Summary
The marginal costs and benefits of converting malaria programmes from a control to an elimination goal are central to strategic decisions, but empirical evidence is scarce. We present a conceptual framework to assess the economics of elimination and analyse a central component of that framework—potential short-term to medium-term financial savings. After a review that showed a dearth of existing evidence, the net present value of elimination in five sites was calculated and compared with effective control. The probability that elimination would be cost-saving over 50 years ranged from 0% to 42%, with only one site achieving cost-savings in the base case. These findings show that financial savings should not be a primary rationale for elimination, but that elimination might still be a worthy investment if total benefits are sufficient to outweigh marginal costs. Robust research into these elimination benefits is urgently needed.
This is the fourth in a Series of four papers about malaria elimination
Introduction
Few dispute that eliminating malaria where technically and operationally feasible is a worthy goal.1 At the same time, many argue that elimination will need substantial additional resources to avert the last few cases and deaths, making this strategy a less efficient means of use of limited health resources than other strategies.2 Yet, others contend that elimination is an attractive investment because of its ability to pay for itself through future cost reductions and to generate substantial non-health benefits.3
So far, this debate has relied largely on anecdotal evidence on the costs and benefits of elimination and on few analyses that were done for the Global Malaria Eradication Program (GMEP) four decades ago.4 Countries currently considering elimination, however, face a different reality. For example, countries must now pursue elimination without the support of a global eradication campaign, and thus have to plan for recurring expenditure to manage the continual importation of new cases from neighbouring countries.5 Since this new reality has substantial implications for finite domestic and international financing, there is urgent need to assemble robust cost data to enable policy makers to make evidence-based judgments about whether and how to pursue elimination. In the long term, the total benefits of elimination will likely outweigh its costs, in addition to contributing to the massive global benefit of eradication. The decision facing policy makers, however, is how to best allocate limited resources in the short term.
Several essential questions about the short-term and medium-term returns of elimination should therefore be immediately pursued, including: what are the total costs of achieving and of sustaining elimination? What additional benefits does elimination generate? And how do these costs and benefits compare with the alternative—a strategy of achieving and maintaining a state of controlled low-endemic malaria, at which it is no longer a major public health problem?6 If policy makers decide to pursue elimination, many countries will face an additional question: how can an elimination programme be sufficiently and sustainably financed in view of political and economic realities that are constraining growth in health financing and shifting attention away from disease-specific investments?
Key messages.
-
•
Comprehensive cost–benefit analysis is important to establish whether and how to pursue malaria elimination. To be relevant to policy makers, this analysis should compare elimination with an alternative of effective malaria control.
-
•
Although there is little information about the marginal costs of elimination and substantial variation between different contexts, data indicate that the cost of achieving elimination will be substantially greater than the cost of control.
-
•
Case studies in five diverse sites show that the potential for elimination to save money in the medium term varies by context. However, the low probability of cumulative cost-savings in these case studies suggests that this metric should not be the main rationale for the pursuit of elimination.
-
•
Absence of cumulative cost-savings does not mean that elimination is an unattractive investment, since total benefits might still outweigh costs. Indirect benefits were not calculated in the case studies, but cumulative costs were often modestly higher than control so marginal benefits would also only need to be modest for elimination to achieve a positive cost–benefit ratio.
-
•
Successful achievement and maintenance of local elimination in the absence of a global eradication campaign will need a paradigm shift in malaria financing. Malaria will need to be viewed as a recurring investment similar to routine immunisation.
-
•
Our findings draw attention to the urgency of undertaking additional robust analyses on the economics of elimination in different contexts, with particular emphasis on quantification of potential benefits.
We seek to establish a conceptual approach and an initial empirical base from which to address these questions. We begin with a framework to assess the economic attractiveness of malaria elimination. After reviewing evidence about elimination costs and benefits, we undertake case studies of several countries to explore a central component of that framework—whether the direct cost savings of elimination will offset initial investment costs. We conclude by identifying financial threats and corresponding imperatives facing malaria-eliminating countries and by proposing priorities for additional future research.
Economics of malaria elimination: a conceptual framework
Methods to assess the comparative value of investing in different health interventions are well established, if imperfect.7 These methods—cost-effectiveness analysis and cost–benefit analysis—have been commonly used to assess malaria control, and extensive published work about their effective execution exists.8 Elimination, however, has several unique attributes that warrant adaptations of standard methods to generate results most relevant to policy.
There are several overarching principles that are central to any economic analysis of elimination. First, and most importantly, is the definition of the alternative to elimination. WHO recommends a null state of disease without intervention as the alternative.9 In practice, most health-economic analyses use a loosely defined status quo, which varies substantially between countries. Such is the case for much of the microeconomic and macroeconomic analyses of malaria interventions so far.7
A null-state alternative can provide important information about the total value of a malaria programme. This information is particularly useful for policy makers to understand the benefits of continued investment in malaria, even when the disease is greatly reduced or absent. However, the main choice facing countries is between elimination and controlled low-endemic malaria, not a retraction of existing interventions or acceptance of partial, suboptimum control (ie, often the status quo).10 As such, the most policy-relevant alternative for economic analysis of elimination is controlled low-endemic malaria. Although seemingly intuitive, this approach is not always used in the existing analysis of elimination and eradication. For example, many of the cost–benefit analyses of efforts to eradicate other infectious diseases have used a complete absence of control as the alternative to eradication.11–13
A second imperative of elimination analysis is the inclusion of postelimination costs. During the GMEP, the expectation was that malaria-related expenditure would stop after elimination.14 However, most successful countries needed to continually invest in surveillance and other interventions.15 Thus, the inclusion of a robust estimate of postelimination interventions in all costing analyses, taking into consideration the potential sharing of costs with other disease programmes, is important.
Lastly, the supranational implications of elimination should be considered. As a country approaches and reaches elimination, its neighbours—and many other countries—benefit from reduced importation of malaria and the corresponding reduction in the risk of outbreaks. These spillover benefits should be incorporated into a complete economic evaluation of elimination.
Cost-effectiveness analysis, which calculates the amount of funding an intervention needs to prevent loss of a standard unit of disease burden, is the most commonly used approach to compare the economic attractiveness of health programmes.8 Both cost-effectiveness analysis and the related cost-minimisation analysis have important parts to play within an elimination context by identifying the optimum level of intervention needed to achieve controlled low-endemic malaria and elimination and prevent reintroduction once malaria-free status is achieved. These analyses are essential for robust economic assessments of elimination and are valuable programme planning interventions.
Cost-effectiveness analysis, however, is likely to be incapable of adequately informing the ultimate choice between elimination and controlled low-endemic malaria. Elimination has been justified on the basis of more than health outcomes—the threats of insecticide resistance and the instability of international malaria funding were the central impetus for the GMEP16—and recent arguments for elimination have focused on the effect on economic growth and international externalities.17,18 Moreover, policy makers often place substantial value on outcomes beyond efficiency in prioritisation of the allocation of health resources;19,20 thus cost–benefit analysis, which enables broader benefits to be translated into a common monetary metric, is a more effective means to inform strategic decisions regarding potential elimination strategies.
