Abstract
Human T-lymphotropic virus type 1 (HTLV-1) p12I localizes to the endoplasmic reticulum and Golgi causing sustained release of calcium, T cell activation, and enhanced expression of several calcium-regulated genes. In recent microarray studies, p300 mRNA was increased in T cells expressing p12I. The co-activator p300 is a key regulator of cellular and viral transcription; however, factors that influence its transcriptional regulation are less well studied. We hypothesized that the transcription of p300 is calcium dependent and that sustained low magnitude increases in intracellular calcium may enhance the transcription of p300. Herein, we report enhanced expression of p300 in T cells by p12I in a calcium-dependent, but calcineurin-independent manner. Sustained low magnitude calcium release induced by ionomycin in T cells was sufficient to increased mRNA and protein levels of p300 resulting in enhanced transcription from a p300-dependent promoter. Promoter analysis of the p300 gene was used to predict calcium-responsive transcription factor binding sites. Using mutant forms of p12I, we demonstrate that ER localization of the viral protein is required to increase p300. In addition, p12I reversed the repression of HTLV-1 LTR-driven transcription by HTLV-1 p30II, a p300-binding protein. HTLV-1 p12I-mediated enhancement of p300 expression represents a novel mechanism of regulation of cellular gene expression by viral proteins. By targeting a ubiquitous second messenger such as calcium, HTLV-1 p12I may regulate the expression of the cellular transcriptional co-activator p300 to modulate viral gene expression and promote lymphocyte survival.
Keywords: Calcium, p300, Retrovirus, HTLV-1, Accessory protein, Transcription
Introduction
p300 is an important regulator of the transcription apparatus in response to a variety of cell signaling pathways that determines cell proliferation, differentiation, and apoptosis. As a transcriptional co-activator, p300 interacts with various cellular and viral promoter elements by bridging together transcription factors or modifying, through histone acetyltransferase activity, the structure of nucleosomal histones (Chan and La Thangue, 2001). A large number of sequence-specific, DNA-binding factors form complexes with p300, including nuclear steroid receptors, c-Jun, Fos, p53, Sap1, Stat1 and Stat2, MyoD, Ets-1, NFκB, HIF1, GATA 1, cMyb, and Smad proteins (Blobel, 2002; Iyer et al., 2004). In addition, p300 interacts with TBP, TFIIB, TFIID, RNA helicase A, CREB, MAP kinase p90rsk, and RNA polymerase II (reviewed in Janknecht, 2002; Goodman and Smolik, 2000; Vo and Goodman, 2001). Several viral proteins also interact with p300, including HTLV-1 p30II and Tax, adenovirus E1A, HIV-1 Vpr and Tat, Kaposi’s sarcoma-associated herpes virus (KSHV) viral interferon regulatory factor protein (vIRF), simian virus 40 large T antigen, HPV E6 and E7, small delta antigen of hepatitis delta virus, Epstein–Barr virus (EBV) nuclear antigen 3C (EBNA3C) and EBNA2, and herpes simplex virion protein-16 (VP16) (Ludlow and Skuse, 1995; Marzio and Giacca, 1999; Goodman and Smolik, 2000; Ali and Decaprio, 2001; Kashanchi and Brady, 2005; Zhang et al., 2001).
p300 mediates the activities of various transcription factors; however, its availability in the cell is limited (Petrij et al., 1995). A variety of cellular proteins are known to compete with each other for binding to p300 (Colgin and Nyborg, 1998). This environment of competition between transcription factors for co-activator binding provides an additional layer of tightly regulated gene expression. Competition between viral and cellular proteins for binding p300 has been reported for HIV, adenovirus, and SV40 (Hottiger et al., 1998; Yang et al., 1996). The activities of p300 are regulated through competitive protein–protein interactions or post-translational modifications such as phosphorylation and acetylation (Chan and La Thangue, 2001; Giordano and Avantaggiati, 1999). However, the transcriptional regulation of p300 remains to be elucidated.
A doubly spliced mRNA within the most distal 3′ end of the HTLV-1 genome encodes both positive regulators of viral gene expression, the Rex and Tax proteins from ORFs III and IV, respectively (reviewed in Younis and Green, 2005; Grassmann et al., 2005; Kashanchi and Brady, 2005). In HTLV-1, both the singly and doubly spliced ORF I mRNAs encode a protein of 12 kDa (p12I) (Ciminale et al., 1992; Koralnik et al., 1993). A unique doubly spliced mRNA from ORF II encodes p30II (Ciminale et al., 1992), whereas a singly spliced mRNA within the same ORF II encodes p13II (Berneman et al., 1992; Koralnik et al., 1993). Nucleotide sequence alleles of p12I including those that would result in specific residue changes associated with protein stability (lysine versus arginine at position aa 88) or truncation mutants have been reported in a minority (<7%) of HTLV-1-infected subjects with HAM/TSP, ATL, and asymptomatic patients (Martins et al., 2002; Furukawa et al., 2004; Iniguez et al., 2006). The influence of these HTLV-1 variants in viral transmission or disease outcomes is unclear. We have reported that ablation of the acceptor splice site for the singly and doubly spliced ORF I mRNA is associated with a reduction of viral infectivity in vivo (Collins et al., 1998) that can be modeled when resting T cells are used as targets in vitro (Albrecht et al., 2000).
p12I localizes to the endoplasmic reticulum and Golgi, where it associates with two resident calcium-binding proteins, calreticulin and calnexin (Ding et al., 2001) causing sustained release of calcium (Ding et al., 2002), leading to activation of nuclear factor of activated T cell (NFAT)-mediated transcription (Albrecht et al., 2002). p12I also binds the calcium/calmodulin-dependent serine/threonine phosphatase, calcineurin (Kim et al., 2003). We have recently reported that p12I expression in Jurkat T cells alters the expression of several calcium-regulated genes (Nair et al., 2005). A surprising finding in this report was the observation that p300 mRNA was increased in Jurkat and primary human T cells during p12I expression (Nair et al.,2005). Based on this, we hypothesized that the transcription of p300 is calcium dependent and that sustained low magnitude increase in intracellular calcium concentration would enhance the transcription of p300.
Herein, we report that HTLV-1 p12I increases the expression of p300 mRNA and protein in T cells. The enhanced p300 expression was calcium dependent, but calcineurin independent. We demonstrate that ionomycin, a well-characterized calcium ionophore that triggers calcium release in Jurkat T cells, causes increased mRNA and protein levels of p300 sufficient to enhanced transcription from p300-dependent reporter genes. Furthermore, this p300-dependent transcriptional activity could be blocked by BAPTA-AM, a known calcium chelator, whereas cyclosporine A, a calcineurin inhibitor, did not have any effect on the p300-dependent transcriptional activity. In addition, using an ER-localization-deficient mutant of HTLV-1 p12I, we demonstrate that ER localization of p12I is required for its ability to increase p300. In a dose-dependent manner, the expression of p12I reversed HTLV-1 p30II-mediated repression of HTLV-1 LTR-driven transcription. As p30II is a p300-binding protein, these data illustrate that HTLV-1 accessory proteins may act together to balance viral expression. Collectively, HTLV-1 p12I appears to regulate the expression of the key cellular transcriptional co-activator p300 through the ubiquitous second messenger calcium to modulate viral gene expression and promote long-term T lymphocyte survival.
Results
HTLV-1 p12I enhances expression of p300
We have reported that the expression of p12I in Jurkat T cells and primary CD4 T lymphocytes is associated with enhanced expression of p300 mRNA and increased p300-mediated transcription in Jurkat T cells (Nair et al., 2005). To test whether expressing p12I in T cells results in increased p300 itself, we performed western immunoblot assays from Jurkat T cells spin infected with recombinant lentiviral vectors. Our data indicated that Jurkat T cells expressing p12I had ~2.1-fold higher protein levels of p300 compared to mock vector-infected control Jurkat T cells (Figs. 1A and B).
Fig. 1.
