM technique was used for quantitative assessment of the learning curve. The CUSUM is the running total of differences between the individual data points and the mean of all data points. Thus, CUSUM can be performed recursively. The CUSUM technique was used for 48 cases that had SCT data available.
First, the cases were ordered chronologically, from the earliest to the latest date of surgery. The CUSUMSCT of the first case was the difference between the SCT for the first case and the mean SCT for all the cases (μSCT). The CUSUMSCT of the second case was the previous case’s CUSUMSCT added to the difference between the SCT for the second case and μSCT. This recursive process continued until CUSUMSCT for the last case was calculated as zero. Because no patient deaths occurred in this series, risk-adjusted CUSUM (RA-CUSUM) [7, 8] was not performed.
Statistical analysis
Statistical analysis was performed using Intercooled Stata version 9 software (StataCorp LP, College Station, TX, USA). For interphase comparisons, the Wilcoxon rank-sum test was used (alpha after Bonferroni correction = 0.0167). Comparisons also were made between phases 1 and 2 combined (learning curve) and phase 3 (competency) using a two-tailed Student’s t-test (alpha = 0.05).
Results
During the study period, 50 patients underwent RALS for rectosigmoid resection. Patient demographics, preoperative diagnoses, surgical procedures, operative characteristics, and postoperative outcomes are summarized in Table 1. The series comprised 23 females (46%) and 27 males (54%) with a mean age of 54.4 years, a mean BMI of 27.8 kg/m2, and a median ASA of 2. Surgery was performed for malignant disease in 22 cases (44%) and benign disease in 28 cases (56%). Of the 50 patients, 25 (50%) underwent AR, 15 had LAR (30%), 6 had APR (12%), and 4 (8%) had RP. The majority of ARs (72%) were performed for diverticular disease, whereas the majority of LARs (66.7%) were performed for malignant disease. All 6 APRs were performed for malignant disease, including 5 for anal cancer. The mean DT was 14 min (range, 6–45 min), the mean SCT was 115.1 min (range, 40–210 min), and the mean OT was 246.1 min (range, 90–540 min). The DT and SCT accounted for 6.3% and 46.8% of the OT, respectively.
Table 1.
Patient demographics, preoperative diagnoses, surgical procedures, operative characteristics, and postoperative outcomes (n = 50, unless otherwise specified)
| Category | Parameter | Mean ± SD | Range | Median |
|---|---|---|---|---|
| Patient characteristics | Age (years) | 54.4 ± 13.1 | 24–82 | 53.5 |
| ASA score | 2.3 ± 0.5 | 2–4 | 2 | |
| BMI (kg/m2) | 27.8 ± 6.3 | 16–49.4 | 26.9 | |
| Gender: 27 male (54%), 23 female (46%) | ||||
| Preoperative | Malignant (n = 22; 44%) | |||
| Diagnosis | Diverticulitis (n = 18, 36%) | |||
| Rectal prolapse (n = 5, 10%) | ||||
| Endometriosis (n = 2, 4%) | ||||
| Other pathology (n = 3, 6%) | ||||
| Surgical | AR (n = 25, 50%) | |||
| Procedure | LAR (n = 15, 30%) | |||
| APR (n = 6, 12%) | ||||
| RP (n = 4, 8%) | ||||
| Intraoperative parameters | DT (min, n = 48) | 14.0 ± 7.7 | 6–45 | 12 |