Considerable, if arguably insufficient, analysis has been done to investigate the costs and benefits of malaria control, and has shown it to be among the most attractive applications of global social investment.7,21,22 However, no comprehensive cost–benefit analysis has been completed for malaria elimination using an alternative of controlled low-endemic malaria.4 Analyses of programmes to eliminate or eradicate other infectious diseases have consistently reported that benefits outweigh costs, but these analyses cannot be directly applied to malaria.23
Effective cost–benefit analysis of elimination will need alternative approaches to assess benefits. Cost–benefit analysis usually focuses on benefits that are closely linked to the health effects of the programme, including reduced public and private expenditure (direct) and increased productivity and educational attainment (indirect).7 These benefits will always be modest for elimination—the main effect on educational attainment, for example, will result from the achievements of the baseline control programme. Elimination might, however, increase the equity of these benefits. The poorest and most vulnerable members of a society are typically the last to benefit from public services such as malaria control and will thus be the primary beneficiaries of an elimination campaign.24 As such, for societies that prioritise distribution in addition to efficiency of health outcomes, equity should be appropriately incorporated into cost–benefit analyses of elimination.25–27
The economic attractiveness of elimination will often depend on two additional classes of benefits: financial and threshold. Financial benefits might be realised if recurring costs fall sufficiently after elimination so that the cumulative cost of the programme is lower than that of controlled low-endemic malaria over the medium-to-long term. In this event, the elimination programme will pay for itself and its benefits will always exceed its costs. Accordingly, this financial analysis is a central component of the economic evaluation of elimination, although not the primary metric, as Mills and colleagues4 have suggested.
Many of the supposed benefits of elimination are only realised once the absolute threshold of malaria-free status is achieved.18 A common argument is that elimination will generate substantial additional economic activity by creation of an environment conducive to direct foreign investment and tourism, among other advantages. Since there is only modest risk of malaria at the baseline state, this argument relies on the assumption that companies and individuals will perceive a disproportionate difference between controlled low-endemic malaria and elimination. Maartens and colleagues28 reported that tourists' perceptions of risk were highly unresponsive to actual changes in malaria transmission in an area of South Africa. This finding is consistent with published work on divergences between actual and perceived risk of infectious disease and their effect on economic behaviour;29,30 however, the existence and magnitude of this effect for malaria elimination has never been studied.
Several other costs and benefits should ideally be included in a comprehensive cost–benefit analysis of elimination, but they are considerably more difficult to quantify. These factors include the potential increase in public confidence in government services and international recognition generated by successful elimination of a tenacious disease.10 Elimination could also strengthen components of the health system as occurred in the USA through the creation of the Centers for Disease Control and Prevention,31 although others contend that the GMEP undermined surveillance and other systems.32
Conversely, substantial political will, domestic and potentially international, will be needed to achieve elimination. Lines and colleagues1 suggest that the risk and consequences of failing to eliminate (eg, resurgence resulting from disillusionment with the programme) should be a prominent factor in elimination decisions and related analyses. However, this approach would affect the cost–benefit ratio of elimination only if the risks posed by elimination are greater than those of controlled low-endemic malaria; there is no evidence to suggest this would be the case.33
Overall, the decision whether or not to pursue elimination is best informed by a comprehensive cost–benefit analysis that compares the potential net benefits of elimination with those of continued controlled low-endemic malaria. Such an exercise should begin with cost-minimisation analysis—which is largely absent despite the volume of resources invested in malaria—to establish the optimum package of interventions with which to achieve both controlled low-endemic malaria and elimination. Ultimately, the economic attractiveness of elimination will rely on financial and threshold non-health benefits, including those yielded by other countries. Robust quantification of those benefits will be complex and time consuming, suggesting that other methods such as cost-effectiveness analysis should be used in the short term. However, in view of the shortcomings of those methods and the informal judgments that drive elimination-related decisions, even a partial application of this cost–benefit analysis framework could produce improvements in debate and decision making.34
Marginal cost of elimination
Methods
Evidence for the potential benefits of elimination is insufficient to meaningfully undertake a full cost–benefit analysis. However, sufficient data exist to enable the partial application of the framework through the quantification of marginal costs and potential cost-savings. Financial comparison alone cannot establish the total value of an elimination programme since, by definition, it excludes the other marginal benefits of an elimination programme. However, it can be a useful interim guide for policy making and a foundation for further research. If elimination is cost saving, and financial benefits alone exceed costs, policy makers should be comfortable pursuing an elimination programme without quantifying additional benefits. If elimination is not cost saving, then gathering the evidence needed to calculate potential additional benefits becomes a priority.
To examine the financial benefits of elimination, we conducted a review of published works and created and analysed datasets from present elimination programmes. To develop the new datasets, relevant institutions and researchers were contacted in 16 countries that are pursuing or that recently achieved elimination. Inclusion criteria included full yearly data from at least two of the key programme phases, sufficient detail to establish costs by intervention, and availability of detailed epidemiological and programmatic information (eg, malaria incidence and intervention coverage) to place costs in context.
Data were identified from eight countries, four of which met the inclusion criteria for this analysis: China (the provinces of Hainan and Jiangsu), Mauritius, Swaziland, and Tanzania (the islands of Zanzibar). Table 1 presents key contextual indicators for these five sites, with additional information about the malaria programmes in each site included in the webappendix.33 Costing data were obtained from a variety of sources in each country, including national health accounts, donor proposals, and informant interviews (webappendix pp 17, 27). Type of data varied accordingly between country and programme phase, falling into three categories: actual expenditure from preceding years; prospective budgets based on expert opinion or recent experience within the country; and prospective budgets derived from mathematical models. For budgetary sources, prospective programmes were reviewed in detail and compared with international guidance and evidence about the technical requirements needed for elimination.
Table 1.
Contextual characteristics of case study focus sites
| Population (thouands)35 | Estimated population at risk (thousands)36,37 | GDP per head (US$)38,39 | Total annual government health expenditure per head (US$; 2006)40 | Plasmodium falciparum inherent transmission risk (R0)36 | Antenatal-care coverage (2007 %)41 | EPI coverage (2007 %)42 | |
|---|---|---|---|---|---|---|---|
| China (Hainan) | 8640 | 8440 | 2802 | 53 | .. | 90·9% | 96·7% |
| China (Jiangsu) | 77 245 | 58 120 | 6467 | 123 | .. | 90·9% | 96·7% |
| Mauritius—elimination (1983–88) | 1021 | 1021 | 2289 | .. | .. | .. | .. |
| Mauritius—present (2009) | 1286 | 1286 | 7146 | 157 | .. | .. | 97·3% |
| Swaziland | 1186 | 228 | 2854 | 114 | 1·6 | 84·8% | 95·0% |
| Tanzania (Zanzibar) | 1221 | 1221 | 496 | 18 | 18·8 | 75·8% | 87·0% |
All indicators are for 2009 except where noted. China and Tanzania indicators are shown for relevant subnational units except antenatal and EPI coverage. ..=data not available. GDP=gross domestic product. EPI=Expanded Program on Immunisation.
For all countries, only provider (ie, the government-led programme) and malaria-specific (ie, excluding general health-system resources) costs were included in the final dataset. Costs in each country were grouped into standardised intervention and expenditure categories (webappendix pp 2–4). For items used partly for non-malaria activities or for several interventions, the cost was apportioned among activities on the basis of the judgments of local staff. All equipment and durable commodities were costed using straight-line depreciation, and all costs were converted into 2008 US dollars with appropriate deflators and exchange rates.
To calculate the potential financial benefits of elimination, robust costing data are needed for three phases of a malaria programme: the baseline of controlled low-endemic malaria, the interruption of transmission (or elimination phase), and the postelimination (or prevention-of-reintroduction phase).43 We assigned each year of data available for each site to one of the three phases. All years after a site began to implement programmatic changes for elimination were included in the elimination phase, with the phase of prevention of reintroduction beginning the year after the end of local transmission. All other years were included in the controlled low-endemic malaria phase.10
On the basis of WHO guidance and modelling outputs, 10 years was assumed to be the time needed to reach elimination (excluding Mauritius, where elimination was actually achieved in 8 years).33,43 When data were not available for this entire period, missing costs (typically for the final 5 years) were extrapolated from available data by inflation of relevant interventions by population growth. A further 15 years of postelimination intervention was then assumed, and this entire 25-year period—a length of time relevant to policy makers and commonly used in cost–benefit analyses of eradication44—was compared with the same number of years of controlled low-endemic malaria by adjustment for population growth. Cumulative costs were discounted at a standard rate of 3%.