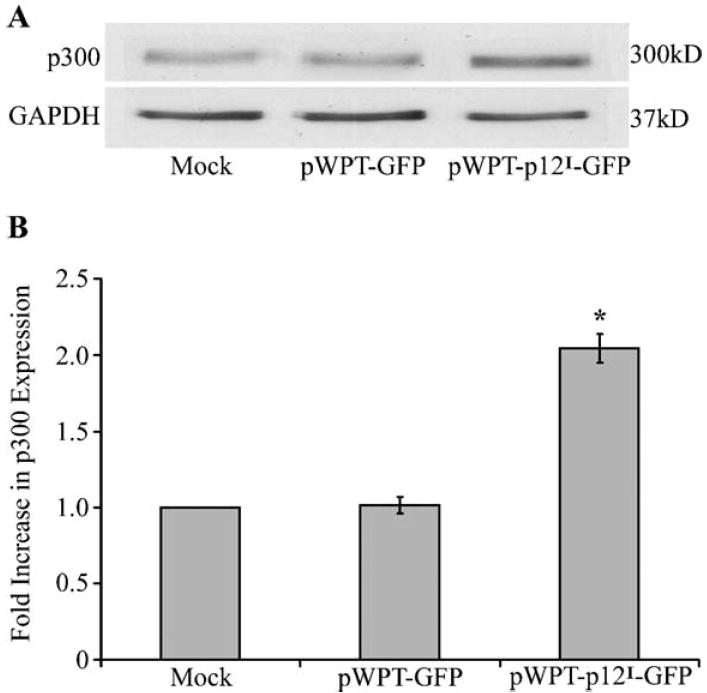
HTLV-1 p12I enhances p300 protein levels in Jurkat T lymphocytes. (A) Jurkat T lymphocytes (2 × 106) were spin infected with lentiviral vectors expressing p12I at an MOI of 5. Total protein was extracted and western immunoblot analysis for the detection of p300 was performed. Jurkat T cells expressing p12I demonstrated increased protein levels of p300. (B) Densitometric analysis of radiograph was performed using Gel pro analyzer software and normalized to GapDH. Jurkat T cells expressing p12I showed ~2.1-fold increased expression of p300. Statistical analysis was performed using Student’s t test, P < 0.05.
Sustained low magnitude increase in intracellular calcium enhances the levels of p300 by increasing its transcription
Previous reports from our laboratory demonstrated that HTLV-1 p12I increases cytosolic calcium by enhancing release from ER stores (Ding et al., 2002). Increased intracellular calcium levels have been extensively studied using calcium ionophores, such as ionomycin (Liu and Hermann, 1978). Ionomycin is routinely used for investigating calcium-mediated T cell activation and proliferation. At high concentrations (0.5–2.0 μM), typically used to study proliferative responses of cells to calcium, ionomycin mediates passive calcium influx by directly inserting into the plasma membrane (Donnadieu et al., 1995). However, at 10- to 100-fold lower concentrations (50–100 nM), ionomycin binds cellular endomembranes and increases cytosolic calcium by depletion of intracellular calcium stores and capacitative calcium entry (Donnadieu et al., 1995; Putney, 1990). Thus, stimulation of T cells with 50–100 nM of ionomycin causes calcium influx similar to HTLV-1 p12I (Ding et al., 2001).
To test if low magnitude increase in intracellular calcium mediated through ionomycin enhances the expression of p300, we performed semi-quantitative RT-PCR on total RNA extracted from Jurkat T cells stimulated with ionomycin. The cells were stimulated with 25, 50, 100, 500 nM, and 1 μM of ionomycin for 36 h before RNA extraction. Our data indicated that p300 expression was increased in a dose-dependent manner up to 100 nM of ionomycin in Jurkat T cells, 1.5, 1.8, and 2.4, respectively (Figs. 2A and B). p300 mRNA were not significantly altered at higher concentrations of ionomycin (500 nM and 1 μM) due to reduced cell viability at these higher concentrations, a known property of high dose ionomycin treatments.
Fig. 2.
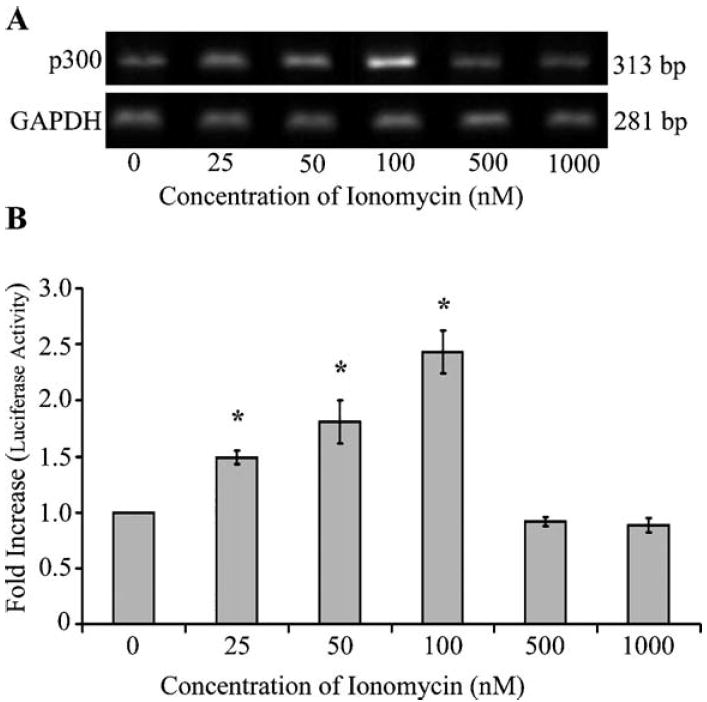
Sustained low magnitude increase in intracellular calcium concentration enhances transcription of p300. (A) Jurkat T cells (1 × 106 per ml) were stimulated with varying concentrations of ionomycin for 36 h. Total cellular RNA was extracted and semi-quantitative RT-PCR was performed to identify the mRNA levels of p300. There was a dose-dependent increase in p300 expression in Jurkat T cells stimulated with low concentrations of ionomycin (25, 50, and 100 nM). (B) Densitometric analysis was performed using alpha imager software and normalized to GAPDH. Dose-dependent increases (up to 2.4-fold) in p300 mRNA were observed in Jurkat T cells stimulated with 100 nM ionomycin. Statistical analysis was performed using Student’s t test, P < 0.05.
To investigate if increased levels of p300 mRNA correlated with a corresponding increase in protein levels of p300, we performed western immunoblot assays using Jurkat T cells stimulated with these same concentrations of ionomycin. Our data indicated a dose-dependent increase in p300 in Jurkat T cells stimulated with these lower concentrations of ionomycin. The fold increase in p300 protein levels in cells treated with 25, 50, and 100 nM of ionomycin were 1.2, 1.6, and 2.4, respectively (Figs. 3A and B). Densitometry values were normalized to constitutively expressed GAPDH in both RT-PCR and western immunoblot assays.
Fig. 3.