| SCT (min, n = 48) | 115.1 ± 46.9 | 40–210 | 112.5 | |
| OT (min) | 246.1 ± 80.7 | 90–540 | 240 | |
| EBL (ml) | 106.9 ± 58.0 | 20–250 | 100 | |
| Short-term postoperative outcome | LOS (days) | 3.5 ± 2.3 | 2–16 | 3 |
ASA American Society of Anesthesiologists, BMI body mass index, AR anterior resection, LAR lower anterior resection, APR abdominoperineal resection, RP rectopexy, DT docking time, SCT surgeon console time, EBL estimated blood loss, OT total operative time, LOS hospital length of stay
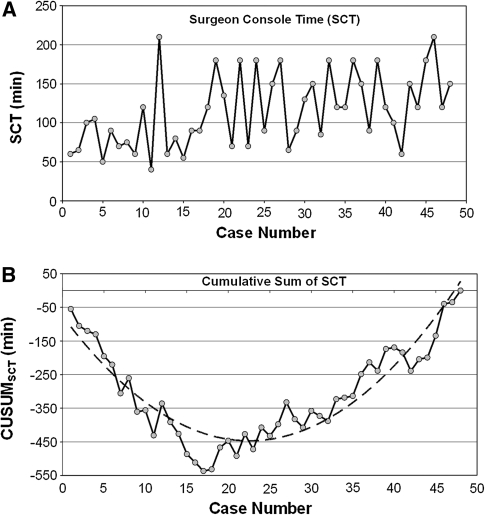
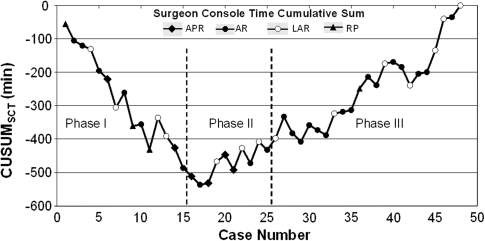
The raw SCT times were plotted in chronological case order (Fig. 1A). The CUSUMSCT learning curve was best modeled as a second-order polynomial (parabola) with equation CUSUMSCT in minutes equal to 0.73 × case number2 − 31.54 × case number − 107.72, which had a high R value of 0.93 (Fig. 1B). The CUSUMSCT learning curve was observed to consist of three unique phases: phase 1 (the initial 15 cases), phase 2 (the middle 10 cases), and phase 3 (the final 23 cases) (Fig. 2).
Fig. 1.
Surgeon console time (SCT). A SCT plotted against case number. B Cumulative sum (CUSUM)SCT plotted against case number (solid line). The dashed line represents the curve of best fit for the plot (a second-order polynomial with equation CUSUMSCT = 0.73 × case number2 − 31.54 × case number − 107.72 (R = 0.93)
Fig. 2.
Three phases of the surgeon console time (SCT) in terms of the cumulative sum (CUSUM) learning curve. The solid diamond represents abdominoperineal resection (APR), and the solid circle represents anterior resection (AR). The open circle represents low anterior resection (LAR), and the solid triangle represents rectopexy (RP)
Comparisons of various parameters between the three phases identified by CUSUMSCT analysis are presented in Table 2. Age, BMI, ASA, and previous surgical history did not differ significantly among the three phases. The proportion of cases involving performance of an anastomosis (AR and LAR) differed significantly between phase 3 and phases 1 and 2 combined (p < 0.008).
Table 2.
Interphase comparisons of patient characteristics and other parameters (mean ± standard deviation)
| Characteristic | Phase 1 (n = 15) |
Phase 2 (n = 10) |
Phase 3 (n = 23) |
Phases 1 and 2 vs Phase 3 (p-value) |
|---|---|---|---|---|
| Age (years) | 51.7 ± 12.5 | 59.0 ± 8.3 | 53.2 ± 14.7 | NS |
| BMI (kg/m2) | 26.8 ± 8.0 | 27.2 ± 4.6 | 28.7 ± 5.7 | NS |
| ASA | 2.5 ± 0.6 | 2.4 ± 0.4 | 2.2 ± 0.4 | NS |
| Female/male | 10:5 | 4:6 | 8:15 | NS |
| Malignant disease (%) | 4 (26.7) | 7 (70) | 9 (39.1) | NS |
| APR (%) | 2 (13.3) | 4 (40) | 0 (0) | <0.013a |
| RP (%) | 3 (20) | 0 (0) | 1 (4.3) | NS |
| LAR and AR (anastomosis) (%) | 10 (66.7) | 6 (60) | 22 (95.7) | <0.008a |
ASA American Society of Anesthesiologists, APR abdominoperineal resection, AR anterior resection, BMI body mass index, LAR low anterior resection, NS not statistically significant, RP rectal prolapse
aStatistically significant
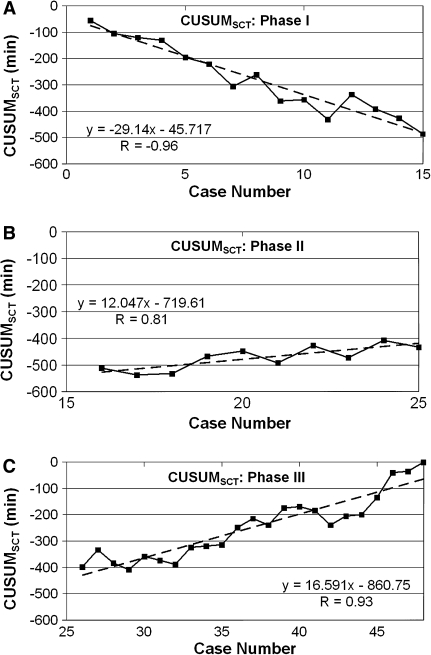