None of the case-study sites had data for all three programme phases. As a result, missing phases were extrapolated from available data. For the Chinese provinces of Hainan and Jiangsu, programmes for prevention of reintroduction were assumed to be equivalent to those in three other provinces (Hebei, Fujian, and Shanxi) that have been successful in maintaining malaria-free status (webappendix pp 18, 19). A yearly average cost of prevention of reintroduction per head at-risk was calculated from the detailed expenditure data for these provinces and applied to the relevant years for Hainan and Jiangsu. For Zanzibar, the costs of the prevention-of-reintroduction phase were derived from the results of mathematical modelling, as described by Cohen and colleagues.33 We assumed that Swaziland would be able to withdraw prevention interventions but would have to keep other costs constant. Lastly, the cost of controlled low-endemic malaria in Mauritius was estimated on the basis of the increases between control and elimination for 20 countries with relevant data reported by Kaser.45
To assess the robustness of conclusions to these assumptions, we did minimum-to-maximum sensitivity analyses on all major assumptions for both controlled low-endemic malaria and elimination by varying yearly costs by intervention category, time to elimination, cost of prevention of reintroduction, discount rate, and period of analysis to the smallest and largest values deemed realistic (webappendix pp 5–7).46 We also did probabilistic analysis by examining the 95% certainty intervals from 50 000 Monte Carlo simulations for each site using triangular distributions for all assumptions.47,48 For these simulations, potential permutations of costs for controlled low-endemic malaria, elimination, and prevention-of-reintroduction phases were calculated repeatedly by randomly drawing values from distributions around each assumption.
Results
Existing evidence
Findings from published work showed few, and no contemporary, evidence for elimination costs. Several studies examined the costs of elimination programmes during the GMEP in few individual countries.49,50 The most comprehensive source of costs from this period examined the 5-year expenditures of most countries participating in the GMEP (table 2). These analyses indicate substantial variation in the per head cost of elimination, even in countries within the same region and with similar populations. However, since these studies do not describe the programme components or the epidemiological context, what contributed to these vast differences in costs is unclear. Data from this era are, therefore, of only modest use for present programme planning in view of the substantial evolution in interventions and strategies.
Table 2.
Per head expenditure on control and elimination by country, 1956–61
|
Control (1956) |
Elimination (1957–61) |
Median increase (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| All countries | Elimination-successful countries | Range (US$) | Mean ($) | Median ($) | All countries | Elimination-successful countries | Range ($) | Mean ($) | Median ($) | ||
| Central and south Asia | 3 | 0 | 0·03–0·30 | 0·19 | 0·25 | 4 | 0 | 0·25–1·02 | 0·52 | 0·41 | 96% |
| East Asia and Pacific | 6 | 0 | 0·08–1·01 | 0·35 | 0·24 | 9 | 1 | 0·09–2·74 | 0·63 | 0·27 | 58% |
| Europe | 3 | 3 | 0·01–1·46 | 0·50 | 0·03 | 4 | 4 | 0·04–0·69 | 0·23 | 0·09 | 256% |
| Latin America and Caribbean | 7 | 3 | 0·13–2·49 | 1·19 | 1·16 | 15 | 4 | 0·07–4·78 | 1·57 | 1·16 | −11% |
| Middle East and north Africa | 10 | 5 | 0·02–1·78 | 0·39 | 0·22 | 14 | 5 | 0·06–2·33 | 0·89 | 0·70 | 154% |
| Sub-Saharan Africa | 4 | 0 | 0·03–0·95 | 0·31 | 0·14 | 4 | 0 | 0·07–2·30 | 0·83 | 0·48 | 249% |
| Total | 33 | 11 | 0·02–2·49 | 0·55 | 0·25 | 50 | 14 | 0·04–2·74 | 0·81 | 0·54 | 87% |
Median increase compares only countries for which cost data were available for both programme phases. Countries were considered to have successfully eliminated malaria if they received WHO certification within 10 years of these data. Adapted from Kaser45 and converted to 2008 US$.
Similarly, comparisons between the costs of control and elimination are scarce. Mills and colleagues4 identified only two studies that explicitly investigate this comparison. Cohn51 concluded that a time-limited elimination campaign in India would have a greater net cost than would ongoing control, whereas Ruberu and colleagues (unpublished) reported the opposite for Sri Lanka.
Data presented by Kaser45 provide further, although still inadequate, insight into this financial comparison. With the exception of countries in Latin America and the Caribbean, there was a substantial median increase in costs between control and elimination. None of these studies, however, are capable of assessing the potential financial benefits of elimination, since they do not include postelimination expenditure.
Lastly, we identified a range of studies purporting to examine the benefits of elimination.52–55 Most of these analyses conclude that elimination programmes generated sizeable economic benefits, with some reporting that benefits substantially exceeded costs.17 However, all the studies use an absence of control as the baseline and are therefore not capable of informing the questions relevant to policy makers.
Case studies
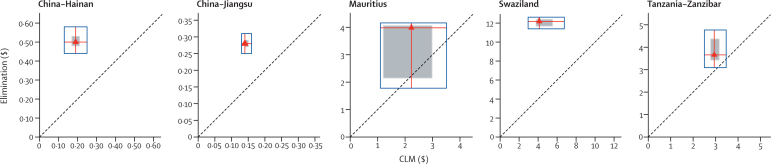
Table 3 presents the average total and per head yearly costs for the available programme phases in each site. Yearly controlled low-endemic malaria costs per head at risk varied greatly between sites, because the programmes differed in their intensity and interventions.4,45 Average costs for elimination are substantially higher than for controlled low-endemic malaria in China, Mauritius, and Swaziland, with increases from 91% to 182% between the phases, but only modestly increased (30%) in Tanzania (Zanzibar). Sensitivity analysis suggests that elimination would be more expensive than would controlled low-endemic malaria under all combinations of assumptions, apart from in Mauritius and Tanzania (Zanzibar; figure 1).
Table 3.
Mean yearly costs and relative financing of malaria programme by phase (in 2008 US$)
| Data type | Years | Average annual costs (US$ thousands) | Average annual per head costs (US$) | Average annual per head (at risk) costs (US$) | % of public expenditure on health | % of malaria programme externally financed | ||
|---|---|---|---|---|---|---|---|---|
| China (Hainan) | ||||||||
| CLM | Actual | 2007–09 | 1632 | 0·19 | 0·20 | 0·4% | 45% | |
| Elimination | Budget (planned) | 2010–14 | 4366 | 0·50 | 0·51 | 1% | 36% | |
| Change (%) | NA | NA | 168% | 159% | 159% | 0·6% | −8% | |
| Prevention of reintroduction | Modelled | 2020–29 | 1106 | 0·12 | 0·12 | NA | NA | |
| Change (%) | NA | NA | −32% | −37% | −39% | NA | NA | |
| China (Jiangsu) | ||||||||
| CLM | Actual | 2007–09 | 8472 | 0·11 | 0·15 | 0·1% | 3% | |
| Elimination | Budget (planned) | 2010–14 | 16 601 | 0·21 | 0·28 | 0·2% | 12% | |
| Change (%) | NA | NA | 96% | 91% | 91% | 0·1% | 9% | |
| Prevention of reintroduction | Modelled | 2020–29 | 7593 | 0·09 | 0·12 | NA | NA | |
| Change (%) | NA | NA | −10% | −18% | −18% | NA | NA | |
| Mauritius | ||||||||
| CLM | Modelled | 1982 | 2470 | 2·19 | 2·19 | NA | NA | |
| Elimination | Actual | 1983–88 | 4356 | 4·28 | 4·28 | NA | 2% | |
| Change (%) | NA | NA | 76% | 95% | 95% | NA | NA | |
| Prevention of reintroduction | Actual | 1990–08 | 2771 | 2·42 | 2·42 | 2% | 1% | |
| Change (%) | NA | NA | 12% | 10% | 10% | NA | −1% | |
| Swaziland | ||||||||
| CLM | Actual | 2004–08 | 987 | 0·87 | 4·51 | 1% | 32% | |
| Elimination | Budget (planned) | 2009–13 | 2984 | 2·45 | 12·72 | 2% | 71% | |
| Change (%) | NA | NA | 202% | 182% | 182% | 1% | 40% | |
| Prevention of reintroduction | Modelled | 2020–29 | 2266 | 1·54 | 7·99 | NA | NA | |
| Change (%) | NA | NA | 130% | 78% | 77% | NA | NA | |
| Tanzania (Zanzibar) | ||||||||
| CLM | Budget (planned) | 2009 | 3908 | 3·01 | 3·01 | 17% | 98% | |
| Elimination | Budget (modelled) | 2010–19 | 4911 | 3·90 | 3·90 | 22% | NA | |
| Change (%) | NA | NA | 26% | 30% | 30% | 5% | NA | |
| Prevention of reintroduction | Modelled | 2020–29 | 3899 | 2·01 | 2·01 | NA | NA | |
| Change (%) | NA | NA | 0% | −33% | −33% | NA | NA | |
CLM=controlled low-endemic malaria. NA=not applicable.