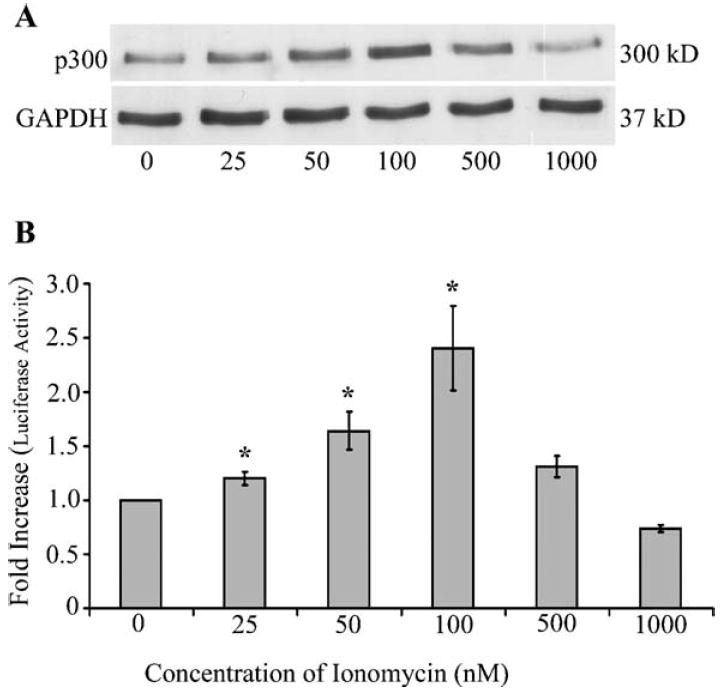
Enhanced mRNA levels of p300 correlates with increased protein levels of p300. (A) Jurkat T cells (1 × 106 per ml) were stimulated with varying concentrations of ionomycin for 60 h. Total protein was extracted and western immunoblot analysis for the detection of p300 protein. There was a dose-dependent increase in p300 expression in Jurkat T cells stimulated with low concentrations of ionomycin (25, 50, and 100 nM). (B) Densitometric analysis of radiograph was performed using Gel pro analyzer software and normalized to GAPDH. Dose-dependent increases (up to 2.5-fold) of p300 were observed in Jurkat T cells stimulated with 100 nM ionomycin. The protein levels were in parallel with mRNA levels observed at similar concentrations of ionomycin. Statistical analysis was performed using Student’s t test, P < 0.05.
Low dose ionomycin enhances p300-mediated transcription
To investigate whether the increased levels of p300 observed during low dose ionomycin treatment resulted in enhanced transcription from p300-dependent promoters, we tested the effect of low dose ionomycin in a VP16-mediated transactivation assay (Nair et al., 2005). Our data indicated that low dose ionomycin elicited a dose-dependent increase in VP16-mediated transactivation (Fig. 4A). Approximately 3.3- to 4.4-fold higher luciferase values were observed at 50 and 100 nM concentrations of ionomycin (Fig. 4A). To further confirm that this increased luciferase activity was specific to increased levels of p300, we co-transfected and adenoviral E1A expressing plasmid, which completely inhibits p300 transcription by directly binding to the transcriptional co-adaptor. Plasmids expressing wild-type E1A or mutants of E1A, including ΔCR1 that contains a mutation in the p300-binding region and ΔCR2 containing a mutation in the retinoblastoma-binding region were transfected with the luciferase reporter plasmid and pM-VP16. Wild-type E1A and the ΔCR2 mutant of E1A inhibited the transactivation of VP16 in a dose-dependent manner (Fig. 4B). Our data indicated up to 82.3% reduction in luciferase activity with wild-type E1A and approximately 70.0% reduction with the ΔCR2 mutant of E1A (Fig. 4B). The ΔCR1 mutant, which is unable to bind p300, did not have any effect on luciferase activity, indicating the p300-dependent nature of VP16-mediated transactivation (Fig. 4B). These findings indicate that low magnitude increases in intracellular calcium enhance p300 expression to functionally significant levels.
Fig. 4.
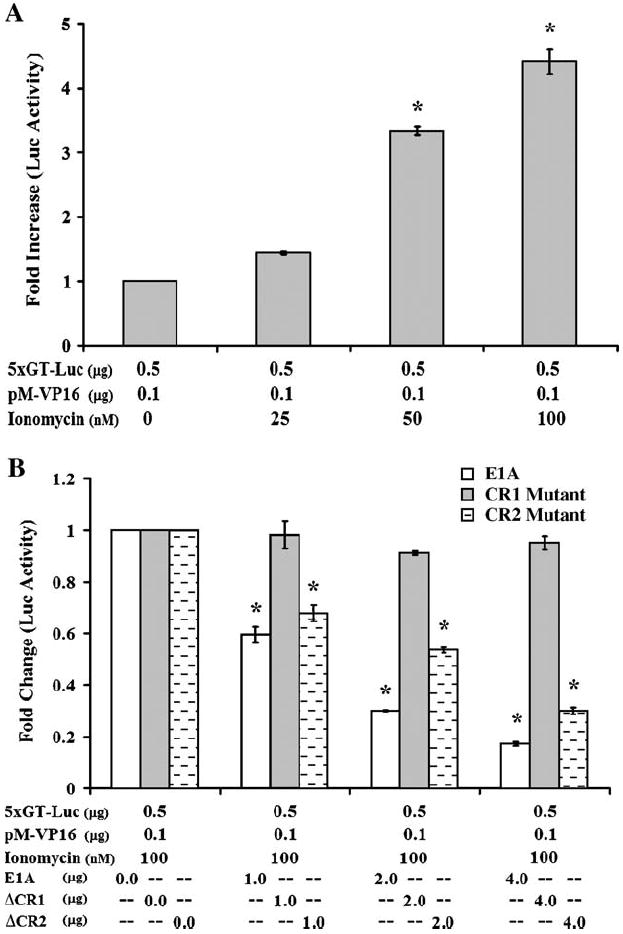
Ionomycin-mediated increase in p300 influences transcription. (A) 2 × 106 Jurkat T cells were transfected with 5xGT-Luc and pM-VP16. The transfected cells were maintained in cRPMI medium containing increasing concentrations of ionomycin. The luciferase activity was tested 60 h post-transfection. A dose-dependent increase in VP16-mediated p300-dependent luciferase activity was observed with increasing concentrations of ionomycin. (B) The increase in VP16-mediated luciferase activity was confirmed to be p300 dependent by transfecting wild-type and mutants of adenoviral E1A protein. Two million Jurkat T cells were transfected with 5xGT-Luc, pM-VP16, and increasing concentrations of wild-type or mutants of adenoviral E1A protein. The transfected cells were incubated in cRPMI medium supplemented with 100 nM ionomycin. ΔCR1 mutant of E1A is incapable of binding p300 whereas ΔCR2 mutant is capable of binding p300 but not retinoblastoma protein. VP16-mediated p300-dependent luciferase activity was inhibited in a dose-dependent fashion in the presence of wild-type as well as ΔCR2 mutant of E1A. No effect on luciferase activity occurred in the presence of ΔCR1 mutant of E1A.
Enhanced expression of p300 is calcium dependent, but calcineurin independent
We then confirmed that p12I enhancement of p300-dependent VP16-driven transactivation was mediated by calcium by transfecting our p12I expression plasmid into Jurkat T cells in the presence of the calcium chelator BAPTA-AM (Fig. 5). In addition, we also stimulated Jurkat T cells with ionomycin with increasing concentrations of BAPTA-AM. p300-dependent VP16-mediated transactivation was inhibited in the presence of BAPTA-AM in a dose-dependent manner in both p12I-transfected and ionomycin-stimulated Jurkat T cells. We observed a 55% reduction in luciferase activity with 10 μM BAPTA-AM in Jurkat T cells expressing p12I and 64% reduction in luciferase activity in Jurkat T cells stimulated with 100 nM ionomycin, respectively (Figs. 5A and B). We then blocked the calcium-dependent phosphatase calcineurin by using cyclosporin A. Calcineurin is the key enzyme required for NFAT translocation to the nucleus. Both p12I-expressing and ionomycin-stimulated Jurkat T cells exhibited no significant difference in luciferase activity with increasing concentrations cyclosporin A up to 200 nm (Figs. 5A and B). BAPTA-AM alone did not have any significant effect on luciferase expression without the presence of calcium. These data were consistent with our previous data and indicated that the expression of p300 is dependent on calcium, but not calcineurin.
Fig. 5.