Interphase comparisons of intraoperative parameters (operative times and EBL) and short-term postoperative outcome (LOS) are presented in Table 3. A significant reduction in DT and EBL was observed between phases 1 and 2 compared with phase 3 (p < 0.0002 and < 0.03, respectively). The mean SCT for the first 15 cases (phase 1: 82.7 ± 41.6 min) was significantly shorter than for the last 23 cases (phase 3: 133.9 ± 40.6 min) (p < 0.001). Figure 3 displays the lines of best fit for the three phases of the SCT learning curve.
Table 3.
Interphase comparisons of intraoperative parameters and short-term outcome
| Operative time | Phase 1 (n = 15) |
Phase 2 (n = 10) |
Phase 3 (n = 23) |
Phases 1 and 2 vs Phase 3 (p-value) |
|---|---|---|---|---|
| DT (min) | 19.5 ± 9.7 | 15.3 ± 6.9 | 10.0 ± 3.0 | <0.0002a |
| SCT (min) | 82.7 ± 41.6 | 120.5 ± 45.6 | 133.9 ± 40.6 | <0.045a |
| OT (min) | 214.0 ± 74.2 | 238.0 ± 71.0 | 269.8 ± 84.2 | <0.048a |
| EBL (ml) | 129.7 ± 70.7 | 112.5 ± 39.5 | 85.9 ± 49.3 | <0.03a |
| LOS (days) | 3.8 ± 3.6 | 3.8 ± 2.1 | 3.1 ± 0.9 | NS |
DT robotic docking time, EBL estimated blood loss, LOS hospital length of stay, NS not statistically significant, OT total operative time, SCT surgeon console time
aStatistically significant
Fig. 3.
Lines of best fit for each phase of the cumulative sum (CUSUM)SCT learning curve. A Phase 1 represents the initial learning curve. B Phase 2 represents the accumulation of additional experience. C Phase 3 represents increasing surgeon competence
Discussion
The learning curve is a graphic representation of the temporal relationship between the surgeon’s mastery of a specifically assigned task and the chronological number of cases performed. The CUSUM technique is a method adopted by the medical profession in the 1970s to analyze the learning curve for surgical procedures [5, 6]. We used the CUSUM method to investigate the learning curve for robotic-assisted (da Vinci® System, Intuitive Surgical, Inc., Sunnyvale, CA) rectosigmoid and rectal surgery in the pelvis for benign and malignant colorectal disease. The focus of this study was on investigating surgeon console time as a surrogate marker for operative competency by dividing operative time into phases shown to correlate with process components of surgeon learning.
Publications investigating the learning curve in robotic surgery have performed their analysis based on chronological cases split into predefined segments (e.g., quartiles), with univariate analysis performed to compare means across segments. For instance, Bell et al. [9] reviewed operative times for 100 consecutive robotic-assisted hysterectomies by dividing the series into 20-case quintiles. Operative times and complication rates decreased over the study period, and maximum improvement was observed after the first quintile. A similarly designed study was reported by Tsao et al. [10], who divided their first 100 cases of robotic-assisted laparoscopic prostatectomy into 25-case quartiles. Total operative time and EBL decreased over the study period, but the most significant improvement in OT and EBL was observed after 25 cases and 50 cases, respectively.