Figure 1.
Comparison of the average yearly cost of elimination with controlled low-endemic malaria per head at risk in five case studies, with sensitivity analysis
Red triangles represent the most likely cost for elimination and controlled low-endemic malaria (CLM), assuming elimination needs 10 years (8 years in Mauritius) and costs are not discounted. Red lines extend vertically to the minimum and maximum costs obtained from sensitivity analysis of elimination costs and horizontally to those for controlled low-endemic malaria, such that all points within the blue box represent potential financial comparisons between the two strategies. The shaded square represents costs that fall within 95% CIs derived from probabilistic simulation. The 45° dotted line shows equal costs for elimination and controlled low-endemic malaria, so that points above that line indicate elimination costs greater than controlled low-endemic malaria and points below indicate elimination costs less than controlled low-endemic malaria. If the entirety of the blue box is above the dotted line, elimination was not identified as less expensive than control in sensitivity analysis.
The composition of each site's programme shows the drivers of the wide variation in cost increases (table 4). The site with the smallest change, Tanzania (Zanzibar), had already incorporated the prevention activities needed for elimination into its controlled low-endemic malaria programme, wheras the Chinese provinces and Swaziland had achieved controlled low-endemic malaria objectives with more modest interventions. Most expenditure in the Chinese provinces and Mauritius was on personnel for both controlled low-endemic malaria and elimination, whereas consumables were the largest cost category in Swaziland and Tanzania (Zanzibar; webappendix p 31).
Table 4.
Summary and relative cost allocation of programmatic interventions by phase and intervention category
|
Prevention |
Diagnosis |
Surveillance |
Management |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Major interventions | Cost per head at risk (US$) | Major interventions | Cost per head at risk (US$) | Major interventions | Cost per head at risk (US$) | Major interventions | Cost per head at risk (US$) | ||
| China (Hainan) | |||||||||
| CLM | 80% ITN coverage in high-risk areas; 80% LLIN coverage in remote areas | 0·06 | Microscopy at all township hospitals; RDTs in remote areas | 0·02 | 60% coverage of passive internet reporting; active surveillance in high-endemic sites; IRS response to outbreaks | 0·02 | 266–528 management staff (0·03–0·06 per head at risk) at provincial, county, and township levels | 0·05 | |
| Elimination | 100% LLIN coverage in high-risk areas | 0·18 | Microscopy at all township hospitals with PCR validation; RDTs in remote areas | 0·03 | 100% coverage of passive internet reporting; active case detection in all endemic sites; screening of febrile cases at borders lower threshold for IRS response to outbreaks | 0·07 | 529–791 management staff (0·06–0·09 per head at risk) at provincial, county, and township levels | 0·16 | |
| Change (%) | 219% | 66% | 240% | 206% | |||||
| China (Jiangsu) | |||||||||
| CLM | Personal protection* | 0·00 | Microscopy at 50% of township hospitals | 0·01 | 50% coverage of passive internet reporting; active surveillance in high-endemic sites; IRS response to outbreaks | 0·04 | 1573–3142 management staff (0·03–0·05 per head at risk) at provincial, county, and township levels | 0·05 | |
| Elimination | Personal protection* | 0·00 | Microscopy at all township hospitals with PCR confirmation | 0·02 | 100% coverage of passive internet reporting; active case detection in all endemic sites; screening of febrile cases at borders; lower threshold for IRS response to outbreaks | 0·10 | 3143–4712 management staff (0·05–0·08 per head at risk) at provincial, county, and township levels | 0·11 | |
| Change (%) | NA | 117% | 138% | 130% | |||||
| Mauritius | |||||||||
| Elimination | 13% IRS coverage; island-wide larviciding | 2·58 | All suspected cases confirmed with microscopy | 0·10 | ABER=7·8%, through border screening and case follow-up | 1·16 | 1032 unskilled labour and field staff; 306 skilled and managerial staff | 0·19 | |
| Prevention of reintroduction | <1% IRS coverage; island-wide larviciding | 1·05 | All suspected cases confirmed with microscopy | 0·09 | ABER=3·4%, almost all through border screening | 0·91 | 266 unskilled labour and field staff; 260 skilled and managerial staff | 0·40 | |
| Change (%) | −57% | −5% | −17% | 117% | |||||
| Swaziland | |||||||||
| CLM | 5% ITN; 30% IRS coverage | 1·63 | 0% cases confirmed | 0·37 | Passive surveillance only | 0·36 | Four central staff | 1·57 | |
| Elimination | 95% ITN; 95% IRS coverage | 3·41 | 95% cases confirmed with RDT or microscopy | 1·82 | Active surveillance around new cases | 3·02 | 12 central staff | 2·99 | |
| Change (%) | 109% | 395% | 733% | 90% | |||||
| Tanzania (Zanzibar) | |||||||||
| CLM | 100% LLIN coverage; 95% IRS coverage reduced to 10% after 2 years | 1·61 | All suspected cases tested with RDTs or microscopy in public facilities | 0·39 | Mobile technology reporting system in all public facilities | 0·53 | 19 core programme staff | 0·28 | |
| Elimination | 100% LLIN coverage; 95% IRS coverage reduced to 25% after 2 years and 10% after 4 years | 1·72 | All fever cases tested with RDTs in public and private facilities with PCR validation | 0·52 | Reporting system extended to private sector; screening of households around all new cases | 0·90 | 85 core programme staff | 0·54 | |
| Change (%) | 6% | 32% | 70% | 95% | |||||
In 2008 US$. CLM=controlled low-endemic malaria. ITN=insecticide-treated bednet. LLIN=longlasting insecticide-treated bednet. RDT=rapid diagnostic test. IRS=indoor residual spraying. NA=not applicable. ABER=annual blood examination rate.
includes measures such as untreated bednets and commercial insecticide sprays. For further information about intervention strategies and costs, see webappendix p 30.
