Summary
Experience gained from the Global Malaria Eradication Program (1955–72) identified a set of shared technical and operational factors that enabled some countries to successfully eliminate malaria. Spatial data for these factors were assembled for all malaria-endemic countries and combined to provide an objective, relative ranking of countries by technical, operational, and combined elimination feasibility. The analysis was done separately for Plasmodium falciparum and Plasmodium vivax, and the limitations of the approach were discussed. The relative rankings suggested that malaria elimination would be most feasible in countries in the Americas and Asia, and least feasible in countries in central and west Africa. The results differed when feasibility was measured by technical or operational factors, highlighting the different types of challenge faced by each country. The results are not intended to be prescriptive, predictive, or to provide absolute assessments of feasibility, but they do show that spatial information is available to facilitate evidence-based assessments of the relative feasibility of malaria elimination by country that can be rapidly updated.
This is the second in a Series of four papers about malaria elimination
Introduction
Substantial progress in reducing the morbidity and mortality caused by malaria worldwide has encouraged the Global Malaria Action Plan to outline a long-term vision for malaria eradication through shorter-term efforts to eliminate the disease1 (throughout, we define eradication and elimination as outlined in the first report in this Series2). 32 of the 99 countries in which malaria is endemic have declared a national policy for malaria elimination or are pursuing spatially progressive elimination within their borders.2
Elimination of malaria would be feasible if the technical, operational, and financial challenges to the permanent interruption of transmission could be overcome.3 Elimination is technically feasible if malaria interventions can be deployed at a sufficiently high coverage to interrupt local malaria transmission and be maintained for a duration sufficient to eliminate the local reservoir of parasites.4,5 Since elimination should be considered a one-way transition from malaria-endemic to non-endemic status, technical feasibility also requires an assessment of the probability of malaria being re-established.6 Technical assessments frame the scale of the operational challenge, itself further defined in terms of the human capital, national infrastructure, and political commitment needed by nations to reach their elimination goals. Definition of overall elimination feasibility requires the simultaneous consideration of technical and operational constraints.
Recent authoritative reviews have provided expert opinion on the feasibility of malaria elimination by region (eg, Schapira and colleagues7). We undertook a complementary approach based on the current understanding of the global spatial epidemiology of malaria and the application of mathematical transmission models to define the technical difficulty of elimination of the disease. Spatial data for indicators related to the operational feasibility of malaria elimination were assembled and, in combination, we attempted to define quantitatively which countries are currently the most and the least feasible candidates for malaria elimination. We undertook technical feasibility analyses for Plasmodium falciparum only because the theoretical modelling framework8–10 and global cartography11,12 for this species are sufficiently well developed. Since the same is not true for Plasmodium vivax,13 we restricted the analysis for this species to operational feasibility. Our approach focused on available indices and did not attempt to predict future changes to technical or operational feasibility. Nor did we consider the costs of elimination, which are addressed in a separate paper in this Series.14 Rather, we present a framework to show combinations of technical and operational constraints to elimination in the 99 malaria-endemic countries in an attempt to provide an objective and contemporary relative ranking between nations, rather than an absolute assessment of feasibility. Our approach represents one of many for the construction of composite indices, and since uncertainties are inherent in the datasets used, our results are not definitive statements on feasibility, but a provisional attempt to provide an indication of the challenges faced by each nation relative to others. Although our primary focus was on the relative feasibility of elimination between countries, the results are relevant to the full range of control, including the relative feasibility of effective national scale-up of interventions for those countries not considering elimination.
Key messages.
-
•
Substantial spatial data and an established modelling framework enable evidence-based, species-specific assessments of the feasibility of malaria elimination for policy makers
-
•
The approach presented aims to identify rate-limiting steps to feasibility of malaria elimination and thus provides the opportunity to objectively assess the relative merits of national malaria intervention plans
-
•
Results for relative elimination feasibility vary between countries when technical or operational aspects of feasibility are considered, highlighting different challenges faced by nations
-
•
Elimination of Plasmodium falciparum from the Americas is most feasible, with a less than 50% reduction in 2007 transmission levels needed continent-wide
-
•
Elimination of P falciparum from African countries is least feasible, with much of west and central Africa needing a more than 90% reduction in 2007 transmission levels to achieve elimination
-
•
Substantial developments in modelling and mapping are needed for estimation of the technical feasibility of eliminating Plasmodium vivax (and Plasmodium malariae, Plasmodium ovale, and Plasmodium knowlesi)
-
•
These relative rankings are an objective and easily updated guide; they should be augmented by detailed country-level feasibility assessments before an elimination campaign is started
Assessment of relative technical feasibility between countries
WHO notes that the 108 countries that have eliminated malaria share two characteristics that are important in measuring technical feasibility (panel 1): malaria originally unstable or of low-grade intermediate stability and an absence of major population movements from neighbouring malarious countries (a low malaria importation rate).3 In this paper, we describe a global map that documents transmission levels of P falciparum. This map serves as a basis for estimation of the technical difficulty of achieving elimination based on the intensity of transmission and for estimating malaria importation rates. We combined these factors to assess the relative technical feasibility of elimination of P falciparum malaria for each endemic country. For each factor, data, detailed methods, and sample sensitivity analyses are provided in webappendix pp 2–25.
Panel 1. Factors affecting the technical and operational feasibility of malaria elimination.
Technical feasibility
-
•
Intensity of endemic transmission
-
•
Malaria importation rate
Operational feasibility
Government stability, effectiveness, and commitment
-
•
Absence of internal and external conflicts
-
•
Good organisational and technical infrastructure
-
•
Firm political and financial commitment to malaria elimination
Health systems
-
•
Fully developed, functional general health services
-
•
High quality of training and personnel
Populations at risk
-
•
Size of total population at risk
-
•
At-risk population as a proportion of total population
-
•
Access to populations at risk
Estimation of intensity of endemic P falciparum transmission
An index of transmission intensity that is directly relevant to an assessment of technical feasibility is the basic reproductive number, R0. This index describes what would happen if a single infectious person were to be introduced into a population with no malaria, malaria immunity, or malaria control; it indicates the expected number of people who would become infected after one parasite generation.15 A closely related measure is the reproductive number under some existing level of control, Rc. R0 is a measure of maximum potential transmission, and if R0 is 1 or greater, then endemic malaria transmission can be sustained. If control measures are sufficient to reduce Rc to less than 1, endemic transmission can be interrupted on timelines that are proportional to Rc.9 The ratio R0/Rc thus gives a simple measure of the total transmission effect size achieved through the combined effects of control, and R0 establishes a threshold on the total transmission effect size required to eliminate malaria through control (panel 2).
Panel 2. Modelling the effects of control on Plasmodium falciparum R0.
Endemicity can be reduced through various forms of control that affect different features of transmission. The effect sizes of different control modes are directly related to the coverage levels achieved. Vector control with insecticide-treated nets (ITNs), indoor residual spraying (IRS), or larvicides reduces transmission by mosquitoes, as measured by vectorial capacity (denoted V).16 ITNs and IRS affect several features of Anopheles spp feeding behaviour, so the transmission effect sizes for adult vector control increase in a highly non-linear way with the proportion of people who own and use a net or the proportion of houses sprayed.17–19 Antimalarial drugs reduce the average duration of the infectious period (denoted D);20 the effect size on transmission increases with the performance of health systems, specifically the proportion of incident malaria infections that are treated and cured. In areas of high transmission, this proportion can be reasonably low because of clinical immunity,21 and in areas of low transmission, mature gametocytes can account for residual transmission.22 Vaccines, if available, could block infections in human beings (b) or mosquitoes (c). Transmission and the effects of control are also related to the degree of biting heterogeneity (α). The relevance of R0 for an assessment of technical feasibility can be illustrated by rewriting the classic formula:
R0=bcVD(1+α)
After some measure of control, transmission intensity reaches a new level that reflects the changes brought about through multiple modes of control:
Rc=b′c′V′D′(1+α′)
The effect sizes of different control modes are generally multiplicative: a 90% reduction achieved through ITNs (ie, V/V′=10) and a 90% reduction achieved through drugs (ie, D/D′=10) would give a 99% overall reduction in transmission intensity (ie, R0/Rc=100). The combined effect of multiple modes of control are lower if biting heterogeneity increases (ie, α/α′<1), which would occur if a subpopulation had no nets and was never treated.23 In practical terms, there is spatial variation in the difficulty of bringing R0 to less than 1 with current control methods. For instance, reducing R0 in areas where exophilic vectors are dominant is likely to be more challenging than doing so in other areas, because of a lower effectiveness of ITNs.