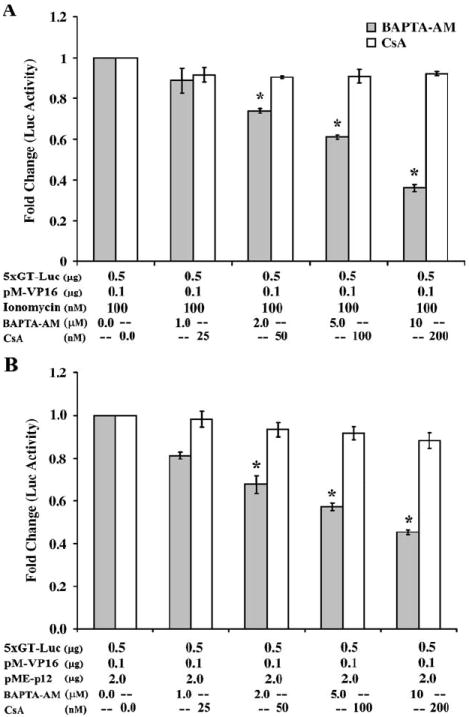
Enhanced expression of p300 is calcium dependent, but calcineurin independent. (A) Jurkat T cells (2 × 106) were transfected with 5xGT-Luc and pM-VP16 and incubated in cRPMI medium supplemented with 100 nM ionomycin. The cells were treated with increasing concentrations of BAPTA-AM or CsA. A dose-dependent reduction in VP16-mediated p300-dependent luciferase activity was noticed in the presence of BAPTA-AM whereas no significant difference in luciferase activity was observed in the presence of CsA. (B) Jurkat T cells (2 × 106) were transfected with 5xGT-Luc, pM-VP16, and pME-p12I and incubated in cRPMI medium. The cells were treated with increasing concentrations of BAPTA-AM or CsA. A dose-dependent reduction in VP16-mediated p300-dependent luciferase activity was evident in the presence of BAPTA-AM, whereas no significant difference in luciferase activity was observed in the presence of CsA.
The mechanisms of transcriptional regulation of p300, including transcription factors that cooperatively bind the p300 promoter, have not been defined. To gain initial insight into predicted transcription factor binding sites in the p300 promoter, we located the mRNA nucleotide sequence for p300 (NM_001429) using a Web-based resource (NCBI nucleotide database). Then proceeding from transcriptional start site of the p300 mRNA to the beginning of the mRNA message, we analyzed 403 nucleotide sequences to predict transcription factor binding sites in the p300 promoter. Nucleotide sequences for consensus or putative binding sites for a number of transcription factor family members are represented in the promoter region of p300. These include transcription factors that are known to respond to calcium signaling such as NF-κB and NFAT (Im and Rao, 2004; Quintana et al., 2005) (Table 1).
Table 1.
Selected putative transcription factor sites in p300 promotera
| Transcription factor | Nucleotide position | Matrix simulation score | Sequence |
|---|---|---|---|
| Ets—family member ELF-2 (NERF1a) | 10–26 | 0.910 | cgaggaGGAAgaggttg |
| Human zinc finger protein ZNF35 | 12–24 | 0.963 | aggaggAAGAggt |
| b zip family, induced by ER damage binds in association with NF-Y | 67–81 | 0.945 | ccgCCACggccggcc |
| STAT6: signal transducer and activator of transcription 6 | 107–125 | 0.963 | gcgaaTTCCcgagaactcg |
| Ikaros 1, potential regulator of lymphocyte differentiation | 112–124 | 0.934 | tctcGGGAattcg |
| HMGI (Y) high-mobility group protein I (Y) factor | 113–125 | 0.953 | gcgAATTcccgag |
| PAX6 paired domain and homeodomain | 172–190 | 0.900 | ggcttgggcCCAGgcccgg |
| Myc-associated zinc finger protein (MAZ) | 188–200 | 0.910 | gtgcGAGGggccg |
| Gut-enriched Krueppel-like factor | 203–217 | 0.924 | agaaaaggtaAGGGc |
| c-Rel | 264–278 | 0.914 | aaaggaacTTCCccc |
| NF-kappaB | 264–278 | 0.964 | ggGGGAagttccttt |
| Zinc finger transcription factor ZBP-89 | 270–292 | 0.971 | acttcccccaCCCCctcgggtgc |
| Erythroid Krueppel like factor (EKLF) | 277–289 | 0.915 | cccgaggGGGTgg |
| Myeloid zinc finger protein MZF1 | 327–333 | 0.985 | gcGGGGa |
| Nuclear factor of activated T cells | 368–378 | 0.975 | cgagGAAAacc |
| Brn-2, POU-III protein class | 391–403 | 0.946 | cggccattttTAATtcttt |
mRNA nucleotide sequence for p300 (NM_001429) obtained from NCBI Web-based resource (http://www.ncbi.nlm.nih.gov/). Preceding from the predicted start site of p300 mRNA, 403 nucleotide sequences were analyzed using commercially available software (Genomatix: http://www.genomatix.de/) to analyze and predict transcription factor binding sites in the promoter region in the p300 gene (chromosome 22, contig-NT_01150.09, Homo sapiens chromosome 22). Base pairs in capital letters denote the core sequence used by MatInspector (Genomatix). Matrix simulation scores with great than 0.90 were selected from 66 total predicted transcription factor binding sites, based from a 1.00 core simulation score of known nucleotide sequence binding sites.
Localization of p12I to ER is required for enhanced expression of p300
Previous studies from our laboratory demonstrated that ER localization of HTLV-1 p12I is critical for the release of calcium its enhancement of NFAT-mediated transcription (Ding et al., 2003). We therefore tested whether ER localization of p12I is required for its ability to enhance the expression of p300. An N- and C-terminal deletion mutant of p12I containing amino acids 15–47 localizes to the nucleus and the ER localization of this mutant requires the addition of an ER targeting signal KKLL (Ding et al., 2003). This mutant allowed us the opportunity to test if ER localization of p12I is required to enhance p300-dependent transactivation by transfecting Jurkat T cells with equal amounts of either the empty vector, wild-type p12I, 15–47 mutant of p12I, or the ER redirected 15-47-KKLL chimeric mutant along with the luciferase reporter and Gal4-VP16 expression plasmids. A marked reduction in the luciferase activity, up to 70%, was observed in Jurkat T cells expressing the 15–47 mutant of p12I, suggesting that ER localization of p12I is critical for its ability to enhance the expression of p300 (Fig. 6). This tenet was further supported by the ability of the ER-targeted 15-47-KKLL mutant of p12I to partially (80% of the wild-type levels) restore the luciferase activity (Fig. 6).
Fig. 6.

Accumulation of p12I to ER is required for enhanced expression of p300. Jurkat T cells (2 × 106) were transfected with 5xGT-Luc, pM-VP16, along with either of the following plasmids—pME control vector, pME-p12I, pME-15-47, or pME-15-47KKLL, and then incubated in cRPMI medium. pME-15-47, which accumulates in the nucleus of transfected cells (Ding et al., 2002), exhibits a marked reduction in the VP16-mediated p300-dependent luciferase activity. The activity was partially restored by redirecting the protein to the ER.
HTLV-1 p12I inhibits the transcriptional repression of p30II on HTLV-1 LTR
p300 plays a crucial role in the regulation of viral gene transcription from HTLV-1 LTR in infected cells by forming complexes with other transcriptional factors (Lemasson et al., 2002). HTLV-1 Tax transactivates LTR-driven transcription of HTLV-1 genes, in part, through its interaction with the p300 (Bex and Gaynor, 1998). In addition, studies from our laboratory have demonstrated that an accessory protein of HTLV-1, p30II, inhibits transcription of viral genes from the HTLV-1 LTR (Zhang et al., 2001). This inhibition involves the interaction of p30II with p300 and making it unavailable for transcriptional co-activation at the viral LTR (Zhang et al., 2001). By transiently transfecting increasing concentrations of pCMV-p300 with constant amounts of pME-p30IIHA plasmid, studies from our laboratory demonstrated that p300 expression reverses the p30II-dependent repression of LTR-luciferase reporter gene activity (Michael et al., submitted for publication).