Additionally, CUSUM analysis has been used to analyze the learning curve in conventional laparoscopic colorectal surgery [11]. Tekkis et al. [1, 12] reported two such series. One of the two studies compared right and left colectomy [12]. The analysis demonstrated a learning curve of 55 cases for right-sided and 62 cases for left-sided colectomy. The median operative time declined with operative experience. The readmission rate and the postoperative complications were not dependent on operative experience. The second study investigated RA-CUSUM in ileal-pouch anal anastomosis [1]. Pouch failure was the primary end-point, and the trainee staff showed an improvement in pouch failure rate after 23 cases. To our knowledge, however, a CUSUM-based approach for the analysis of the robotic learning curve in colorectal cases has yet to be reported.
Our study used the CUSUM method to investigate the learning curve in RALS. The study report describes the experience at a single institution by the same surgeon (E.M.H.), who has been performing minimally invasive colorectal surgery since 2002. The SCT was analyzed in depth because it represents the surgeon’s time at the robotic console and, based on pairwise correlation analysis, it correlated strongly with OT (R = 0.76; p < 0.00001).
We chose CUSUM analysis because meaningful conclusions cannot be drawn from raw data plotted by chronological cases (Fig. 1A). The CUSUMSCT graph (Fig. 1B) shows the variance from the mean on a case-by-case basis, yielding a parabolic curve with three distinct phases from which correlates of the learning curve can be assessed (Fig. 2). The large magnitude of the R values (–0.96, 0.81, and 0.93, respectively) in Fig. 3 for the line of best fit in each phase indicates the unique components of the surgeon’s learning curve for RALS colorectal procedures. The negative slope in phase 1 indicates shorter SCTs during this learning curve phase (lower with respect to the mean SCT over all cases). The positive slope in phases 2 and 3 indicates longer SCTs (greater with respect to the mean SCT over all cases), which are necessary for the performance of more complicated cases taken on with increased surgeon competence.
We believe the learning curve entails the surgeon’s mastery of three important and unique facets of robotic-assisted technology: (1) overcoming the loss of tensile and tactile feedback by recognizing visual cues with regard to tension and manipulation of the tissues, (2) conceptualizing the spatial relationships of robotic instruments outside the active field of view to manipulate and reposition safely without direct visualization, and (3) mentally visualizing the spatial relationships of the robotic arms and cart (and blinded to these external movements) while operating at the console, thereby minimizing external clashing and optimizing maneuverability and range of motion. To facilitate the acquisition of such unique facets in a safe and stepwise fashion, we believe it is important for a surgeon to acquire expert laparoscopic skills before transitioning to the robotic approach.
Phase 1 represents the initial learning curve phase, found to include 15 cases. An additional 10 cases comprises phase 2, which represents the accumulation of additional experience once the initial learning curve has been achieved. In our series, we found that the intraoperative complication rate was not dependent on operative experience. However, two recognized thermal injuries occurred during phase 1, the learning curve. The phase 2 plateau represents increased competence with the robotic technology. Our results showed the expected decline in SCT (phase 1) followed by a plateau (phase 2), as seen in typical learning curve studies.
The increased operative time in the post-learning period (phase 3) was attributed to a greater proportion of more technically challenging cases in phase 3 as well as to an increased fraction of morbidly obese patients and those with low pelvic malignancies. For instance, significantly more procedures required anastomosis (LAR and AR) in phase 3 than in phases 1 and 2, in which a greater proportion of APRs and RPs were performed. Furthermore, the male-to-female ratio was reversed between phases 1 and 3. Specifically, phase 1 had twice as many females as males, whereas phase 3 had 1.9 times as many males as females. The male pelvis is considered to be more narrow and confined than the female pelvis, thereby limiting visibility and affecting outcomes in colorectal procedures of the deep pelvis [13]. Despite this trend of a longer OT, however, EBL was significantly diminished in phase 3, and the LOS was not significantly longer. This was also found in a study in which a longer OT during laparoscopic sigmoid colectomy was not associated with an increased complication rate or a longer LOS [14].
This study, using CUSUM analysis, identified three unique phases of the learning curve in the field of robotic-assisted laparoscopic colorectal surgery. The data suggest that after a learning curve phase of 15 to 25 cases, the surgeon may achieve a higher level of competence and consider offering this approach to patients presenting with more complicated cases. Overcoming the learning curve involves mastery of the visual cues as well as both the internal and external spatial relationships unique to the robotic approach.