The first step in an assessment of technical feasibility is the estimation of R0 and the intervention coverage levels required to reduce Rc to less than 1. Unfortunately, field estimates of R0 are generally not available.15 The parasite rate (PR, the prevalence of malaria infection in a sampled population) is commonly measured, however, and assemblies of this metric have been used to build the first evidence-based global map of P falciparum PR (PfPR) endemicity.12 Mathematical models provide a method for estimation of PfR0 from PfPR.24,25 The resulting estimates are uncertain because the underlying estimates of PfPR are uncertain and because additional uncertainty surrounds assumptions of biting heterogeneity and malaria immunity used in the model translation of PfPR to PfRc.10 This method was used to create a global map of PfRc (figure 1) from the global PfPR map in 2007.12
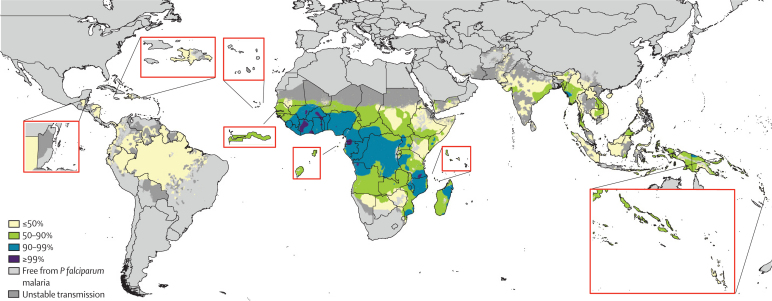
Figure 1.
Categorical map of Plasmodium falciparum reproductive number, PfRc, indicating the extent to which transmission needs to be reduced for elimination
Map highlights areas that would require transmission reductions of less than 50% (PfRc 1–10), 50–90% (PfRC 10–50), 90–99% (PfRC 50–100), or greater than 99% (PfRc>100) to eliminate P falciparum malaria. Unstable transmission is as defined in reference 11.
This new map reflects the levels of malaria control in 2007, such as the use of insecticide-treated nets26 and earlier drug policy changes from failing drugs to artemisinin-based combination therapies.27 The map therefore describes PfRc, the level of additional control effort required to achieve elimination, in the absence of imported infections. The map, when grouped heuristically into four stable transmission classes (figure 1), suggests that, technically, P falciparum malaria could be eliminated in most of the world if malaria transmission could be reduced by 90%. The timelines over which this eradication could be achieved depend on endemicity and intervention coverage levels, and have been described elsewhere.9,24 In most of Africa and a few areas outside of Africa, a 90–99% reduction in transmission intensity would be needed for successful elimination. Areas in which elimination is most challenging are those in west and central Africa with very high endemicity.
Measurement of technical feasibility requires estimates of PfR0, and not only PfRc. Although estimation of PfR0 from PfRc is theoretically possible, it would require contemporary maps of coverage levels for all malaria control interventions, which are not available. The PfRc estimates presented in figure 1 represent imperfect measures of absolute PfR0; however, they are likely to represent realistic relative estimates. Moreover, use of the only alternative historical global endemicity map28 had little to no effect on the results (webappendix pp 2–6). Population-weighted mean values of transmission intensity per country were then calculated from the map in figure 1 (webappendix pp 2–4).
P vivax
Our methods were developed on the basis of an understanding of the epidemiology of P falciparum. The relatively poor understanding of the epidemiology of P vivax13 means that the extent to which P falciparum transmission models will translate is unclear because P vivax infection dynamics are complicated by a dormant liver-stage infection with hypnozoites, which can cause relapsing blood stage infections several months to years after the infectious bite.29 The frequency of these dormant infections, the timing and number of relapses, and the geographical variation in these factors are poorly quantified.13 When these factors are combined, describing P vivax R0 (PvR0) in simple models becomes much more difficult. More fundamentally, global maps of P vivax endemicity have not yet been published and P vivax cannot be easily eliminated with the same methods shown to be successful against P falciparum.30 Near-term elimination strategies will require a focus on tackling the reservoir of infection in the livers of the human population with primaquine (contraindicated in those who are glucose-6-phosphate dehydrogenase deficient), although methods such as sufficiently sensitive rapid diagnostic or community-level tests for glucose-6-phosphate dehydrogenase deficiency do not exist.13,31 Therefore, strategies for the elimination of malaria are likely to be species-specific, and feasibility assessments must acknowledge this parasitological diversity. We therefore considered P falciparum and P vivax separately and did not assess feasibility of elimination of Plasmodium malariae, Plasmodium ovale, or Plasmodium knowlesi because of the dearth of information about their distribution, abundance, and public health importance.
Estimation of imported rates of P falciparum malaria
As local endemicity is reduced, the importance of imported malaria in sustaining transmission increases.32 Moreover, after R0 has decreased to less than 1 and malaria has been eliminated from a region, importation becomes the primary concern. Risk of importation can be defined as the rate at which infected and infectious hosts are imported into a country per year. Typically, anophelines fly short distances, so human carriage of parasites constitutes the main risk.6,33–35
Quantification of human movement temporally and spatially, and the resulting importation rate of malaria, is essential if feasibility of elimination is to be assessed.6,36 Ideally, data at the range of scales relevant to malaria transmission are required and methods are being developed to provide these,37,38 but such data are rarely available on a global scale. The release of a bilateral database of international migration39 provides one valuable source of information about the relative sizes of population movements between nations. In accordance with approaches outlined elsewhere,36 we linked these data with per-country population-weighted measures of PfPR to create an index that accounted for both the relative size of incoming population movement to a country and the P falciparum endemicity in the country of origin. The index is high when a country has high migrant flow from predominantly high P falciparum endemicity countries (webappendix pp 7–8).
Relative technical feasibility of P falciparum elimination
Examination of the relative technical feasibility of malaria elimination between endemic countries requires simultaneous consideration of endemic transmission and rates of imported malaria. Thus, transmission and imported malaria indicators for each country were analysed together by use of the partially ordered set approaches described in panel 3, table 1, and webappendix pp 28–29.
Panel 3. Combining indicators.
Composite or summary measures are widely used in public policy and health policy debate,40,41 and summarise a broad range of outcomes in a single statistic. Few experts would claim that these measures show everything of importance and no universally accepted scientific rules are in place for their construction,40 yet because they are comprehensive, concise, and easy to communicate, they have become an established part of health policy. Such measures are discussed in further detail in webappendix pp 26–29.
Briefly, after testing and assessment of different indicators and methods of combining indicators, we used the partially ordered sets (posets) approach42 with an aim of minimising subjectivity in the analyses presented. Outputs of the posets methodology include ranked groups of countries, with countries assigned to each group only when there is clear information to do so. For instance, consider the simplified example of comparing the feasibility of Plasmodium falciparum elimination from nations solely on the basis of baseline transmission intensity, political stability, and health-care spending. Table 1 presents the values and ranks (in parentheses) for these factors for five countries.
The conventional average rank column shows the resulting composite scores when the mean of ranks is calculated. When comparing the Dominican Republic with Burundi, Somalia, and Equatorial Guinea, it is clear that the Dominican Republic should be ranked as more feasible for elimination because the values for all three factors are more favourable. However, when comparing, for instance, Equatorial Guinea with Burundi, it can be seen that Burundi has the lower transmission intensity, but Equatorial Guinea is more politically stable and has higher levels of spending on health.
By assuming each factor is equally important, conventional average ranking suggests that elimination of P falciparum is more feasible for Equatorial Guinea (average rank 3·3) than it is for Burundi (average rank 3·7), when, in reality, the relative importance of R0 levels in making this assessment is neither known nor agreed upon and might represent a more important factor. In cases like this where conflicting evidence exists, or the relative effect of the evidence on the rankings is unknown, the posets approach makes no judgment on the importance of each indicator in determination of elimination feasibility.
Creation of the final poset mean rankings involves calculation of all possible ranking combinations of the countries that satisfy these conservative associations—ie, the Dominican Republic must always be ranked as more feasible than Burundi, Somalia, and Equatorial Guinea. For instance, one possible ranking from most to least feasible is 1, Dominican Republic; 2, Ghana; 3, Burundi; 4, Equatorial Guinea; 5, Somalia. Taking a mean of all combinations produces the poset average rankings, which quantify the relative differences between countries as a single index. Finally, the posets approach enables the sensitivities of the calculated relative technical, operational, and overall feasibility rankings to the removals and additions of the different indicators to be examined and quantified by the total number of country rank changes that each produced.
Table 1.
Example of selected indices and ranking methods for five countries
| R0 | Political stability | Health-care spending | Conventional mean rank | Poset mean rank | |
|---|---|---|---|---|---|
| Dominican Republic | 0·19 (1) | 0·12 (2) | 356 (1) | 1·3 | 1·2 |
| Burundi | 16·44 (3) | −1·42 (4) | 17 (4) | 3·7 | 4·0 |
| Ghana | 59·25 (4) | 0·22 (1) | 93 (3) | 2·7 | 3·0 |
| Somalia | 5·16 (2) | −3·01 (5) | 0 (5) | 4·0 | 4·0 |
| Equatorial Guinea | 79·81 (5) | −0·16 (3) | 282 (2) | 3·3 | 4·0 |
Numbers in parentheses are ranks. See panel 3 for discussion. R0=basic reproductive number.