Because p12I has the ability to increase the levels of p300, we hypothesized that p12I expression would reverse the inhibitory effect of p30II on HTLV-1 LTR-driven transcription. To test our hypothesis, we transfected increasing amounts of our pME-p12IHA plasmid concurrently with a constant amount of pME-p30IIHA plasmid in the presence of an HTLV-1 LTR-responsive reporter gene. Our data indicated that p12I expression reverses the p30II-dependent repression of LTR-luciferase reporter gene activity in a dose-dependent manner (Fig. 7). We did not observe alterations in the cellular localization of p30II in the presence of p12I (data not shown). To test if the p12I block of p30II repression of LTR reporter gene activity was p300 dependent, we co-transfected an adenoviral E1A expression plasmid along with pME-p12IHA and pME-p30IIHA plasmids and demonstrated that the expression of E1A blocked the effect of p12I on p30II-mediated LTR repression (Fig. 7). These data further confirmed that HTLV-1 p12I inhibits the transcriptional repression of p30II on the HTLV-1 LTR in a p300-dependent manner and establishes that HTLV-1 accessory proteins have the capacity to act cooperatively to modulate viral gene expression.
Fig. 7.

HTLV-1 p12I partially inhibits the transcriptional repression of p30II on HTLV-1 LTR. (A) Jurkat T cells (2 × 106) were transfected with pTRE-Luc, pME-p30II and increasing concentrations of pME-p12I and then incubated in cRPMI medium. A dose-dependent increase in luciferase activity was observed with increasing concentrations of p12I. (B) To confirm that the increased luciferase activity from pTRE-Luc was dependent on increased levels of p300, we transfected adenoviral E1A protein with the above-described plasmids. These data indicated a marked reduction in luciferase activity in the presence of E1A.
Discussion
HTLV-1, a human deltaretrovirus associated with lymphoproliferative disorders, encodes nonstructural or “accessory” proteins that modulate viral and cellular gene expression by interaction with p300 or through calcium-dependent mechanisms (Michael et al., 2004; Albrecht and Lairmore, 2002). One such protein encoded in pX ORF I of HTLV-1 is p12I, which localizes to the endoplasmic reticulum and Golgi, where it associates with two resident calcium-binding proteins, calreticulin and calnexin (Ding et al., 2001), causing sustained release of calcium (Ding et al., 2002) leading to activation of nuclear factor of activated T cell (NFAT)-mediated transcription (Albrecht et al., 2002). We have reported recently that p12I expression in Jurkat T cells alters the expression of several calcium-regulated genes (Nair et al., 2005). Unexpectedly, we observed that p300 mRNA was increased in T cells during p12I expression (Nair et al., 2005). We therefore hypothesized that the transcription of p300 is calcium dependent and that sustained low magnitude increase in intracellular calcium concentration would enhance the transcription of p300. Our data presented herein is the first to demonstrate that p300, an important co-activator of transcription, is responsive to sustained low magnitude increases in intracellular calcium. Because p300 is limited in cells, the expression of p300 in long-lived lymphocytes, such as memory T cells, would be particularly important for their survival. Interestingly, HTLV-1 has been demonstrated to target memory T cells (Yoshie et al., 2002; Lal et al., 1992). We demonstrate that ER localization of p12I is required for its ability to increase p300. In addition, our data indicate that p12I reverses the repression of HTLV-1 LTR-driven transcription by HTLV-1 p30II. HTLV-1 p12I-mediated enhancement of p300 expression represents a novel mechanism of regulation of cellular gene expression by viral proteins. By increasing calcium-mediated signaling HTLV-1 p12I enhances the expression of p300, which would modulate viral gene expression and favor cell survival. Collectively, our data provide new insight into HTLV-1-mediated gene expression and calcium signaling as a mechanism of transcriptional regulation of p300.
Calcium is a universal and highly versatile intracellular messenger, which plays a critical role in many varied biological processes such as cell proliferation, cell cycle, transcription, signal transduction, and apoptosis (Lewis, 2001). Calcium fluxes in mammalian cells are achieved by altering the amplitude and spatial distribution of intracellular calcium (Berridge et al., 1999, 2000; Bootman et al., 2001). Calcium mobilization in lymphocytes is accomplished by binding of inositol 1,4,5-trisphosphate (IP3) to its receptor in the ER membrane and subsequent rapid but transient release of Ca2+ from ER stores (Berridge, 1993). Alternatively, sustained extracellular Ca2+ influx across the plasma membrane, by store-operated or capacitative Ca2+ entry, is activated by depletion of intracellular Ca2+ stores and operated through store-operated Ca2+ channels (SOC) or calcium release-activated Ca2+ channels (CRAC) (Clapham, 1995; Parekh and Penner, 1997; Putney, 1990).
Based on DNA microarray analysis, Feske et al. (2001) demonstrated that Ca2+ signals modulate the expression of various genes involved in transcription, including c-Myc, c-Jun, CREM, NFAT4, FosB, E2F3, IRF-1, IRF-2, NF-IL3A, Fra-2, FLI1, and SMBP2. Calcium-dependent activation of a wide variety of transcription factors, such as NFAT, NFκB, Elk-1, Nur77, AP-1, ATF-2, and CREB, occurs through calmodulin-dependent protein kinases and phosphatases (Aramburu et al., 2000; Rao et al., 1997; Tokumitsu et al., 1995). Whereas a small transient spike of Ca2+ increase by store depletion activates signaling pathways and transcription factors such as NFκB and JNK (Dolmetsch et al., 1997), capacitative calcium entry and a sustained Ca2+ increase are necessary to activate other transcription factors, such as NFAT (Dolmetsch et al., 1997, 1998; Li et al., 1998).
To date, p300 has not been identified as a calcium-responsive gene. In addition, the transcriptional regulation of p300 and detailed examination of transcription complexes operative in regulating p300 have not been reported. We used the mRNA nucleotide sequence to predict transcription factor binding sites in the p300 promoter. Our analysis indicates that the p300 promoter contains binding sites for a number of transcription factor family members known to respond to calcium signaling such as NF-κB and NFAT (Im and Rao, 2004; Quintana et al., 2005), suggesting that calcium signaling has a role in replenishing limiting amounts of cellular p300.
Typically, the role of calcium in various signal transduction pathways has been investigated using relatively high concentrations (0.5–2.0 μM) of calcium ionophores, such as ionomycin, for short periods of time (6–18 h). This stimulation condition simulates calcium-dependent T cell activation upon antigen binding to the T cell receptor; however, such stimulation protocols do not simulate the effect of prolonged stimulation with calcium. Higher concentrations of ionomycin are cytotoxic, limiting the possibility of long-term stimulation. This might explain why previous studies did not identify p300 as a calcium-regulated gene. At low concentrations (50–100 nM), ionomycin binds cellular endomembranes and increases cytosolic calcium concentration by depletion of intracellular calcium stores and capacitative calcium entry (Donnadieu et al., 1995; Putney, 1990). Low concentrations of ionomycin therefore appear to simulate the effect of HTLV-1 p12I, which causes sustained increases in intracellular calcium concentration (Ding et al., 2002).
A number of viruses encode proteins that modulate cellular Ca2+ homeostasis to regulate various aspects of viral pathogenesis (Ganem, 2001). Examples include hepatitis B virus X protein (HBx), which activates Ca2+ signaling through mitochondria to influence HBV replication (Bouchard et al., 2001), and strains of rotavirus that encode NSP4, a nonstructural glycoprotein that increases the cytosolic calcium in infected cells (Tian et al., 1995). In addition, Kaposi’s sarcoma-associated herpesvirus (KSHV) mitochondrial protein K7 targets CAML, a cellular Ca2+-modulating protein to increase cytosolic Ca2+ allowing the completion of lytic replication (Feng et al., 2002). Coxsackievirus protein 2B induces the influx of extracellular Ca2+ and releases Ca2+ from ER stores, modifying plasma membrane permeability to facilitate virus release (van Kuppeveld et al., 1997). HIV-1 Nef causes atypical IP3R triggering of plasma membrane calcium influxes, independent of intracellular calcium stores (Manninen and Saksela, 2002). Furthermore, calcium plays a critical role in the replication cycles or pathogenesis of other viral infections including poliovirus, cytomegalovirus, vaccinia, and measles (Ruiz et al., 2000).