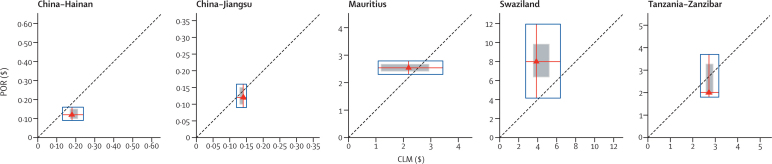
Figure 2 shows the actual (Mauritius) and assumed (other sites) average yearly cost of prevention of reintroduction compared with an equivalent period of controlled low-endemic malaria. Postelimination reductions were estimated to generate yearly savings in the Chinese provinces and in Tanzania (Zanzibar), whereas yearly expenditures for prevention of reintroduction in Mauritius and Swaziland did not fall below controlled low-endemic malaria in the base case. However, the sensitivity analysis showed that these findings were not robust against different combinations of assumptions in any of the sites, including Swaziland and Tanzania (Zanzibar), where the greatest differences between controlled low-endemic malaria and elimination were reported.
Figure 2.
Comparison of average yearly cost of prevention of reintroduction with controlled low-endemic malaria per head at risk in five case studies, with sensitivity analysis
Red triangles represent the most likely cost for prevention of reintroduction (POR) and controlled low-endemic malaria (CLM), assuming prevention of reintroduction is maintained for 10 years and costs are not discounted. Red lines extend vertically to the minimum and maximum costs obtained from sensitivity analysis of prevention-of-reintroduction costs and horizontally to those for controlled low-endemic malaria, such that all points within the blue box represent potential financial comparisons between the two strategies. The shaded square represents costs that fall within 95% CIs derived from probabilistic simulation. The 45° dotted line shows equal costs for prevention of reintroduction and controlled low-endemic malaria, so that points above that line indicate prevention-of-reintroduction costs greater than controlled low-endemic malaria and points below indicate prevention-of-reintroduction costs less than controlled low-endemic malaria. If a blue box crosses the dotted line, prevention of reintroduction was less expensive than controlled low-endemic malaria under certain combinations of assumptions.
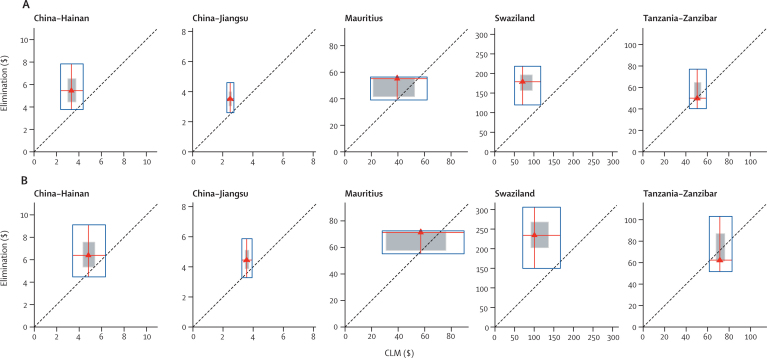
Figure 3 presents the results of the analysis of the net-present value of the costs of elimination relative to controlled low-endemic malaria in each site. Elimination is not expected to be cumulatively cost-saving in China, Mauritius, and Swaziland over the full 50-year period, with the total cost of elimination ranging from 25% (China [Jiangsu] and Mauritius) to 133% (Swaziland) greater than controlled low-endemic malaria on average. By contrast, cumulative elimination costs in Tanzania (Zanzibar) were modestly lower than controlled low-endemic malaria in both the 25-year and 50-year periods.
Figure 3.
Comparison of present value of elimination with controlled low-endemic malaria per head at a risk over 25 years (A) and 50 years (B) in five case studies, with sensitivity analysis
Red triangles represent the most likely cost for elimination and controlled low-endemic malaria (CLM), assuming a 3% discount rate. Red lines extend vertically to the minimum and maximum costs obtained from sensitivity analysis of elimination costs and horizontally to those for controlled low-endemic malaria, such that all points within the blue box represent potential financial comparisons between the two strategies. The shaded square represents costs that fall within 95% CIs derived from probabilistic simulation. The 45° dotted line shows equal costs for elimination and controlled low-endemic malaria, so that points above that line indicate elimination costs greater than controlled low-endemic malaria and points below indicate elimination costs less than controlled low-endemic malaria. If a blue box crosses the dotted line, elimination was cumulatively less expensive than controlled low-endemic malaria under specific combinations of assumptions.
The minimum-to-maximum sensitivity analysis resulted in a wide range of possible values for both controlled low-endemic malaria and elimination, qualitatively changing core findings in all sites. However, the probabilistic analysis indicated that the likely range of values will be much more narrow. The probability that elimination would be cost-saving is small in China (Hainan [0·2%] and Jiangsu [0·1%]) and absent in Swaziland over the full 50-year period. Mauritius had higher, although still modest, probabilities of 5·3% over 25 years and 9·6% over 50 years, whereas Tanzania (Zanzibar) had the greatest chance of cost-savings of 10·0% and 42·0%, respectively. Removing the discount rate did not qualitatively change findings over a 25-year period, but resulted in elimination costs falling modestly below controlled low-endemic malaria in China (Hainan [19%] and Jiangsu [11%]) over 50 years. The time to elimination, cost of prevention of reintroduction, and cost of prevention interventions accounted for most of the variation in the majority of sites, with the assumed cost of controlled low-endemic malaria responsible for most of the variation in Mauritius (webappendix pp 7, 8).
Financial feasibility of elimination
This analysis shows that achieving and sustaining elimination will need substantial funding, and that some countries will need intensive support from the international community to achieve that goal. Consistently securing sufficient financing will be a challenge for many countries. Health programmes in developing countries are constantly disrupted by donor unpredictability.56 And financial volatility is not unique to foreign donors: national governments, including those of the USA and other industrialised countries, cause financial volatility that jeopardise the success of domestic health programmes.57–59
Financial unpredictability might be especially acute for elimination programmes. Donors are increasingly results driven, seeking concrete and compelling effects from their investment. As a result, elimination programmes, ironically, might be victims of their own success, with donors shifting funds to programmes with seemingly greater potential effects as malaria cases approach zero. Moreover, the long-term nature of elimination programmes contrasts with governments' and donors' typical short-term funding cycles and goals. The implications of this unpredictability are similarly severe; experience has shown that disruptions in financing can lead to rapid resurgence in malaria.60
The success of elimination in many countries will thus be contingent on mitigation of the risk of unpredictable financing, which will need a paradigm shift in the way that malaria funding is considered and allocated. Instead of a “quick win” solution that warrants rapid scale-up and implies the ability for subsequent withdrawal,61 malaria interventions will need to be viewed as a continuous expenditure even when the disease is absent, such as with routine immunisation, until global eradication is achieved. Quantification of the health and economic benefits of continuing spending can ease that shift. For wealthier countries, policy makers' understanding of that new paradigm might be sufficient to ensure sustained financing, although even the USA uses alternative financing methods to hedge against political risk for its own priority health issues.62,63
Poorer countries, however, will probably need additional solutions to protect against donor volatility. Mechanisms to increase the sustainability of health and development aid have been frequently debated, but have led to marginal improvements at best.64 As such, instead of seeking a universal solution, the global malaria community will need to help countries to identify pragmatic approaches through a mix of long-term donor commitments, increased national contributions, and targeted innovative mechanisms. Increased emphasis on the regional and global benefits of elimination might also provide a partial solution, by securing sustained support from countries with a direct self-interest in the success of the malaria-eliminating country (ie, by reducing malaria importation).
In the end, however, there is no substitute for sustained political will in ensuring financial sustainability. Apart from in the poorest countries, governments that are truly committed to elimination can find the necessary resources. Countries such as Lebanon and Taiwan were poorer than many current malaria-eliminating countries at the time when they achieved elimination, but have successfully maintained malaria-free status after the withdrawal of donor support.10,50,65 Thus, financial feasibility is, in effect, political feasibility. As a result, the final crucial step in the assessment of elimination is for a government, with use of rigorous and clear assessment of the expected costs, benefits, and timelines, to establish whether they are prepared to take the often oneorous steps to achieve and sustain that goal. With more poor countries exploring elimination, the international community should also now make the same judgment.