Mean rankings for relative technical feasibility are shown in table 2 and figure 2. Additional outputs are presented in webappendix p 30. The combination of low population-weighted R0 and low levels of movement from other countries endemic for P falciparum malaria result in Belize, Suriname, and Bolivia being ranked as more technically feasible for P falciparum elimination relative to the other P falciparum malaria-endemic countries. By contrast, because of widespread high endemic transmission intensities and substantial incoming movement from surrounding west African countries with high levels of transmission, Côte d'Ivoire, Burkina Faso, and Nigeria are the least technically feasible candidates for elimination. Figure 3 shows that our estimates for relative technical feasibility resemble current patterns of P falciparum transmission intensity,12 with more countries in the Americas and Asia showing a lower average ranking than those in sub-Saharan Africa. Exceptions to the rule are related to patterns of human movement. For example, although transmission of P falciparum in Thailand is relatively low overall, high levels of movement from surrounding countries with higher transmission increase its malaria importation rate relative to other countries. This situation reduces the estimates of overall relative technical feasibility of P falciparum elimination to an average ranking that is similar to that of islands such as Madagascar and São Tomé and Príncipe, which have higher levels of baseline transmission but much lower rates of P falciparum importation than does Thailand.
Table 2.
Mean rankings for estimated relative levels of technical, operational, and overall elimination feasibility by parasite species for malaria-endemic countries
| P falciparum/P vivax endemic |
Mean feasibility rankings |
||||
|---|---|---|---|---|---|
| P falciparum, technical | P falciparum, operational | P falciparum, overall | P vivax, operational | ||
| WHO Regional Office for Africa | |||||
| Algeria* | .. | .. | .. | .. | .. |
| Angola | Both | 59·34 | 78·46 | 76·50 | 91·20 |
| Benin | Both | 75·56 | 42·50 | 42·50 | 27·43 |
| Botswana | Both | 32·81 | 4·47 | 9·44 | 13·71 |
| Burkina Faso | Both | 83·90 | 42·50 | 42·50 | 48·00 |
| Burundi | Both | 52·70 | 28·33 | 28·33 | 72·00 |
| Cameroon | Both | 71·72 | 68·00 | 70·83 | 27·43 |
| Cape Verde* | Pf | .. | .. | .. | .. |
| Central African Republic | Both | 68·00 | 79·69 | 78·46 | 89·14 |
| Chad | Both | 50·09 | 81·60 | 80·53 | 90·00 |
| Comoros | Both | 28·33 | 42·50 | 42·50 | 64·00 |
| Congo | Both | 81·53 | 70·83 | 68·00 | 80·00 |
| Côte d'Ivoire | Both | 83·90 | 78·46 | 76·50 | 80·00 |
| Democratic Republic of the Congo | Both | 78·01 | 80·00 | 80·95 | 90·35 |
| Equatorial Guinea | Both | 68·30 | 51·00 | 42·50 | 48·00 |
| Eritrea | Both | 25·50 | 28·33 | 28·33 | 82·29 |
| Ethiopia | Both | 52·42 | 78·46 | 77·27 | 91·43 |
| Gabon | Both | 78·58 | 48·57 | 42·50 | 48·00 |
| Ghana | Both | 83·79 | 30·91 | 42·50 | 13·71 |
| Guinea | Both | 82·76 | 76·50 | 76·50 | 76·80 |
| Guinea-Bissau | Both | 47·22 | 74·38 | 72·86 | 64·00 |
| Kenya | Both | 66·11 | 42·50 | 68·00 | 48·00 |
| Liberia | Both | 82·07 | 78·46 | 76·50 | 80·00 |
| Madagascar | Both | 41·75 | 72·86 | 56·67 | 88·62 |
| Malawi | Both | 74·10 | 42·50 | 42·50 | 13·71 |
| Mali | Both | 71·45 | 74·38 | 76·50 | 80·00 |
| Mauritania | Both | 45·65 | 28·33 | 28·33 | 24·00 |
| Mozambique | Both | 62·63 | 68·00 | 68·00 | 16·00 |
| Namibia | Both | 51·56 | 28·33 | 28·33 | 17·45 |
| Niger | Both | 66·79 | 77·27 | 76·50 | 76·80 |
| Nigeria | Both | 83·88 | 42·50 | 42·50 | 26·18 |
| Rwanda | Both | 58·44 | 8·50 | 28·33 | 13·71 |
| São Tomé and Príncipe | Both | 40·65 | 5·31 | 10·63 | 4·00 |
| Senegal | Both | 67·11 | 42·50 | 42·50 | 10·67 |
| Sierra Leone | Both | 76·85 | 72·86 | 70·83 | 36·00 |
| Somalia | Both | 37·46 | 81·96 | 77·92 | 93·91 |
| South Africa | Both | 66·52 | 12·14 | 28·33 | 27·43 |
| Sudan | Both | 81·08 | 79·69 | 78·93 | 92·44 |
| Swaziland | Both | 31·06 | 7·08 | 17·00 | 11·29 |
| Tanzania | Both | 79·01 | 70·83 | 68·00 | 53·33 |
| The Gambia | Both | 58·98 | 21·25 | 28·33 | 9·60 |
| Togo | Both | 81·60 | 56·67 | 56·67 | 51·69 |
| Uganda | Both | 73·03 | 70·83 | 68·00 | 54·86 |
| Zambia | Both | 72·25 | 63·75 | 56·67 | 57·60 |
| Zimbabwe | Both | 66·69 | 42·50 | 63·75 | 64·00 |
| WHO Regional Office for the Americas | |||||
| Argentina | Pv | .. | .. | .. | 2·00 |
| Belize | Both | 2·00 | 5·00 | 5·31 | 19·20 |
| Bolivia | Both | 3·40 | 17·00 | 21·25 | 72·00 |
| Brazil | Both | 22·55 | 4·25 | 4·72 | 32·00 |
| Colombia | Both | 7·97 | 9·44 | 10·63 | 41·14 |
| Costa Rica | Pv | .. | .. | .. | 10·67 |
| Dominican Republic | Pf | 10·08 | 3·40 | 4·72 | .. |
| Ecuador | Both | 9·60 | 28·33 | 21·25 | 72·00 |
| El Salvador | Pv | .. | .. | .. | 3·31 |
| French Guiana† | Both | 18·89 | 34·00 | 42·50 | 21·33 |
| Guatemala | Both | 3·45 | 18·89 | 5·67 | 64·00 |
| Guyana | Both | 3·40 | 56·67 | 42·50 | 67·20 |
| Haiti | Pf | 12·88 | 70·83 | 42·50 | .. |
| Honduras | Both | 3·86 | 3·86 | 4·47 | 41·14 |
| Mexico | Pv | .. | .. | .. | 16·00 |
| Nicaragua | Both | 6·75 | 21·25 | 5·67 | 64·00 |
| Panama | Both | 13·19 | 10·63 | 14·17 | 32·00 |
| Paraguay | Pv | .. | .. | .. | 32·00 |
| Peru | Both | 9·11 | 24·29 | 21·25 | 64·00 |
| Suriname | Both | 1·00 | 7·08 | 5·00 | 38·40 |
| Venezuela | Both | 3·86 | 12·14 | 17·00 | 72·00 |
| WHO Regional Office for the Eastern Mediterranean | |||||
| Afghanistan | Both | 15·11 | 75·56 | 70·83 | 91·64 |
| Djibouti | Both | 14·78 | 4·05 | 5·67 | 48·00 |
| Iran | Both | 9·30 | 4·47 | 7·08 | 14·77 |
| Iraq | Pv | .. | .. | .. | 64·00 |
| Pakistan | Both | 54·34 | 72·86 | 70·83 | 90·00 |
| Saudi Arabia | Both | 34·33 | 2·93 | 21·25 | 2·09 |
| Yemen | Both | 34·30 | 81·14 | 77·27 | 93·26 |
| WHO Regional Office for Europe | |||||
| Azerbaijan | Pv | .. | .. | .. | 19·20 |
| Georgia | Pv | .. | .. | .. | 4·00 |
| Kyrgyzstan | Pv | .. | .. | .. | 5·33 |
| Tajikistan | Both | 5·31 | 28·33 | 21·25 | 64·00 |
| Turkey | Pv | .. | .. | .. | 6·86 |
| Uzbekistan | Pv | .. | .. | .. | 11·29 |
| WHO Regional Office for Southeast Asia | |||||
| Bangladesh | Both | 50·09 | 28·33 | 42·50 | 80·00 |
| Bhutan | Both | 16·40 | 28·33 | 42·50 | 64·00 |
| Burma | Both | 41·56 | 79·33 | 75·56 | 93·26 |
| India | Both | 68·26 | 42·50 | 42·50 | 64·00 |
| Indonesia | Both | 28·33 | 56·67 | 56·67 | 90·00 |
| Nepal | Both | 19·19 | 42·50 | 42·50 | 72·00 |
| North Korea | Pv | .. | .. | .. | 32·00 |
| Sri Lanka | Both | 24·52 | 14·17 | 21·25 | 19·20 |
| Thailand | Both | 37·95 | 17·00 | 21·25 | 19·20 |
| Timor-Leste | Both | 16·74 | 28·33 | 28·33 | 72·00 |
| WHO Western Pacific Region | |||||
| Cambodia | Both | 33·71 | 42·50 | 28·33 | 57·60 |
| China | Both | 7·73 | 14·17 | 21·25 | 32·00 |
| Laos | Both | 24·68 | 76·50 | 72·86 | 85·33 |
| Malaysia | Both | 41·75 | 7·73 | 17·00 | 32·00 |
| Papua New Guinea | Both | 9·30 | 77·92 | 56·67 | 91·20 |
| Philippines | Both | 38·64 | 21·25 | 21·25 | 60·00 |
| South Korea | Pv | .. | .. | .. | 6·86 |
| Solomon Islands | Both | 23·45 | 72·86 | 70·83 | 68·57 |
| Vanuatu | Both | 5·15 | 17·00 | 17·00 | 38·40 |
| Vietnam | Both | 16·40 | 10·63 | 17·00 | 27·43 |
Lowest values=most feasible. Highest values=least feasible. Plasmodium falciparum (Pf) and Plasmodium vivax (Pv) endemic classifications are defined in Guerra et al.11,43 Technical feasibility is assessed by combining data for baseline transmission with data for imported malaria. Operational feasibility is assessed by combining data for governance, data for health systems, and data for populations at risk.