Our studies indicated that ablation of the acceptor splice site for the singly and doubly spliced ORF I mRNA in context to an infectious clone of HTLV-1 is associated with a reduction of viral infectivity in vivo (Collins et al., 1998) that can be model when resting T cells are used as targets in vitro (Albrecht et al., 2000). In addition, nucleotide sequence alleles of p12I including those that would result in specific residue changes associated with protein stability (lysine versus arginine at position aa 88) or truncation mutants have been reported in a minority (<7%) of HTLV-1-infected subjects with HAM/TSP, ATL, and asymptomatic patients (Martins et al., 2002; Furukawa et al., 2004; Iniguez et al., 2006). In this minority subset of infected persons, it appears that the presence of a lysine residue at position 88 of the protein is not a specific marker for HAM/TSP (Martins et al., 2002) and that truncations of p12I (e.g., premature stop codons leading to p12I variants from 82 to 86 amino acids in length) do not preclude transmission (Furukawa et al., 2004; Iniguez et al., 2006). These findings are consistent with our data using exogenously express truncation mutants of p12I, which retain the ability to localized to subcellular compartments, and elicited calcium-mediated NFAT activation of T cells (Ding et al., 2002; Kim et al., 2003).
p300 forms complexes with other transcription factors at the HTLV-1 promoter and plays a critical role in the regulation of HTLV-1 transcription in infected T cells (Lemasson et al., 2002). HTLV-1 Tax activates the HTLV-1 LTR through its interaction with p300 (Bex and Gaynor, 1998; Kashanchi et al., 1998; Jiang et al., 1999) and directly interacts with p300 in an acetyltransferase/activator complex (Harrod et al., 2000). We have demonstrated that HTLV-1 p30II binds p300 at the highly conserved KIX region (Zhang et al., 2001). Intriguingly, the KIX domain is also the binding site of p300 for HTLV-1 Tax. At lower concentrations, p30II activates HTLV-1 LTR-mediated transcription and at higher concentrations, p30II represses LTR-mediated transcription (Zhang et al., 2000). In addition, p30II was able to disrupt CREB-Tax-p300 complexes bound to the viral 21-bp TRE repeats (Zhang et al., 2001). Thus, HTLV-1 p30II and Tax appear to compete with each other in modulating the transcriptional activity from the LTR, possibly through competitive binding to p300 (Michael et al., submitted for publication). Our data present herein indicate that p12I blocks the repression LTR-driven transcription by HTLV-1 p30II. These findings indicate that p12I-mediated enhancement of p300 expression may not only regulate cellular gene expression to promote lymphocyte survival, but may also cooperate with other viral proteins to regulate viral gene expression.
Overall, our study demonstrates that HTLV-1 p12I enhances the expression of p300, an important but limiting co-activator of transcription in T cells. Thus, HTLV-1 p12I alters calcium-dependent cell signaling to promote lymphocyte survival, a characteristic of HTLV-1-associated lymphoproliferative disorders. In addition, we demonstrate that p12I has the capacity to reverse the transcriptional repression of p30II on the HTLV-1 LTR in a p300-dependent manner, demonstrating that HTLV-1 accessory proteins have the capacity to act cooperatively to modulate viral gene expression.
Materials and methods
Cells and plasmids
Jurkat Tcells (clone E6-1, catalog # TIB-152, American Type Culture Collection) were maintained in RPMI 1640 media (Invitrogen) supplemented with 15% FBS, 100 μg/ml streptomycin/penicillin, 2 mM l-glutamine, and 10 mM HEPES (Invitrogen). The pME-18S and pME-p12I plasmids (Mulloy et al., 1996) were provided by G. Franchini (National Cancer Institute, National Institutes of Health). The pME-p12I plasmid expresses the fusion protein of HTLV-1 p12I tagged with the influenza hemagglutinin (HA1) tag. Generation of p12I truncation mutants in the pME-18S vector was previously described (Ding et al., 2001). Mutant p12I15-47KKLL, which was constructed by inserting an ER targeting (Gomord et al., 1999; Plemper et al., 2001) KKLL sequence, has been described previously (Ding et al., 2003). Plasmid p5XGT-TATA-Luc (P. Quinn, The Pennsylvania State University, Hershey, PA), contains five tandem Gal4 DNA-binding sequences upstream of a TATA box, derived from positions −264 to +11 of the phosphoenolpyruvate carboxykinase (PEPCK) gene in a luciferase reporter gene plasmid. The pRSV-β-Gal, 12SE1A, 12SE1A-ΔCR1, and 12SE1A-ΔCR2 (T. Kouzarides, University of Cambridge, Cambridge, UK) have been described previously (Zhang et al., 2000, 2001). The pTRE-Luc plasmid and pRSV-β-Gal have been described previously (Zhang et al., 2000, 2001). pME-p30IIHA plasmid, which was created by cloning the p30II sequence from HTLV-1 molecular clone, ACH with downstream influenza hemagglutinin (HA1) tag, into pME-18S plasmid (G. Franchini, National Cancer Institute) between 5′ EcoRI and 3′ NotI sites, has been described previously (Michael et al., submitted for publication). The lentiviral vector system for expression of HTLV-1p12I has been described (Nair et al., 2005).
Stimulation of Jurkat T cells
To identify the optimal concentration and time frame for calcium-mediated enhancement of the transcription of p300, Jurkat T cells were stimulated with 25, 50, 100, and 500 nM and 1 μM ionomycin. To identify mRNA levels of p300, RT-PCR was performed between 36 and 48 h post-stimulation, whereas western blot and luciferase assays were performed at 60 h post-stimulation. The calcium chelator BAPTA-AM [glycine, N,N-1,2-ethanediylbis(oxy-2,1-phenylene)-bis-N-2-(acetyloxy) methoxy-2-oxoethyl]-[bis(acetyloxy)methyl ester] (Molecular Probes) and calcineurin inhibitor cyclosporin A (Sigma) were added to Jurkat T cells 24 h post-transfection or post-stimulation with ionomycin for 1 h at 37 °C. Transfected cells were washed and resuspended in cRPMI, whereas cells stimulated with ionomycin were resuspended in cRPMI containing appropriate concentrations of ionomycin.
Semi-quantitative RT-PCR for p300
Total cellular RNA was isolated from Jurkat T lymphocytes stimulated with ionomycin, using RNAqueous as described by the manufacturer (Ambion). One microgram RNA was reversed transcribed to cDNA as described by the manufacturer (Reverse Transcription system, Promega). cDNA from 100 ng of total RNA was then PCR amplified with AmpliTaq DNA polymerase (Perkin Elmer) using primers specific for p300 and GAPDH. The PCR primers are as follows: p300: GTAGCCTAAAAGACAATTTTCCTTG (forward), ATGTCAACCATCTGCACCAGTA (reverse); and GAPDH: TGCACCACCAACTGCTTAG (forward), GAGGCAGGGATGATGTTC (reverse). PCR was performed at multiple cycles to maintain the amplification in a linear range. PCR products were separated by agarose gel electrophoresis and densitometric analysis was performed using alpha imager spot densitometry (Alpha Innotech) as described previously (Albrecht et al., 1998). Densitometric values for p300 were normalized using the values for GAPDH. Statistical analysis was performed using Student’s t test, P < 0.05. DNA contamination tested in samples by performing a control with no reverse transcriptase.
Analysis of p300 promoter
From the National Center for Biological Information (NCBI) Web-based resource (http://www.ncbi.nlm.nih.gov/), the mRNA nucleotide sequence for p300 (NM_001429) was obtained. Preceding from the predicted start site of p300 mRNA, 403 nucleotide sequences were analyzed using commercially available software (Genomatix: http://www.genomatix.de/) to analyze and predict transcription factor binding sites in the promoter region in the p300 gene (Chromosome 22, contig-NT_01150.09, Homo sapiens chromosome 22).