Conclusions
Overall, this analysis indicates that the initial investment needed to achieve elimination varies greatly between countries and contexts, but is greater than the cost of control. Although the case studies find similar wide variation in the cumulative costs of elimination and controlled low-endemic malaria, they suggest that policy makers should not view the generation of substantial short-term or medium-term cost-savings as a rationale for elimination until more robust evidence is available to suggest otherwise. These findings do not mean that elimination is a poor investment. A complete economic assessment of elimination includes a range of additional benefits that are not quantified here.66 In view of the dearth of evidence for these benefits, to conclude whether the total cost–benefit ratio would be negative or positive is not possible at present, even in a setting with high comparative costs—such as Swaziland. Where the total cost of elimination is modestly higher than controlled low-endemic malaria (China and Mauritius), additional benefits would need to be similarly modest to produce a positive cost–benefit ratio.
The low probability of elimination generating cost-savings in most of the case studies is driven mainly by two basic factors: discounting of future costs and the initial increased cost of elimination. In the Chinese provinces, the assumed reduction in postelimination expenditures results in substantial yearly savings compared with controlled low-endemic malaria. However, discounting diminishes the value of those savings, preventing them from fully offsetting the initial increased investments to reach elimination. Tanzania (Zanzibar) has similar yearly postelimination cost reductions as the Chinese provinces, but is the only site to produce cumulative savings despite discounting. This difference is caused by the substantially lower increase between controlled low-endemic malaria and the initial elimination investment in Tanzania (Zanzibar); many of the estimated elimination requirements are already incorporated into its controlled low-endemic malaria programme, whereas the Chinese provinces and Swaziland need to make major new investments in several interventions. The probability of cost-savings was less sensitive to the time needed to achieve elimination than other factors, such as the cost of prevention, as a result of the large range of possible values assumed for those factors and the short duration (6–14 years) of the assumed elimination phase.
Wide variations in epidemiological and economic context probably explain much of the difference in controlled low-endemic malaria and elimination costs. Tanzania (Zanzibar), with its high transmission potential and weak health system, has had to implement extensive interventions to achieve controlled low-endemic malaria, whereas China (Jiangsu) has done so without any vector control (webappendix p 32).67 Conversely, China (Jiangsu) must address the more tenacious Plasmodium vivax parasite whereas Tanzania's (Zanzibar) burden is predominantly Plasmodium falciparum, which could have substantial cost implications during the elimination phase. Variable programme efficiency might also contribute to differences in costs between sites and phases—some costs are derived from donor budgets, which experience suggests differ greatly from actual need and expenditure. This variability draws attention to the importance of establishing the optimum amount of expenditure for both controlled low-endemic malaria and elimination; that is, the cost of the minimum package of interventions needed to achieve these states, as opposed to the cost of a programme that generally seeks to maximise scale-up of all interventions. Such comprehensive cost-minimisation analysis is challenging but feasible and is critical to enabling accurate assessment of the economics of elimination.68
Although the public health community is focused on comparative cost–benefit ratios, the main metric of interest for most local policy makers is affordability. Here too, the case studies show substantial diversity. The investments needed to achieve elimination will account for a small proportion of public expenditure on health in the Chinese provinces, which, along with confirmation of technical feasibility, is probably the main driver of the government's recent decision to pursue elimination in those areas.69 By contrast, Tanzania (Zanzibar) will need to allocate nearly a fifth of estimated public health resources, whereas Swaziland will need to consider increased malaria spending in the context of its massive HIV/AIDS burden. Malaria financing in these sites, however, is mainly provided by external sources, indicating that the questions of whether and how to make the tradeoffs required to pursue elimination will need to be answered by international donors in addition to national governments.
Substantial caution should be used when interpreting case-study results. The programmes we examined are not optimised to achieve elimination, and whether or not they will be sufficient to interrupt malaria transmission (with the exception of Mauritius) is unknown. We also excluded costs borne by the general health system and individuals, which might vary between controlled low-endemic malaria and elimination, and do not consider potential sharing of costs (eg, surveillance) between malaria and other disease programmes. Lastly, we do not assume any changes in costs as a result of external factors despite the long period of analysis. Key factors that could decrease ongoing costs include reduced transmission potential (eg, through economic development), lower importation of parasites from neighbouring countries, and integration of the malaria programme into the health system, whereas those that could increase costs include development of drug or insecticide resistance. However, only changes in importation will differentially affect controlled low-endemic malaria and elimination costs.
Great care should also be used in extrapolation of findings to other countries since these sites, which include one of the world's largest economies and several islands, are not representative of most malaria-endemic countries, and our findings show that the costs of control and elimination programmes vary greatly between countries because of a range of factors. Nevertheless, these case studies represent the only such examination of the potential cost-savings of elimination so far and can serve as an interim guide for countries until they are able to undertake robust economic analyses in their local context. Above all, our results accentuate the urgency for thorough research into the additional benefits of elimination. With elimination unlikely to be warranted on its financial returns alone, full cost–benefit analysis is essential to guide relevant policy decisions. For such robust analyses to be possible, the massive gap in empirical evidence must be closed by pursuit of specific research priorities (panel).
Panel. Priorities for research on the economics of malaria elimination.
Costs
-
•
Determination of an optimum sustained control level and corresponding intervention strategies and costs to establish a consistent baseline
-
•
Marginal-cost curves for key elimination-related interventions, including vector control, surveillance, and border screening
-
•
Additional comprehensive actual expenditures of contemporary elimination programmes in differing eco-epidemiological settings
-
•
Additional actual expenditures on the prevention of reintroduction by countries with differing transmission potential and importation rates
-
•
Detailed estimates of the magnitude and timing of malaria-specific costs that are absorbed into the health system after elimination across different settings
Benefits
-
•
Retrospective and prospective quantification of direct and indirect health benefits of elimination in different eco-epidemiological and socioeconomic settings
-
•
Marginal curves of key indirect benefits of malaria reduction, including educational attainment, agricultural productivity, and fertility to identify potential threshold benefits
-
•
Presence and magnitude of potential threshold benefits of achieving elimination, notably for tourism and foreign direct investment, in different settings
-
•
Equity of direct and indirect benefits of elimination compared with scale-up of control
-
•
Magnitude of the regional and international financial benefits of elimination from a specific country
-
•
Retrospective effect of malaria-elimination programmes on health systems and other disease programmes
Financing
-
•
Further robust quantification of the health and economic benefits of sustaining financing for malaria at low levels of disease
-
•
Quantification and mapping of baseline transmission potential in all endemic countries to enable targeting of financing based on the risk of resurgence
-
•
Analysis of national, regional, and global instruments to mitigate the risk of financial volatility on elimination programmes
Acknowledgments
Acknowledgments
We thank the many other individuals who contributed to this work, particularly Simon Kunene, Abdullah Ali, and the members of the Swaziland and Zanzibar National Malaria Control Programmes for their inspiring analysis and pursuit of malaria elimination; Colin Boyle, Chris Cotter, Clara Kim, Angela Ni, Yuan Sun, Mitsuru Toda, Shanqing Wang, and Huayun Zhou for their invaluable contributions. The work of the Global Health Group of the University of California, San Francisco, on malaria elimination is supported by grants from the Bill & Melinda Gates Foundation and ExxonMobil. The Clinton Health Access Initiative acknowledges support from the Global Health Group for their work on malaria elimination in southern Africa. We are also grateful for the financial support from the Bill & Melinda Gates Foundation to the Clinton Health Access Initiative and the Institute of Health Metrics and Evaluation, University of Washington. JGK was supported in part by NIH/NCRR UCSF-CTSI Grant Number UL1 RR024131. Lastly, we are grateful to members of the Malaria Elimination Group for their guidance on the material in this paper.