Acknowledgments
Disclosures
Malak B. Bokhari, Chirag B. Patel, Diego I. Ramos-Valadez, Madhu Ragupathi, and Eric M. Haas have no conflicts of interest or financial ties to disclose.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Tekkis PP, Fazio VW, Lavery IC, Remzi FH, Senagore AJ, Wu JS, Strong SA, Poloneicki JD, Hull TL, Church JM. Evaluation of the learning curve in ileal pouch-anal anastomosis surgery. Ann Surg. 2005;241:262–268. doi: 10.1097/01.sla.0000152018.99541.f1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Taylor RH, Funda J, Eldridge B, Gomory S, Gruben K, LaRose D, Talamini M, Kavoussi L, Anderson J. A telerobotic assistant for laparoscopic surgery. IEEE Eng Med Biol. 1995;14:279–288. doi: 10.1109/51.391776. [DOI] [Google Scholar]
- 3.Weber PA, Merola S, Wasielewski A, Ballantyne GH. Telerobotic-assisted laparoscopic right and sigmoid colectomies for benign disease. Dis Colon Rectum. 2002;45:1689–1694. doi: 10.1007/s10350-004-7261-2. [DOI] [PubMed] [Google Scholar]
- 4.Hashizume M, Tsugawa K. Robotic surgery and cancer: the present state, problems, and future vision. Jpn J Clin Oncol. 2004;34:227–237. doi: 10.1093/jjco/hyh053. [DOI] [PubMed] [Google Scholar]
- 5.Chaput de Saintonge DM, Vere DW (1974) Why don’t doctors use CUSUMs? Lancet 1:120–121 [DOI] [PubMed]
- 6.Wohl H. The CUSUM plot: its utility in the analysis of clinical data. N Engl J Med. 1977;296:1044–1045. doi: 10.1056/NEJM197705052961806. [DOI] [PubMed] [Google Scholar]
- 7.Biswas P, Kalbfleisch JD. A risk-adjusted CUSUM in continuous time based on the Cox model. Stat Med. 2008;27:3382–3406. doi: 10.1002/sim.3216. [DOI] [PubMed] [Google Scholar]
- 8.Steiner SH, Cook RJ, Farewell VT, Treasure T. Monitoring surgical performance using risk-adjusted cumulative sum charts. Biostatistics. 2000;1:441–452. doi: 10.1093/biostatistics/1.4.441. [DOI] [PubMed] [Google Scholar]
- 9.Bell MC, Torgerson JL, Kreaden U. The first 100 da Vinci hysterectomies: an analysis of the learning curve for a single surgeon. S D Med. 2009;62(91):93–95. [PubMed] [Google Scholar]
- 10.Tsao AK, Smaldone MD, Averch TD, Jackman SV. Robot-assisted laparoscopic prostatectomy: the first 100 patients—improving patient safety and outcomes. J Endourol. 2009;23:481–484. doi: 10.1089/end.2008.0241. [DOI] [PubMed] [Google Scholar]
- 11.Delaney CP, Lynch AC, Senagore AJ, Fazio VW. Comparison of robotically performed and traditional laparoscopic colorectal surgery. Dis Colon Rectum. 2003;46:1633–1639. doi: 10.1007/BF02660768. [DOI] [PubMed] [Google Scholar]
- 12.Tekkis PP, Senagore AJ, Delaney CP, Fazio VW. Evaluation of the learning curve in laparoscopic colorectal surgery: comparison of right-sided and left-sided resections. Ann Surg. 2005;242:83–91. doi: 10.1097/01.sla.0000167857.14690.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seike K, Koda K, Oda K, Kosugi C, Shimizu K, Miyazaki M. Gender differences in pelvic anatomy and effects on rectal cancer surgery. Hepatogastroenterology. 2009;56:111–115. [PubMed] [Google Scholar]
- 14.Scheer A, Martel G, Moloo H, Sabri E, Poulin EC, Mamazza J, Boushey RP (2009) Laparoscopic colon surgery: does operative time matter? Dis Colon Rectum 52:1746–1752 [DOI] [PubMed]