Western immunoblot assays
Western immunoblot assays for the detection of p300 were performed as described previously (Zhang et al., 2000, 2001). Briefly, cells were lysed in RIPA buffer containing phosphate-buffered saline, 1% Nonidet P-40, 0.5% sodium deoxycholate, and 0.1% sodium dodecyl sulfate (SDS). Cell lysates were prepared by centrifugation at 14,000 rpm (Beckman) for 20 min at 4 °C. Equal amounts of proteins were mixed with Laemmli buffer (62.5 mM Tris [pH 6.8], 2% SDS, 10% glycerol, 0.2% bromophenol blue, 100 mM dithiothreitol). After boiling for 5 min, samples were electrophoresed through 5% polyacrylamide gels. The fractionated proteins were transferred to nitrocellulose membranes (Amersham Pharmacia Biotechnology) at 100 V for 1 h at 4 °C. Membranes were then blocked in Tris-buffered saline containing 5% nonfat milk and 0.1% Tween 20. p300 was detected with the rabbit anti-p300 primary antibody (Santa Cruz Biotechnology, catalog # SC-584), followed by an anti-rabbit immunoglobulin G (IgG)–horseradish peroxidase-conjugated goat antibody (Upstate). GAPDH, used as a normalization control, was detected using goat anti-GAPDH primary antibody (Santa Cruz) followed by an anti-goat immunoglobulin G (IgG)–horseradish peroxidase-conjugated donkey antibody (Upstate). Blots were developed using an enhanced chemiluminescence detection system (NEN Life Science). Densitometric analysis of radiograph was performed using Gel pro analyzer software (Media Cybernatic Inc.) and normalized to GAPDH. Protein detection was optimized to test proteins in the linear range of expression by testing at various protein concentrations and exposure times. Statistical analysis was performed using Student’s t test, P < 0.05.
Reporter gene assays
Unless otherwise mentioned, all the transfections of Jurkat T cells were performed using Superfect transfection reagent according to manufacturer’s instructions (Qiagen). Jurkat T lymphocytes (2 × 106) were transfected with 500 ng of 5xGT-luc, 100 ng of pM-VP 16,500 ng of pRSV-β-Gal in the presence or absence of increasing amounts of wild-type or mutant 12sE1A, and stimulated with appropriate amounts of ionomycin. When the effect of HTLV-1 p12I on p300 expression was tested, either pME-p12IHA or pME-18s were transfected with the above described plasmids in the absence if ionomycin. To test the effect of p12I on HTLV-1 p30II-mediated LTR repression, 0.2 μg of pTRE-Luc reporter plasmid was co-transfected with 1.2 μg pME-p30IIHA and increasing concentrations (0.0, 0.6, 1.2, and 2.4 μg) of pME-p12IHA using Lipofectamine Plus (Invitrogen). To test if the rescue of p30II-mediated HTLV-1 LTR repression by p12I is p300 dependent, 1.0 μg E1A expression plasmid was also co-transfected. As an internal control for transfection efficiency, 0.1 μg of pRSV-β-Gal (Invitrogen) was used in each transfection. pME-18S was used as carrier DNA to equalize DNA concentrations for each transfection.
At 60 h post-transfection, cells were lysed with a commercial buffer (Promega) at room temperature for 15 min. Twenty microliters of each lysate was used to test luciferase reporter gene activity using an enhanced luciferase assay kit according to the manufacturer’s protocol (Promega). Staining with 5-bromo-4-chloro-3-indolyl-beta-d-galactopyranoside (X-Gal) (Sigma) and counting β-Gal-expressing cells were performed to normalize the transfection efficiency. The final concentration of ionomycin and BAPTA-AM used was chosen after multiple pilot experiments using different concentrations of these reagents. BAPTA-AM alone did not have any significant effect on luciferase expression without the presence of calcium. Data were expressed as mean of normalized luciferase activity in arbitrary light units (ALU) with standard error (SE) from a minimum of triplicate experiments. Statistical analysis was performed using Student’s t test, P < 0.05.
Acknowledgments
The authors thank H. Hiraragi, S. J. Kim, P. Green, and L. Mathes for suggestions and critical review of the manuscript, and G. Franchini for sharing valuable reagents. This work was supported by NIH grants CA100730 and RR14324 awarded to Dr. Michael Lairmore and CA-70529 from the NCI, awarded through the OSU Comprehensive Cancer Center.
References
- Albrecht B, Lairmore MD. Critical role of human T-lymphotropic virus type 1 accessory proteins in viral replication and pathogenesis. Microbiol Mol Biol Rev. 2002;66:396–406. doi: 10.1128/MMBR.66.3.396-406.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrecht B, Collins ND, Newbound GC, Ratner L, Lairmore MD. Quantification of human T-cell lymphotropic virus type 1 proviral load by quantitative competitive polymerase chain reaction. J Virol Methods. 1998;75:123–140. doi: 10.1016/s0166-0934(98)00087-1. [DOI] [PubMed] [Google Scholar]
- Albrecht B, Collins ND, Burniston MT, Nisbet JW, Ratner L, Green PL, Lairmore MD. Human T-lymphotropic virus type 1 open reading frame I p12(I) is required for efficient viral infectivity in primary lymphocytes. J Virol. 2000;74:9828–9835. doi: 10.1128/jvi.74.21.9828-9835.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrecht B, D’Souza CD, Ding W, Tridandapani S, Coggeshall KM, Lairmore MD. Activation of nuclear factor of activated T cells by human T-lymphotropic virus type 1 accessory protein p12(I) J Virol. 2002;76:3493–3501. doi: 10.1128/JVI.76.7.3493-3501.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali SH, Decaprio JA. Cellular transformation by SV40 large T antigen: interaction with host proteins. Semin Cancer Biol. 2001;11:15–23. doi: 10.1006/scbi.2000.0342. [DOI] [PubMed] [Google Scholar]
- Aramburu J, Rao A, Klee CB. Calcineurin: from structure to function. Curr Top Cell Regul. 2000;36:237–295. doi: 10.1016/s0070-2137(01)80011-x. [DOI] [PubMed] [Google Scholar]
- Berneman ZN, Gartenhaus RB, Reitz MS, Blattner WA, Manns A, Hanchard B, Ikehara O, Gallo RC, Klotman ME. Expression of alternatively spliced human T-lymphotropic virus type 1 pX mRNA in infected cell lines and in primary uncultured cells from patients with adult T-cell leukemia/lymphoma and healthy carriers. Proc Natl Acad Sci U S A. 1992;89:3005–3009. doi: 10.1073/pnas.89.7.3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge MJ. Inositol trisphosphate and calcium signalling. Nature. 1993;361:315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- Berridge M, Lipp P, Bootman M. Calcium signalling. Curr Biol. 1999;9:R157–R159. doi: 10.1016/s0960-9822(99)80101-8. [DOI] [PubMed] [Google Scholar]
- Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol. 2000;1:11–21. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]
- Bex F, Gaynor RB. Regulation of gene expression by HTLV-I tax protein. Methods. 1998;16:83–94. doi: 10.1006/meth.1998.0646. [DOI] [PubMed] [Google Scholar]
- Blobel GA. CBP and p300: versatile coregulators with important roles in hematopoietic gene expression. J Leukocyte Biol. 2002;71:545–556. [PubMed] [Google Scholar]
- Bootman MD, Collins TJ, Peppiatt CM, Prothero LS, Mackenzie L, de Smet P, Travers M, Tovey SC, Seo JT, Berridge MJ, Ciccolini F, Lipp P. Calcium signalling—An overview. Semin Cell Dev Biol. 2001;12:3–10. doi: 10.1006/scdb.2000.0211. [DOI] [PubMed] [Google Scholar]