Contributors
OS conceived, planned, and undertook the analysis and wrote the paper. JMC did the analysis and contributed to the report. MSH collected and analysed data and contributed to the report. JLC, JGK, SBas, SBar, RGAF, and DTJ helped conceive the research and contributed to the analysis. LT, BZ, QG, LZ, AT, and SA collected and managed data and contributed to the analysis. JU managed the data and contributed to the analysis.
Conflicts of interest
OS, JMC, JLC, AT, JU, and LZ work within or assist the malaria programme at the Clinton Health Access Initiative, which is supporting malaria elimination in southern Africa. MSH and RGAF work at the Global Health Group of the University of California, San Francisco, CA, USA. MSH helps coordinate the joint Asia Pacific Malaria Elimination Network secretariat. The Global Health Group exists in part to support countries that are embarked on an evidence-based pathway towards elimination. SBas serves as a principal advisor to the UN Secretary General's Special Envoy for Malaria who has been charged to coordinate efforts to end deaths from malaria including seeking funding to fight the disease. LT, BZ, QG, and SA are actively involved in the management of elimination or prevention of reintroduction programmes. OS, MSH, SBas, LT, DTJ, and RGAF are members of the Malaria Elimination Group. DTJ also co-chairs Global Fund and WHO committees related to malaria. JGK consults with a bednet manufacturer. The findings and conclusions in the report are those of the authors and do not necessarily represent the views of their employing organisations nor of the sources of funding.
Web Extra Material
References
- 1.Lines J, Schapira A, Smith T. Tackling malaria today. BMJ. 2008;337:435–437. doi: 10.1136/bmj.a869. [DOI] [PubMed] [Google Scholar]
- 2.Kahn JG, Basu S, Boyle C. Financing elimination. In: Feachem RGA, Phillips AA, Targett GAT, editors. Shrinking the malaria map: a prospectus on malaria elimination. The Global Health Group, Global Health Sciences, University of California, San Francisco; San Francisco: 2009. [Google Scholar]
- 3.Feachem RGA, Phillips AA, Targett GAT, editors. Shrinking the malaria map: a prospectus on malaria elimination. The Global Health Group, Global Health Sciences, University of California, San Francisco; San Francisco: 2009. [Google Scholar]
- 4.Mills A, Lubell Y, Hanson K. Malaria eradication: the economic, financial and institutional challenge. Malar J. 2008;7:11. doi: 10.1186/1475-2875-7-S1-S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moonen B, Cohen JM, Snow RW. Operational strategies to achieve and maintain malaria elimination. Lancet. 2010 doi: 10.1016/S0140-6736(10)61269-X. published online Oct 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohen JM, Smith DL, Moonen B, Snow RW. How absolute is zero? An evaluation of historical and current definitions of malaria elimination. Malar J. 2010;9:213. doi: 10.1186/1475-2875-9-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mills A, Shillcutt S. Challenge paper on communicable diseases. In Copenhagen Consensus Center. Copenhagen Consensus 2004; Copenhagen: 2004. [Google Scholar]
- 8.Jamison DT. Cost effectiveness analysis: concepts and applications. In: Detels R, McEwen J, Beaglehole R, Tanaka H, editors. Oxford Textbook of Public Health: Volume 2, The Methods of Public Health. fifth edn. Oxford University Press; Oxford: 2009. pp. 767–782. [Google Scholar]
- 9.Edejer TT, Baltussen R, Adam T. WHO guide to cost-effectiveness analysis. World Health Organization; Geneva: 2003. [Google Scholar]
- 10.Feachem RGA, Phillips AA, Hwang J. Shrinking the malaria map: progress and prospects. Lancet. 2010 doi: 10.1016/S0140-6736(10)61270-6. published online Oct 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bart KJ, Foulds J, Patriarca P. Global eradication of poliomyelitis: benefit-cost analysis. Bull World Health Organ. 1996;74:35–45. [PMC free article] [PubMed] [Google Scholar]
- 12.Khan MM, Ehreth J. Costs and benefits of polio eradication: a long-run global perspective. Vaccine. 2003;21:7–8. doi: 10.1016/s0264-410x(02)00584-4. [DOI] [PubMed] [Google Scholar]
- 13.Kim A, Ruiz-Tiben E. Cost-benefit analysis of the dracunculiasis eradication campaign. World Bank; Washington DC: 1997. [Google Scholar]
- 14.WHO . World Health Organization Expert Committee on Malaria: Technical Report Series No. 123. Sixth Report. World Health Organization; Geneva: 1957. [PubMed] [Google Scholar]
- 15.Najera JA. Malaria control: achievements, problems, and strategies. World Health Organization; Geneva: 1999. [PubMed] [Google Scholar]
- 16.Yekutiel P. The Global Malaria Eradication Campaign. In: Yekutiel P, editor. Eradication of infectious diseases: a critical study. S. Karger; Basel, Switzerland: 1980. pp. 34–88. [Google Scholar]
- 17.Pampana E. A textbook of malaria eradication. Oxford University Press; London: 1969. [Google Scholar]
- 18.Moonen B, Barrett S, Tulloch J, Jamison DT. Shrinking the malaria map: a prospectus on malaria elimination. The Global Health Group, Global Health Sciences, University of California, San Francisco; San Francisco: 2009. Making the decision. [Google Scholar]
- 19.Ottersen T, Mbilinyi D, Maestad O, Norheim OF. Distribution matters: Equity considerations among health planners in Tanzania. Health Policy. 2008;85:218–227. doi: 10.1016/j.healthpol.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 20.Jehu-Appiah C, Baltussen R, Acquah C. Balancing equity and efficiency in health priorities in Ghana: the use of multicriteria decision analysis. Value Health. 2008;1:1081–1087. doi: 10.1111/j.1524-4733.2008.00392.x. [DOI] [PubMed] [Google Scholar]
- 21.Lomborg B. Global Crises, Global Solutions. Cambridge University Press; Cambridge: 2009. [Google Scholar]
- 22.Goodman CA, Mills AJ. The evidence base on the cost-effectiveness of malaria control measures in Africa. Health Policy Plan. 1999;14:301–312. doi: 10.1093/heapol/14.4.301. [DOI] [PubMed] [Google Scholar]
- 23.Barrett S. Eradication versus control: the economics of global infectious disease policies. Bull World Health Organ. 2004;82:683–688. [PMC free article] [PubMed] [Google Scholar]
- 24.Castro-Leal F, Dayton J, Demery L, Mehra K. Public spending on health care in Africa: do the poor benefit? Bull World Health Organ. 2000;78:66–74. [PMC free article] [PubMed] [Google Scholar]
- 25.Cleary S. Trade-offs in scaling up HIV treatment in South Africa. Health Policy Plan. 2010;25:99–101. doi: 10.1093/heapol/czp068. [DOI] [PubMed] [Google Scholar]
- 26.Bleichrodt H, Diecidue E, Quiggin J. Equity weights in the allocation of health care: the rank-dependent QALY model. J Health Econ. 2004;23:157–171. doi: 10.1016/j.jhealeco.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 27.Ong KS, Kelaher M, Anderson I, Carter R. A cost-based equity weight for use in the economic evaluation of primary health care interventions: case study of the Australian Indigenous population. Int J Equity Health. 2009;8:34. doi: 10.1186/1475-9276-8-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maartens F, Sharp B, Curtis B, Mthembu J. The impact of malaria control on perceptions of tourists and tourism operators concerning malaria prevalence in KwaZulu-Natal, 1999/2000 versus 2002/2003. J Travel Med. 2007;14:96–104. doi: 10.1111/j.1708-8305.2006.00086.x. [DOI] [PubMed] [Google Scholar]
- 29.Smith RD. Responding to global infectious disease outbreaks: lessons from SARS on the role of risk perception, communication and management. Soc Sci Med. 2006;63:3113–3123. doi: 10.1016/j.socscimed.2006.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brug J, Aro AR, Richardus JH. Risk perceptions and behaviour: towards pandemic control of emerging infectious diseases. Int J Behav Med. 2009;16:3–6. doi: 10.1007/s12529-008-9000-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Humphreys M. Kicking a dying dog. DDT and the demise of malaria in the American South, 1942–1950. Isis. 1996;87:1–17. doi: 10.1086/357400. [DOI] [PubMed] [Google Scholar]
- 32.Litsios S. Which way for malaria control and epidemiological services? World Health Forum. 1993;14:43–52. [PubMed] [Google Scholar]
- 33.ZMCP Malaria elimination: a feasibility assessment. Zanzibar malaria control program. 2009. http://www.malariaeliminationgroup.org/sites/default/files/MalariaEliminationZanzibar.pdf (accessed March 15, 2010).