Data are limited or too few cases exist for these countries, hence the discrepancies with Feachem et al.2
French Guiana is an overseas region of France and not a separate country, but is listed here since it has endemic malaria transmission.
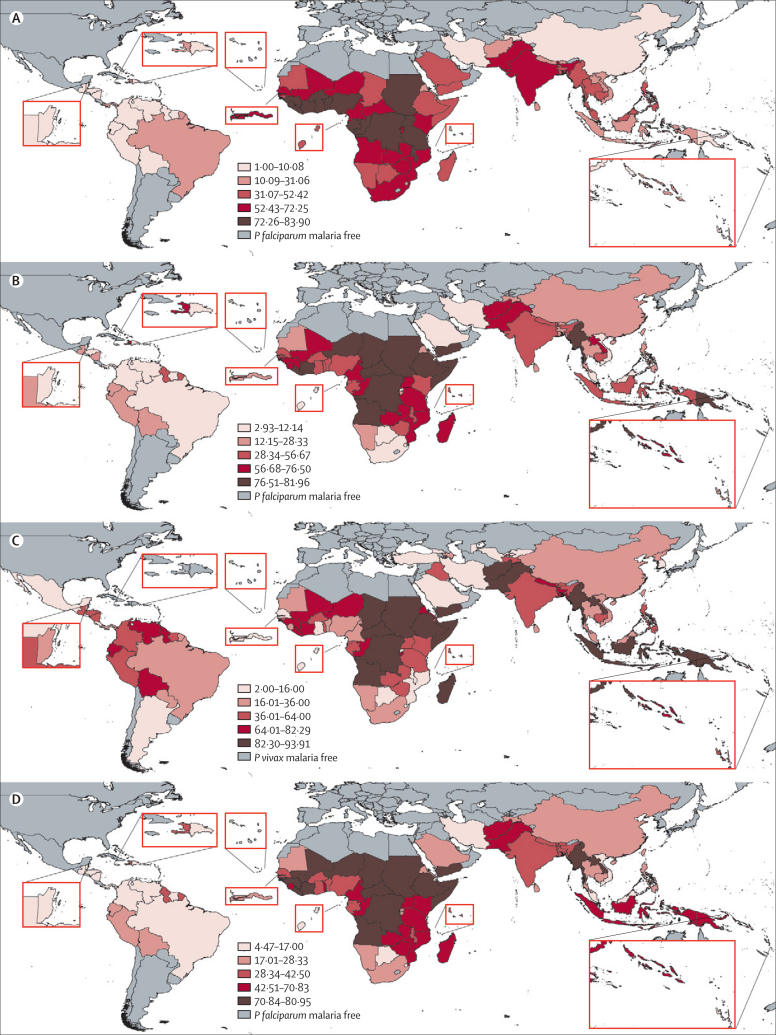
Figure 2.
Quintiles of relative elimination feasibility rankings between malaria-endemic countries
(A) Mapped quintiles of mean rankings for Plasmodium falciparum relative technical feasibility. (B) Mapped quintiles of mean rankings for P falciparum relative operational feasibility. (C) Mapped quintiles of mean rankings for Plasmodium vivax relative operational feasibility. (D) Mapped quintiles of mean rankings for P falciparum overall relative elimination feasibility. In each map, the dark red sections and largest numbers represent the lowest feasibility.
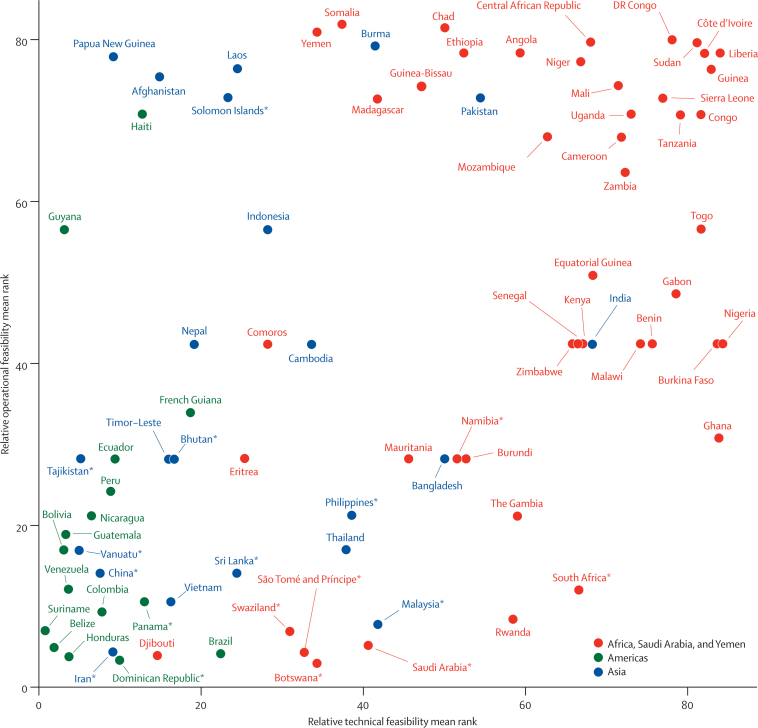
Figure 3.
A scatterplot of the mean rankings for estimated Plasmodium falciparum relative technical feasibility versus those for P falciparum relative operational feasibility
The positioning of countries on the plot highlights the ranking defined different challenges faced relative to other countries. Low mean rank values equate to countries being more feasible than others, whereas high values represent low feasibility. DR=Democratic Republic. *Country that has declared a national policy for malaria elimination or is persuing spatially progressive elimination within its borders.
Assessment of relative operational feasibility between countries
Operational feasibility of malaria elimination assesses whether the interventions needed to achieve and sustain elimination can be implemented under local financial, demographic, political, and health-system constraints.4,44 We do not consider the financial capacity of countries to mount elimination campaigns, which currently remains poorly defined in terms of needs and financing mechanisms, and is examined by Sabot and colleagues in the fourth paper in this Series.14
The 108 countries that have eliminated malaria share important characteristics (panel 1) that can be measured as a proxy of operational feasibility, and which can be grouped into factors relating to governance (panel 4), health systems (panel 5), and populations at risk (panel 6). The panels outline how each of these three factors affect the ability to eliminate malaria and how datasets representing each factor can be assembled for malaria-endemic countries at the national scale. Additional details are provided in webappendix pp 9–25. Although these datasets are often available only at the national level, within-country differences exist that might affect feasibility of elimination (eg, decentralised health systems); therefore, control strategies need to be adapted to subnational situations. Moreover, many of these datasets represent simple point-estimate surrogates for complex factors, and ideally, methods to quantify their uncertainties should be developed and integrated into any future assessments of elimination feasibility.
Panel 4. Indicators of malaria elimination feasibility—government stability, effectiveness, and commitment.
Political stability
Malaria elimination requires political stability and an absence of conflict. Examples of where armed conflict and civil unrest have hindered intervention efforts include Tajikistan, where reported cases increased from less than 200 to nearly 30 000 owing to the disruption of malaria control during the civil war (1992–97),45 and Colombia, where near-elimination was prevented by civil strife restricting access to large malarious areas.46 The World Bank Political Stability and Absence of Violence Index47 is a measure of the “perceptions of the likelihood that the government will be destabilized or overthrown by possibly unconstitutional and/or violent means, including domestic violence and terrorism”. Low scores indicate that citizens cannot count upon continuity of government policy or the ability to peacefully select and replace those in power. These data were obtained for every Plasmodium falciparum and Plasmodium vivax malaria-endemic country for 2008,48 and thus do not capture more recent changes, such as the Haitian earthquake, the decline in stability in Madagascar, post-election violence in Kenya, or the end of conflict in northern Sri Lanka.
Government effectiveness
Strong and effective organisation and infrastructure are required to achieve elimination. This infrastructure includes the capacity to implement and run a near-perfect surveillance system within a robust health system, provide effective information and education programmes, construct a legal framework adapted to the needs of an elimination agenda, and facilitate excellent interagency, community, and cross-border collaboration. These are difficult factors to measure, since the organisational and technical infrastructure set up to eliminate malaria will be constructed once a decision to eliminate the disease has been made. Moreover, government efficiency in terms of health has been shown to be related to the degree of decentralisation of the health system,49 enabling the tailoring of interventions to regions, but quantification of this association remains challenging. The World Bank Government Effectiveness Index47 is a measure of “the quality of public service provision, the quality of the bureaucracy, the competence of public servants, and the independence of the civil service from political pressures”. Undertaking malaria elimination in a country has been shown to require effective organisation and infrastructure of the highest order, and thus this index provides valuable information about the ability of a nation to achieve this level. We obtained these data for every P falciparum and P vivax malaria-endemic country for 2008.48
Health expenditure
Governmental commitment to elimination is a difficult factor to measure objectively and harder still to predict for the decades of intervention that a malaria elimination plan requires.50 Nevertheless, existing data for public health spending by both the government and the private health sector provides an indicator of how committed a nation is, both financially and politically, to the health of its citizens, and this should be a useful proxy correlate to a commitment to malaria elimination. To ensure comparability of expenditure between countries, accounting for both population size and different costs, we acquired data for total expenditure on health per head (US$) at average exchange rates for the most recent year available (2006).51
Panel 5. Indicators of malaria elimination feasibility—health systems.
A health system includes all organisations and people whose primary role it is to promote, restore, or maintain health.51 The strength of the basic health system within a country is integral to elimination of a disease. The system needs to be capable of providing near-universal access to high-quality diagnosis and treatment.44 Information about measures that are specifically relevant for malaria elimination (access to treatment, levels of decentralisation, diagnosis quality, drug supply, and health management information system quality) are generally unavailable, unreliable, or incomplete.51,52 The lack of reliable data for surveillance system efficiencies is particularly striking,53 since without effective case detection, elimination remains impossible to achieve or document. Most well reported health statistics are fairly static measures, such as physicians or hospital beds per head,51 which do not inform on the process or effect of the health system.49 As a proxy for health-system performance, we used two types of statistics that are proven to have direct effects on health outcomes, sensitive to health-system improvement or deterioration, and relatively precise, verifiable, and objective compared with other candidate statistics.
Immunisation coverage
Data for immunisation coverage can serve as an indicator of a health system's capacity to deliver essential services to the most vulnerable members of a population and has been shown to be significantly related to health-worker density.54 Immunisation is a health output with a strong effect on child morbidity, child mortality, and permanent disability.55 The usefulness of immunisation coverage is not simply as a measure of the implementation of one health intervention, but as a proxy for the overall performance of the health system in terms of supporting priority health interventions.56 Although comparability over time is sometimes limited,57 coverage statistics of vaccinations included within the Expanded Programme on Immunization (EPI) are widely reported and provide relatively precise and objective measures of performance compared with other statistics available. For every Plasmodium falciparum and Plasmodium vivax malaria-endemic country, we obtained coverage statistics for 2008 on eight immunisations from UNICEF.58 Sensitivity and other analyses were done to ascertain the effects of the range of vaccination coverage statistics on the results (webappendix pp 13–18). Ultimately, this approach resulted in the average coverage percentages for the third dose of diphtheria and tetanus toxoid with pertussis vaccine, the measles-containing vaccine, and the third dose of polio vaccine for each country being used.
Coverage of antenatal care
Antenatal care represents a second essential service to vulnerable populations and is a health output with a strong effect on maternal mortality.59 Moreover, it is a sensitive indicator which, by being measured yearly, provides timely evidence of the state of current services. Whereas EPI coverage indicates the ability of a health system to deliver services, antenatal care coverage (ANCC) provides an indicator of health-system access and use. ANCC represents the percentage of women who used antenatal care provided by skilled health personnel for reasons related to pregnancy at least once during pregnancy, as a percentage of livebirths in 2000–08. We explored the effects of choosing an alternative maternal health indicator (the percentage of births attended by skilled personnel in 2000–08) on our results, but few changes were noted (webappendix pp 18–20).
Panel 6. Indicators of malaria elimination feasibility—populations at risk.
The feasibility of elimination will depend not only on the number of people at risk, but also on what proportion of the total national population these make up, which affects the ability of the government to deal with eliminating malaria transmission in these populations. Moreover, the difficulty in accessing these populations at risk presents a further obstacle to success in achieving elimination.
Country totals and proportions at risk
Numbers of people at risk of stable Plasmodium falciparum for each country in 2007 have been estimated previously.11 Identical approaches were used to estimate numbers at risk for Plasmodium vivax.43 From these data, we calculated the proportion of the total population of each country living in areas of stable P falciparum and P vivax transmission.
Accessing populations at risk
Gridded estimates of time taken to reach the nearest substantial settlement by use of land or water-based travel are available globally60 (webappendix pp 24–25). These estimates provide a comparable basis for measuring the per-country accessibility of populations at risk of P falciparum or P vivax malaria from their nearest substantial settlement, where primary health facilities are based and from which intervention and control efforts are coordinated and launched. We calculated the mean population-weighted travel time for those living in areas of stable P falciparum and P vivax transmission to provide indicators of the relative level of difficulty that will be faced in accessing populations at risk of P falciparum and P vivax malaria in a country.
Relative operational feasibility
Rankings for relative operational feasibility suggest that Somalia, Chad, Yemen, and the Democratic Republic of the Congo, which all show mean ranks greater than 80 (relatively low feasibility), will face substantial operational difficulties in achieving P falciparum elimination compared with the other P falciparum malaria-endemic countries (table 2, figure 2, webappendix p 31). Most countries with relatively high operational feasibility of elimination are among the most economically developed in the three continental regions, such as the Dominican Republic, Saudi Arabia, Brazil, and Iran, or those with small, fairly accessible populations at risk, such as Honduras, Djibouti, and Belize. The countries with rankings indicating the highest operational feasibility come from a wide geographical extent and include countries in sub-Saharan Africa. For example, the relatively high spending on health per head and the lower absolute numbers of people at risk in Botswana, Swaziland, and Rwanda mean that, on an operational basis, these countries have advantages over many other P falciparum malaria-endemic countries in terms of feasibility of elimination.
In many Asian countries, operational feasibility of P vivax elimination is lower than that for P falciparum. Populations at risk of stable P vivax transmission in countries such as Afghanistan, Burma, and Indonesia, are substantially larger than those in most sub-Saharan African countries. Moreover, the wider extent of stable transmission of P vivax than of P falciparum in countries such as Peru, Nepal, and Bolivia means that many more people are at risk, and often in regions of poor accessibility, making elimination less feasible operationally. Many countries that are ranked as having the highest operational feasibility of P vivax elimination are those with no transmission of P falciparum, including South Korea, Georgia, Argentina, and Turkey, all of which are among the most politically stable and high-income malaria-endemic countries.
Assessment of relative overall feasibility of P falciparum elimination between countries
Our more complete understanding of the factors determining the technical feasibility of P falciparum elimination means that we can combine the full set of national indicators described to estimate overall average rankings for feasibility of elimination and examine the relative sizes of the technical and operational challenges facing each country, within the confines of the datasets used. Table 2 shows the average rankings for overall feasibility and figure 2 maps these by quintile. Additional results are shown in webappendix pp 33–34; the sensitivity scores presented provide an indication that imported malaria rates and accessibility of populations at risk are the two factors to which the rankings are most sensitive. This finding highlights that, overall, these factors most constrain the greatest number of countries from changing feasibility rank up or down.
Figure 3 shows the relative magnitude of technical and operational challenges each country must overcome to achieve elimination of P falciparum. As emphasised previously, we do not consider the financial aspects of feasibility of elimination in this report.
Discussion
Socioeconomic development and the fight against malaria has profoundly shaped the geographical distribution of the disease in the past century.12,61,62 Major international agencies and many governments are now aiming for elimination of the disease.3,63 The decision to move to an elimination agenda within a country is complex and the consequences of an ill-informed decision are serious.5,64 Examination of lessons learned from past successes and failures can provide valuable insights into feasibility of elimination. We have attempted to quantify factors shared by countries that have successfully eliminated malaria, and have used objective methods for assessing relative feasibility between countries. The global evidence base, modelling framework, and epidemiological understanding of P falciparum malaria are better than those for P vivax, and this is reflected in the completeness of analyses presented for each parasite.