- 34.Malam MS. Malaria eradication–FG to distribute 63 million nets. 2009 November 19. http://allafrica.com/stories/200911160754.html (accessed March 15, 2010).
- 35.Population Division of the Department of Economic and Social Affairs of the United Nations Secretariat, World Population Prospects: The 2008 Revision.
- 36.Tatem AJ, Smith DL, Gething PW, Kabaria CW, Snow RW, Hay SI. Ranking of elimination feasibility between malaria-endemic countries. Lancet. 2010 doi: 10.1016/S0140-6736(10)61301-3. published online Oct 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.China Annual Malaria Report, 2. Ministry of Health; Beijing: 2009. [Google Scholar]
- 38.International Monetary Fund World Economic Outlook Database. International Monetary Fund; Washington DC: 2009. [Google Scholar]
- 39.China Statistical Yearbook 2008. National Bureau of Statistics of China; China: 2008. [Google Scholar]
- 40.Lu C, Schneider MT, Gubbins P. Public financing of health in developing countries: a cross-national systematic analysis. Lancet. 2010;375:1375–1387. doi: 10.1016/S0140-6736(10)60233-4. [DOI] [PubMed] [Google Scholar]
- 41.WHO . World Health Statistics. World Health Organization; Geneva: 2009. [Google Scholar]
- 42.UNICEF . Immunization Summary. UNICEF; New York: 2009. [Google Scholar]
- 43.WHO . Malaria elimination: a field manual for low and moderate endemic countries. World Health Organization; Geneva: 2007. [Google Scholar]
- 44.Thompson KM, Tebbens RJ. Eradication versus control for poliomyelitis: an economic analysis. Lancet. 2007;369:1363–1371. doi: 10.1016/S0140-6736(07)60532-7. [DOI] [PubMed] [Google Scholar]
- 45.Kaser MC. Observations on the likely economic efficiency of the malaria eradication program. In: Barlow R, editor. The economic effects of malaria eradication. University of Michigan School of Public Health; Michigan: 1968. pp. 145–167. [Google Scholar]
- 46.Briggs A, Sculpher M, Buxton M. Uncertainty in the economic evaluation of health care technologies: the role of sensitivity analysis. Health Econ. 1994;3:95–104. doi: 10.1002/hec.4730030206. [DOI] [PubMed] [Google Scholar]
- 47.Robberstad B, Strand T, Black R. Cost-effectiveness of zinc as adjunct therapy for acute childhood diarrhea in developing countries. Bull World Health Organ. 2004;82:523–531. [PMC free article] [PubMed] [Google Scholar]
- 48.Akumu AO, English M, Scott JAG, Griffiths UK. Economic evaluation of delivering Haemophilus influenzae type b vaccine in routine immunization services in Kenya. Bull World Health Organ. 2007;85:511–518. doi: 10.2471/BLT.06.034686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Griffith ME. Financial implications of surveillance in India and other countries. Bull Nat Soc India Malaria Other Mosquito-borne Dis. 1961;9:385–411. [Google Scholar]
- 50.De Zulueta J, Muir DA. Malaria eradication in the near east. Trans R Soc Trop Med Hyg. 1972;66:679–695. doi: 10.1016/0035-9203(72)90082-x. [DOI] [PubMed] [Google Scholar]
- 51.Cohn E. Assessing the costs and benefits of anti-malaria programs: the Indian experience. Am J Public Health. 1973;63:1086–1096. doi: 10.2105/ajph.63.12.1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bleakley H. Malaria eradication in the Americas: a retrospective analysis of childhood exposure. Chicago; University of Chicago: 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cutler D, Fung W, Kremer M, Singhal M, Vogl T. Mosquitoes: The long-term effects of malaria eradication in India. NBER Work Pap Ser. 2007:13539. [Google Scholar]
- 54.Lucas AM. Malaria eradication and educational attainment: evidence from Paraguay and Sri Lanka. Am Econ J Appl Econ. 2010;2:46–71. doi: 10.1257/app.2.2.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ashraf QH, Lester A, Weil DN. When does improving health raise GDP? NBER Work Pap Ser. 2008:14449. doi: 10.1086/593084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Celasun O, Walliser J. Predictability of Aid: Do Fickle Donors Undermine Aid Effectiveness. Econ Policy. 2008;23:545–594. [Google Scholar]
- 57.Johnson KA, Sardell A, Richards B. Federal immunization policy and funding: A history of responding to crises. Am J Prev Med. 2000;19:1–14. doi: 10.1016/s0749-3797(00)00210-5. [DOI] [PubMed] [Google Scholar]
- 58.Jackson S, Sleigh AC, Liu XL. Cost of malaria control in China: Henan's consolidation programme from community and government perspectives. Bull World Health Organ. 2002;80:653–659. [PMC free article] [PubMed] [Google Scholar]
- 59.Soeung SC, Grundy J, Maynard J, Brooks A. Financial sustainability planning for immunization services in Cambodia. Health Policy Plan. 2006;21:302–309. doi: 10.1093/heapol/czl012. [DOI] [PubMed] [Google Scholar]
- 60.Najera JA, Kouznetsov RL, Delacollette C. Malaria epidemics, detection and control forecasting and prevention. World Health Organization Division of Control of Tropical Diseases; Geneva: 1998. [Google Scholar]
- 61.Sachs J. Achieving the millennium development goals—the case of malaria. NEJM. 2005;352:115–117. doi: 10.1056/NEJMp048319. [DOI] [PubMed] [Google Scholar]
- 62.Lazzari S. The black lung excise tax on coal. Congressional Research Service Report; Washington DC: 2004. [Google Scholar]
- 63.Drake B, Tieman AM. National childhood vaccine injury act of 1986: using tax revenue to assure vaccine availability. Public Budgeting Finance. 1994;14:54–64. [Google Scholar]
- 64.Lane C, Glassman A. Smooth and predictable aid for health: A role for innovative financing? Brookings Institution; Washington DC: 2009. [Google Scholar]
- 65.Yip K. Malaria eradication: the Taiwan experience. Parasitologia. 2000;42:117–126. [PubMed] [Google Scholar]
- 66.Feachem RGA, Sabot O. A new global malaria eradication strategy. Lancet. 2008;371:1633–1635. doi: 10.1016/S0140-6736(08)60424-9. [DOI] [PubMed] [Google Scholar]
- 67.Bhattarai A, Ali AS, Kachur SP. Impact of artemisinin-based combination therapy and insecticide-treated nets on malaria burden in Zanzibar. PLoS Med. 2007;4:e309. doi: 10.1371/journal.pmed.0040309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Barrett S, Hoel M. Optimal disease eradication. Environ Dev Econ. 2007;12:627–652. [Google Scholar]
- 69.Ministry of Health . From Control to Elimination: A revised national malaria strategy 2010–2015. China Ministry of Health; Beijing: 2010. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.