The results present initial steps towards providing a quantitative and contemporary overview of the feasibility of malaria elimination. The findings are not intended to be prescriptive or predictive, but simply to show one approach to formalising the conclusions that can be drawn from the global data assembled. The motivation for this work was not to target individual nations, nor to impose upon them strategic plans, or replace more subjective strategic documents. Moreover, the rankings show an approach to deriving a theory-based summary of existing evidence, drawn together with methods designed to minimise subjectivity. Composite indicators and rankings can be generated by several approaches, including those that define variable weights on the basis of simple averages, previous studies, or expert opinion, or that create composite indicators through principal components or factor or cluster analyses,40 with each likely to produce slightly different results. Alternatives to partially ordered sets and ranking should be explored, dependent upon aims. These alternatives could include hierarchical threshold-based approaches that provide definitive answers on feasibility, or clustering approaches that define factors or country groupings for costing priorities. Furthermore, technical and operational feasibility represent core components of low stable endemic control. Thus, adaptations of composite indicator approaches based on spatial databases, such as the one used in this study, can potentially be used to consider threats posed to effective control and elimination. These methods can facilitate analysis of ranking sensitivity with incorporation of new variables, combinations of existing variables, and the handling of uncertainties in different factors. This flexibility is important because our assessment and quantification of the important factors, and the choices inherent in compiling national summary values, will not be universally endorsed. For this reason their values are given in full in webappendix pp 36–47 for wider scrutiny, together with sensitivity analyses that examine the effects of inclusion of alternative variables.
The global situation
The results of our analyses suggest that the Americas have the greatest potential for elimination of P falciparum; the top five countries in which elimination is most feasible are all American, with the rest of the region's countries ranked in the top 50% (figure 2). Moreover, with the exception of operational challenges facing Haiti and Guyana, all countries in the Americas are situated in the most favourable corner of figure 3 for elimination feasibility. Forest and forest-fringe malaria dominates in the Americas65 and elimination in many countries depends on the feasibility of P falciparum elimination in the Amazon basin. This trend was not shown during the Global Malaria Eradication Program, but the challenge is now reduced through economic development, improved health systems, accessibility and, paradoxically, deforestation. Instability and the beleaguered health system in Haiti are a substantial challenge, as is accessing isolated populations in Brazil, Guyana, and Peru. Our analysis suggests that, relative to the rest of the world, many countries in the Americas are well placed operationally to tackle P vivax, with the relatively high feasibility rankings for Argentina, El Salvador, and Mexico (figure 2) reflecting their national elimination aims (table 2). Examples of exceptions are Venezuela and Ecuador, where high proportions of the total populations reside in inaccessible areas of stable P vivax transmission.
Many countries, compared with others, still face important obstacles before elimination of malaria can be considered. Countries in the top right-hand corner of figure 3, with relatively low feasibility in figure 2, face much larger technical and operational challenges than do the remaining countries. Countries showing the lowest feasibility of P vivax elimination in figure 2 face more operational barriers for dealing with P vivax. Most of the countries facing the greatest obstacles are in west and central Africa, where health-care deficiencies and high P falciparum transmission are compounded by substantial cross-border population movements, political instability, and poor governance. In east Africa, the political instability in Somalia and the poor health care and inaccessibility of many populations in Ethiopia make elimination difficult compared with most other malaria-endemic countries. Elsewhere in sub-Saharan Africa, most countries are ranked as relatively more feasible candidates for elimination of P falciparum malaria, because they share factors with those countries that have successfully eliminated the disease—eg, low R0 values in Mauritania, the estimated low levels of imported malaria to Madagascar, accessible populations at risk in Ghana, and low numbers at risk in Burundi. These characteristics are currently counter-balanced by factors that constitute a barrier to elimination: poor access to populations at risk in Mauritania, large numbers at risk of P falciparum and P vivax in Madagascar, high baseline P falciparum transmission intensity and imported P falciparum malaria rates in Ghana, and political instability in Burundi. Within Africa, P falciparum elimination by use of existing methods seems to be more feasible for countries such as Botswana, Djibouti, and Swaziland. An important obstacle to elimination in these three countries is likely to be the influx of parasite carriers from neighbouring countries, so regional initiatives36,66 will be crucially important to the elimination prospects of individual nations.
With the exception of politically volatile countries such as Pakistan, Yemen, and Afghanistan, Asian countries are ranked as more feasible than are those of sub-Saharan Africa for all P falciparum elimination feasibility assessments and for the operational feasibility of P vivax elimination. Some countries remain outliers in figure 3, with the political situations and health systems of Yemen, Afghanistan, and Pakistan resulting in estimates of relatively low feasibility, suggesting that transition to elimination planning in the short term is more unlikely in these countries than it is for other Asian countries. P vivax is most widespread and prevalent in Asia and it is here that the need for an evidence base and transmission modelling is strongest. Figure 2 highlights that in southeast Asia, elimination of P vivax is likely to pose a greater challenge than is elimination of P falciparum. Substantial populations at risk of stable P vivax transmission in Burma, Indonesia, and Laos (many of which are in locations that are difficult to access) contribute to low operational feasibility of P vivax elimination compared with other countries. In the Middle East, a joint programme for malaria elimination in Saudi Arabia and Yemen has been initiated.67 Thus, although national-level indicators point towards poor elimination prospects for Yemen, regional plans for a malaria-free Arabian Peninsula might alter these assessments substantially. Further east, imported cases from Afghanistan and Pakistan are likely to represent the sole major obstacle to the feasibility of malaria elimination in Iran. Political instability in Afghanistan and Sri Lanka represent barriers to elimination relative to other countries. However, the recent subsidence in violence in Sri Lanka and successful transmission reduction68 are likely to further upgrade the nation's average ranking when the relevant indices are updated. Despite increasing wealth and development in India, pockets of high P falciparum transmission, widespread P vivax transmission, low investment in health, and a huge population at risk, as well as the problem of urban malaria transmission from Anopheles stephensi,69 probably make elimination less feasible in India than it is in most Asian countries. Elsewhere, China, Malaysia, Sri Lanka, the Philippines, Timor-Leste, Thailand, Cambodia, and Vietnam all fall into the top 30 countries for overall feasibility of P falciparum elimination in table 2, suggesting that elimination of this parasite might be more feasible in these countries than in most P falciparum malaria-endemic countries. All these nations face potential difficulties because of the importation of parasite carriers; the areas of highest transmission in many of these countries lie along the borders with neighbouring countries with higher rates of transmission, such as Burma and Laos. These obstacles again point to the benefits of regional initiative development,36 and the recently established Asia Pacific Malaria Elimination Network70 augers well for coordinating control and elimination operations in countries with different, but interlinked, prospects for elimination.
Improving elimination feasibility assessments
The decision to embark on elimination is multifactorial and is often not evidence based. Strategic assessments of elimination feasibility within a country or region require sophisticated analyses of information at spatial resolutions not easily summarised for global comparisons. A rigorous and structured study that covers quantitative and qualitative assessments of past and present epidemiology, human movement patterns, health-system adaptation, and financial sustainability should be undertaken.5 Moreover, advances in epidemiological theory, spatial analysis, and mathematical modelling can provide one framework for answering questions pertinent to elimination.
The abundance of spatial data now available enables data-driven decisions to provide evidence-based, species-specific assessments of elimination feasibility for policy makers. This activity was not possible at the time of the Global Malaria Eradication Program. However, there are still some substantial gaps in knowledge, data, and methods that need to be addressed before sophisticated and robust assessments of elimination feasibility can be undertaken on a global scale. First, comparable data for factors that are key to elimination feasibility are missing at a global level, and efforts should be directed to their measurement to improve the robustness of global assessments. These efforts include, as a first level of improvement, estimates of the spatial distributions of P vivax, P ovale, P malariae, and P knowlesi transmission intensities; the global distributions, densities, and bionomics of Anopheles species;71 the level of health system decentralisation by country; and financial capacities14 of malaria-endemic countries. Furthermore, information about the effect of different factors as barriers to elimination would enable increasingly informed weightings to enhance the value of assessments. There is an understanding that the political situation in a country can change and the rapid scale-up of interventions is reducing transmission levels globally. In view of these dynamics, any analyses of elimination feasibility based on such information will require regular updating as new data become available. Second, uncertainty exists in the derivation of each of these factors, and those used in this analysis, yet for most of them this uncertainty is not quantified. Estimation of these uncertainties should be a priority. Finally, as discussed previously, although we have endeavoured to use a straightforward method aimed at summarising the evidence in a non-subjective, relative, and theoretical manner, alternative approaches and methods should also be explored,40 especially those that can incorporate the rigorous handling of uncertainty and assess feasibilities at subnational levels. The reconciliation of national and global feasibility assessments with appropriate strategies for action is important for both national malaria control programmes and the international community. Improving assessments derived from a spatial evidence base should also remain a priority.
Acknowledgments
Acknowledgments
We thank Carlos Guerra, Anand Patil, Will Temperley, and Rosalind Howes for supplying P vivax-related datasets, and Nicholas Campiz for help with data processing. We also thank Kevin Baird, Marcel Tanner, Dean Jamison, Anja Bibby, and the Malaria Elimination Group for extensive comments on the manuscript. We also acknowledge the support of the Kenyan Medical Research Institute (KEMRI). This report is published with the permission of the director of KEMRI. AJT and DLS are supported by a grant from the Bill & Melinda Gates Foundation (#49446) and acknowledge (with SIH) funding support from the RAPIDD programme of the Science and Technology Directorate, Department of Homeland Security, and the Fogarty International Center, US National Institutes of Health. SIH is a Wellcome Trust Senior Research Fellow (#076951) and the grant supports PWG and CWK. RWS is a Wellcome Trust Principal Research Fellow (#079080) and acknowledges the support of the Kenyan Medical Research Institute (KEMRI). This work forms part of the output of the Malaria Atlas Project, principally funded by the Wellcome Trust, UK.
Adapted from reference 3.
R0=basic reproductive number. Rc=reproductive number under some existing level of control.
R0=basic reproductive number.
Contributors
AJT was responsible for the design of the study; gathering, collation, preparation, analysis, and interpretation of the data; and wrote the final report with SIH. CWK was responsible for gathering and checking the data and map creation. SIH and RWS conceived the work and SIH undertook the overall scientific management. DLS, RWS, PWG, and SIH were all involved in the interpretation of data and writing of the final report.
Conflicts of interest
AJT, DLS, RWS, and SIH serve as members of the Malaria Elimination Group. RWS has received funding from Novartis for chairing meetings of national control programmes in Africa and has received a research grant from Pfizer. PWG and CWK declare that they have no conflicts of interest. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of their employing organisations nor of the sources of funding.
Web Extra Material
References
- 1.Roll Back Malaria . Global Malaria Action Plan. Roll Back Malaria Partnership; Geneva: 2008. http://www.rollbackmalaria.org/gmap/ (accessed Sept 12, 2010). [Google Scholar]
- 2.Feachem RGA, Phillips AA, Hwang J. Shrinking the malaria map: progress and prospects. Lancet. 2010 doi: 10.1016/S0140-6736(10)61270-6. published online Oct 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO Global malaria control and elimination: report of a technical review. 2008. http://malaria.who.int/docs/elimination/MalariaControlEliminationMeeting.pdf (accessed Sept 12, 2010).
- 4.Feachem RGA, Phillips AA, Targett GA, editors. Shrinking the malaria map: a prospectus on malaria elimination. The Global Health Group, Global Health Sciences, University of California; San Francisco: 2009. [Google Scholar]
- 5.Pampana E. A textbook on malaria eradication. Oxford University Press; Oxford: 1969. [Google Scholar]
- 6.Prothero RM. Population movements and problems of malaria eradication in Africa. Bull World Health Organ. 1961;24:405–425. [PMC free article] [PubMed] [Google Scholar]
- 7.Schapira A. Prospects for eradication and elimination of malaria: annexe 2. Opportunities, obstacles and risks for elimination of Plasmodium falciparum malaria in different countries and regions of the world with currently existing tools. Department for International Development; London: 2007. [Google Scholar]
- 8.Smith DL, Dushoff J, Snow RW, Hay SI. The entomological innoculation rate and Plasmodium falciparum infection in African children. Nature. 2005;438:492–495. doi: 10.1038/nature04024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith DL, Hay SI. Endemicity response timelines for Plasmodium falciparum elimination. Malar J. 2009;8:87. doi: 10.1186/1475-2875-8-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith DL, McKenzie FE, Snow RW, Hay SI. Revisiting the basic reproductive number for malaria and its implications for malaria control. PLoS Biol. 2007;5:e42. doi: 10.1371/journal.pbio.0050042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guerra CA, Gikandi PW, Tatem AJ. The limits and intensity of Plasmodium falciparum transmission: implications for malaria control and elimination worldwide. PLoS Med. 2008;5:e38. doi: 10.1371/journal.pmed.0050038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hay SI, Guerra CA, Gething PW. World malaria map: Plasmodium falciparum endemicity in 2007. PLoS Med. 2009;6:e1000048. doi: 10.1371/journal.pmed.1000048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mueller I, Galinski MR, Baird JK. Key gaps in the knowledge of Plasmodium vivax, a neglected human malaria parasite. Lancet Infect Dis. 2009;9:555–566. doi: 10.1016/S1473-3099(09)70177-X. [DOI] [PubMed] [Google Scholar]
- 14.Sabot O, Cohen JM, Hsiang MS. Costs and financial feasibility of malaria elimination. Lancet. 2010 doi: 10.1016/S0140-6736(10)61355-4. published online Oct 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dietz K. The estimation of the basic reproduction number for infectious diseases. Stat Methods Med Res. 1993;2:23–41. doi: 10.1177/096228029300200103. [DOI] [PubMed] [Google Scholar]
- 16.Smith DL, McKenzie FE. Statics and dynamics of malaria infection in Anopheles mosquitoes. Malar J. 2004;3:13. doi: 10.1186/1475-2875-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Killeen GF, Smith TA. Exploring the contributions of bednets, cattle, repellents and insecticides to malaria control: a deterministic model of mosquito host-seeking behaviour and mortality. Trans R Soc Trop Med Hyg. 2007;101:867–880. doi: 10.1016/j.trstmh.2007.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Le Menach A, Takala S, McKenzie FE. An elaborated feeding cycle model for reductions in vectorial capacity of night-biting mosquitoes by insecticide-treated nets. Malar J. 2007;6:10. doi: 10.1186/1475-2875-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Macdonald G. Epidemiological basis of malaria control. Bull World Health Organ. 1956;15:613–626. [PMC free article] [PubMed] [Google Scholar]
- 20.Eastman RT, Fidock DA. Artemisinin-based combination therapies: a vital tool in efforts to eliminate malaria. Nat Rev Microbiol. 2009;7:864–874. doi: 10.1038/nrmicro2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Okell LC, Drakeley CJ, Bousema T, Whitty CJM, Ghani AC. Modelling the impact of artemisinin combination therapy and long-acting treatments on malaria transmission intensity. PLoS Med. 2008;5:e226. doi: 10.1371/journal.pmed.0050226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lawpoolsri S, Klein EY, Singhasivanon P. Optimally timing primaquine treatment to reduce Plasmodium falciparum transmission in low endemicity Thai-Myanmar border populations. Malar J. 2009;8:159. doi: 10.1186/1475-2875-8-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koella JC. On the use of mathematical models of malaria transmission. Acta Trop. 1991;49:1–25. doi: 10.1016/0001-706x(91)90026-g. [DOI] [PubMed] [Google Scholar]
- 24.Smith DL, Hay SI, Noor AM, Snow RW. Predicting changing malaria risk after expanded insecticide-treated net coverage in Africa. Trends Parasitol. 2009;25:511–516. doi: 10.1016/j.pt.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith DL, Smith TA, Hay SI. Measuring malaria for elimination. In: Feachem RGA, Phillips AA, Targett GA, editors. Shrinking the malaria map: a prospectus on malaria elimination. The Global Health Group, University of California—Santa Cruz Global Health Sciences; San Francisco: 2009. [Google Scholar]
- 26.Noor AM, Mutheu JJ, Tatem AJ, Hay SI, Snow RW. Insecticide-treated net coverage in Africa: mapping progress in 2000–07. Lancet. 2009;373:58–67. doi: 10.1016/S0140-6736(08)61596-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.WHO World malaria report. 2008. http://www.who.int/malaria/wmr2008 (accessed Sept 12, 2010).
- 28.Lysenko AJ, Semashko IN. Geography of malaria. A medico-geographic profile of an ancient disease. In: Lebedew AW, editor. Academy of Sciences USSR; Moscow: 1968. pp. 25–146.http://www.rollbackmalaria.org/docs/lysenko/lysenko.pdf (in Russian). (accessed Sept 12, 2010). [Google Scholar]
- 29.Garnham PCC. Malaria parasites of man: life-cycles and morphology (excluding ultrastructure) In: Wernsdorfer WH, McGregor I, editors. Malaria: principles and practice of malariology. Churchill Livingstone; Edinburgh: 1988. pp. 61–96. [Google Scholar]
- 30.Sattabongkot J, Tsuboi T, Zollner GE, Sirichaisinthop J, Cui L. Plasmodium vivax transmission: chances for control? Trends Parasitol. 2004;20:192–198. doi: 10.1016/j.pt.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 31.Baird JK. Neglect of Plasmodium vivax malaria. Trends Parasitol. 2007;23:533–539. doi: 10.1016/j.pt.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 32.Cosner C, Beier JC, Cantrell RS. The effects of human movement on the persistence of vector-borne diseases. J Theor Biol. 2009;258:550–560. doi: 10.1016/j.jtbi.2009.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sivagnanasundaram C. Reproduction rates of infection during the 1967–68 P vivax epidemic in Sri Lanka (Ceylon) J Trop Med Hyg. 1973;76:83–86. [PubMed] [Google Scholar]
- 34.Hammadi D, Boubidi SC, Chaib SE. Malaria in Algerian Sahara. Bull Soc Pathol Exot. 2009;102:185–192. (in French). [PubMed] [Google Scholar]
- 35.Julvez J, Mouchet J, Ragavoodoo C. Historical epidemiology of malaria in the archipelago of the Mascarenes. Ann Soc Belg Med Trop. 1990;70:249–261. (in French). [PubMed] [Google Scholar]
- 36.Tatem AJ, Smith DL. International population movements and regional Plasmodium falciparum malaria elimination strategies. Proc Natl Acad Sci USA. 2010;107:12222–12227. doi: 10.1073/pnas.1002971107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stoddard ST, Morrison AC, Vazquez-Prokopec GM. The role of human movement in the transmission of vector-borne pathogens. PLoS Neglected Trop Dis. 2009;3:e481. doi: 10.1371/journal.pntd.0000481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tatem AJ, Qiu Y, Smith DL, Sabot O, Ali AS, Moonen B. The use of mobile phone data for the estimation of the travel patterns and imported Plasmodium falciparum rates among Zanzibar residents. Malar J. 2009;8:287. doi: 10.1186/1475-2875-8-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Parsons CR, Skeldon R, Walmsley TL, Winters LA. Quantifying international migration: a database of bilateral migrant stocks. World Bank Policy Research Working Paper 2007; 4165. http://www-wds.worldbank.org/external/default/WDSContentServer/IW3P/IB/2007/03/06/000016406_20070306151900/Rendered/PDF/wps4165.pdf (accessed Sept 12, 2010).
- 40.Nardo M, Saisana M, Saltelli A, Tarantola S, Hoffman A, Giovannini E. Handbook on contructing composite indicators: methodology and user guide. OECD; Paris: 2005. [Google Scholar]
- 41.Patil AP, Taillie C. Multiple indicators, partially ordered sets, and linear extensions: Multi-criterion ranking and prioritization. Environ Ecol Stat. 2004;11:199–228. [Google Scholar]
- 42.Schröder BSW. Ordered sets: an introduction. Birkhäuser; Boston: 2003. [Google Scholar]
- 43.Guerra CA, Howes RE, Patil AP. The international limits and population at risk of Plasmodium vivax transmission in 2009. PLoS Neglected Trop Dis. 2010;4:e774. doi: 10.1371/journal.pntd.0000774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yekutiel P. Eradication of infectious diseases: a critical study. In: Klinkenburg MA, editor. Contributions to epidemiology and biostatistics. Karger; Basel: 1980. [Google Scholar]
- 45.Matthys B, Sherkanov T, Karimov SS. History of malaria control in Tajikistan and rapid malaria appraisal in an agro-ecological setting. Malar J. 2008;7:217. doi: 10.1186/1475-2875-7-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rojas W, Penaranda F, Echavarria M. Strategies for malaria control in Colombia. Parasitol Today. 1992;8:141–144. doi: 10.1016/0169-4758(92)90287-c. [DOI] [PubMed] [Google Scholar]
- 47.Kaufmann D, Kraay A, Mastruzzi M. Governance matters VII: aggregate and individual governance indicators. World Bank Policy Research Working Paper 2008; 4654. http://www-wds.worldbank.org/external/default/WDSContentServer/IW3P/IB/2008/06/24/000158349_20080624113458/Rendered/PDF/wps4654.pdf (accessed Sept 12, 2010).
- 48.World Bank . Worldwide governance indicators. The World Bank; Washington DC: 2009. http://info.worldbank.org/governance/wgi/index.asp (accessed Sept 12, 2010). [Google Scholar]
- 49.de Savigny D, Adam T. Systems thinking for health systems strengthening. World Health Organization; Geneva: 2009. [Google Scholar]
- 50.Hay SI, Smith DL, Snow RW. Measuring malaria endemicity from intense to interrupted transmission. Lancet Infect Dis. 2008;8:369–378. doi: 10.1016/S1473-3099(08)70069-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.World Health Organization . World health statistics. World Health Organization; Geneva: 2009. http://www.who.int/whosis/whostat/EN_WHS09_Full.pdf (accessed Sept 12, 2010). [Google Scholar]
- 52.Backman G, Hunt P, Khosla R. Health systems and the right to health: an assessment of 194 countries. Lancet. 2008;372:2047–2085. doi: 10.1016/S0140-6736(08)61781-X. [DOI] [PubMed] [Google Scholar]
- 53.Rudan I, Lawn J, Cousens S. Gaps in policy-relevant information on burden of disease in children: a systematic review. Lancet. 2005;365:2031–2040. doi: 10.1016/S0140-6736(05)66697-4. [DOI] [PubMed] [Google Scholar]
- 54.Anand S, Bärnighausen T. Health workers and vaccination coverage in developing countries: an econometric analysis. Lancet. 2007;369:1277–1285. doi: 10.1016/S0140-6736(07)60599-6. [DOI] [PubMed] [Google Scholar]
- 55.Ehreth J. The global value of vaccination. Vaccine. 2002;21:596–600. doi: 10.1016/s0264-410x(02)00623-0. [DOI] [PubMed] [Google Scholar]
- 56.Bos E, Batson A. Using immunization coverage rates for monitoring health sector performance: measurement and interpretation issues. World Bank; Washington DC: 2000. [Google Scholar]
- 57.Murray CJ, Shengelia B, Gupta N, Moussavi S, Tandon A, Thieren M. Validity of reported vaccination coverage in 45 countries. Lancet. 2003;362:1022–1027. doi: 10.1016/S0140-6736(03)14411-X. [DOI] [PubMed] [Google Scholar]
- 58.UNICEF . Immunization summary. UNICEF; New York, NY: 2009. http://www.who.int/whosis/whostat/EN_WHS09_Full.pdf (accessed Sept 12, 2010). [Google Scholar]
- 59.Alvarez JL, Gil R, Hernandez V, Gil A. Factors associated with maternal mortality in sub-Saharan Africa: an ecological study. BMC Public Health. 2009;9:462. doi: 10.1186/1471-2458-9-462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Uchida H, Nelson A. Accessibility model and population estimates. Background paper for the World Development Report 2009. World Bank; Washington DC: 2008. [Google Scholar]
- 61.Hay SI, Guerra CA, Tatem AJ, Noor AM, Snow RW. The global distribution and population at risk of malaria: past, present, and future. Lancet Infect Dis. 2004;4:327–336. doi: 10.1016/S1473-3099(04)01043-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gething PW, Smith DL, Patil AP, Tatem AJ, Snow RW, Hay SI. Climate change and the global malaria recession. Nature. 2010;465:342–346. doi: 10.1038/nature09098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Feachem R, Sabot O. A new global malaria eradication strategy. Lancet. 2008;371:1633–1635. doi: 10.1016/S0140-6736(08)60424-9. [DOI] [PubMed] [Google Scholar]
- 64.Yekutiel P. Problems of epidemiology in malaria eradication. Bull World Health Organ. 1960;22:669–683. [PMC free article] [PubMed] [Google Scholar]
- 65.de Castro MC, Monte-Mor RL, Sawyer DO, Singer BH. Malaria risk on the Amazon frontier. Proc Natl Acad Sci USA. 2006;103:2452–2457. doi: 10.1073/pnas.0510576103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ministry of Health and Social Services. Republic of Namibia Eight countries launch cross-border effort to eliminate malaria. 2009. http://www.globalhealthsciences.ucsf.edu/pdf/e8_press_release_mar0309.pdf (accessed Sept 12, 2010).
- 67.Meleigy M. Arabian Peninsula states launch plan to eradicate malaria. BMJ. 2007;334:117. doi: 10.1136/bmj.39097.499641.4E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rajakaruna RS, Alifrangis M, Amerasinghe PH, Konradsen F. Pre-elimination stage of malaria in Sri Lanka: assessing the level of hidden parasites in the population. Malar J. 2010;9:25. doi: 10.1186/1475-2875-9-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Akhtar R, Dutt AK, Wadhwa V. Malaria resurgence in urban India: lessons from health planning strategies. In: Akhtar R, Dutt AK, Wadhwa V, editors. Malaria in south Asia: eradication and resurgence during the second half of the twentieth century. Springer; Netherlands: 2010. pp. 141–155. [Google Scholar]
- 70.University of California. San Francisco Asia Pacific Malaria Elimination Network (APMEN) 2009. http://www.globalhealthsciences.ucsf.edu/GHG/apmen/index.aspx (accessed Sept 12, 2010).
- 71.Hay SI, Sinka ME, Okara RM. Developing global maps of the dominant Anopheles vectors of human malaria. PLoS Med. 2010;7:e1000209. doi: 10.1371/journal.pmed.1000209. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.