- Bouchard MJ, Wang LH, Schneider RJ. Calcium signaling by HBx protein in hepatitis B virus DNA replication. Science. 2001;294:2376–2378. doi: 10.1126/science.294.5550.2376. [DOI] [PubMed] [Google Scholar]
- Chan HM, La Thangue NB. p300/CBP proteins: HATs for transcriptional bridges and scaffolds. J Cell Sci. 2001;114:2363–2373. doi: 10.1242/jcs.114.13.2363. [DOI] [PubMed] [Google Scholar]
- Ciminale V, Pavlakis GN, Derse D, Cunningham CP, Felber BK. Complex splicing in the human T-cell leukemia virus (HTLV) family of retroviruses: novel mRNAs and proteins produced by HTLV type I. J Virol. 1992;66:1737–1745. doi: 10.1128/jvi.66.3.1737-1745.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapham DE. Calcium signaling. Cell. 1995;80:259–268. doi: 10.1016/0092-8674(95)90408-5. [DOI] [PubMed] [Google Scholar]
- Colgin MA, Nyborg JK. The human T-cell leukemia virus type 1 oncoprotein Tax inhibits the transcriptional activity of c-Myb through competition for the CREB binding protein. J Virol. 1998;72:9396–9399. doi: 10.1128/jvi.72.11.9396-9399.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins ND, Newbound GC, Albrecht B, Beard JL, Ratner L, Lairmore MD. Selective ablation of human T-cell lymphotropic virus type 1 p12I reduces viral infectivity in vivo. Blood. 1998;91:4701–4707. [PubMed] [Google Scholar]
- Ding W, Albrecht B, Luo R, Zhang W, Stanley JR, Newbound GC, Lairmore MD. Endoplasmic reticulum and cis-Golgi localization of human T-lymphotropic virus type 1 p12(I): association with calreticulin and calnexin. J Virol. 2001;75:7672–7682. doi: 10.1128/JVI.75.16.7672-7682.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding W, Albrecht B, Kelley RE, Muthusamy N, Kim S, Altschuld RA, Lairmore MD. Human T lymphotropic virus type 1 p12(I) expression increases cytoplasmic calcium to enhance the activation of nuclear factor of activated T cells. J Virol. 2002;76:10374–10382. doi: 10.1128/JVI.76.20.10374-10382.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding W, Kim SJ, Nair AM, Michael B, Boris-Lawrie K, Tripp A, Feuer G, Lairmore MD. Human T-cell lymphotropic virus type 1 p12(I) enhances interleukin-2 production during T-cell activation. J Virol. 2003;77:11027–11039. doi: 10.1128/JVI.77.20.11027-11039.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolmetsch RE, Lewis RS, Goodnow CC, Healy JI. Differential activation of transcription factors induced by Ca2+ response amplitude and duration. Nature. 1997;386:855–858. doi: 10.1038/386855a0. [DOI] [PubMed] [Google Scholar]
- Dolmetsch RE, Xu K, Lewis RS. Calcium oscillations increase the efficiency and specificity of gene expression. Nature. 1998;392:933–936. doi: 10.1038/31960. [DOI] [PubMed] [Google Scholar]
- Donnadieu E, Bismuth G, Trautmann A. The intracellular Ca2+ concentration optimal for T cell activation is quite different after ionomycin or CD3 stimulation. Pflugers Arch. 1995;429:546–554. doi: 10.1007/BF00704160. [DOI] [PubMed] [Google Scholar]
- Feng P, Park J, Lee BS, Lee SH, Bram RJ, Jung JU. Kaposi’s sarcoma-associated herpesvirus mitochondrial K7 protein targets a cellular calcium-modulating cyclophilin ligand to modulate intracellular calcium concentration and inhibit apoptosis. J Virol. 2002;76:11491–11504. doi: 10.1128/JVI.76.22.11491-11504.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feske S, Giltnane J, Dolmetsch R, Staudt LM, Rao A. Gene regulation mediated by calcium signals in T lymphocytes. Nat Immunol. 2001;2:316–324. doi: 10.1038/86318. [DOI] [PubMed] [Google Scholar]
- Furukawa Y, Usuku K, Izumo S, Osame M. Human T cell lymphotropic virus type I (HTLV-I) p12I is dispensable for HTLV-I transmission and maintenance of infection in vivo. AIDS Res Hum Retroviruses. 2004;20:1092–1099. doi: 10.1089/aid.2004.20.1092. [DOI] [PubMed] [Google Scholar]
- Ganem D. Virology. The X files—One step closer to closure. Science. 2001;294:2299–2300. doi: 10.1126/science.1067850. [DOI] [PubMed] [Google Scholar]
- Giordano A, Avantaggiati ML. p300 and CBP: partners for life and death. J Cell Physiol. 1999;181:218–230. doi: 10.1002/(SICI)1097-4652(199911)181:2<218::AID-JCP4>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Gomord V, Wee E, Faye L. Protein retention and localization in the endoplasmic reticulum and the Golgi apparatus. Biochimie. 1999;81:607–618. doi: 10.1016/s0300-9084(99)80118-7. [DOI] [PubMed] [Google Scholar]
- Goodman RH, Smolik S. CBP/p300 in cell growth, transformation, and development. Genes Dev. 2000;14:1553–1577. [PubMed] [Google Scholar]
- Grassmann R, Aboud M, Jeang KT. Molecular mechanisms of cellular transformation by HTLV-1 tax. Oncogene. 2005;24:5976–5985. doi: 10.1038/sj.onc.1208978. [DOI] [PubMed] [Google Scholar]
- Harrod R, Kuo YL, Tang Y, Yao Y, Vassilev A, Nakatani Y, Giam CZ. p300 and p300/cAMP-responsive element-binding protein associated factor interact with human T-cell lymphotropic virus type-1 tax in a multi-histone acetyltransferase/activator-enhancer complex. J Biol Chem. 2000;275:11852–11857. doi: 10.1074/jbc.275.16.11852. [DOI] [PubMed] [Google Scholar]
- Hottiger MO, Felzien LK, Nabel GJ. Modulation of cytokine-induced HIV gene expression by competitive binding of transcription factors to the coactivator p300. EMBO J. 1998;17:3124–3134. doi: 10.1093/emboj/17.11.3124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im SH, Rao A. Activation and deactivation of gene expression by Ca2+/calcineurin-NFAT-mediated signaling. Mol Cells. 2004;18:1–9. [PubMed] [Google Scholar]
- Iniguez AM, Gastaldello R, Gallego S, Otsuki K, Vicente AC. HTLV-1 p12(I) protein sequences from South America: truncated proteins and common genetic signatures. AIDS Res Hum Retroviruses. 2006;22:466–469. doi: 10.1089/aid.2006.22.466. [DOI] [PubMed] [Google Scholar]
- Iyer NG, Ozdag H, Caldas C. p300/CBP and cancer. Oncogene. 2004;23:4225–4231. doi: 10.1038/sj.onc.1207118. [DOI] [PubMed] [Google Scholar]
- Janknecht R. The versatile functions of the transcriptional coactivators p300 and CBP and their roles in disease. Histol Histopathol. 2002;17:657–668. doi: 10.14670/HH-17.657. [DOI] [PubMed] [Google Scholar]
- Jiang H, Lu H, Schiltz RL, Pise-Masison CA, Ogryzko VV, Nakatani Y, Brady JN. PCAF interacts with tax and stimulates tax transactivation in a histone acetyltransferase-independent manner. Mol Cell Biol. 1999;19:8136–8145. doi: 10.1128/mcb.19.12.8136. published erratum appears in Mol Cell Biol 2000 Mar;20(5):1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashanchi F, Brady JN. Transcriptional and post-transcriptional gene regulation of HTLV-1. Oncogene. 2005;24:5938–5951. doi: 10.1038/sj.onc.1208973. [DOI] [PubMed] [Google Scholar]
- Kashanchi F, Duvall JF, Kwok RP, Lundblad JR, Goodman RH, Brady JN. The coactivator CBP stimulates human T-cell lymphotrophic virus type I tax transactivation in vitro. J Biol Chem. 1998;273:34646–34652. doi: 10.1074/jbc.273.51.34646. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Ding W, Albrecht B, Green PL, Lairmore MD. A conserved calcineurin-binding motif in human T lymphotropic virus type 1 p12I functions to modulate nuclear factor of activated T cell activation. J Biol Chem. 2003;278:15550–15557. doi: 10.1074/jbc.M210210200. [DOI] [PubMed] [Google Scholar]
- Koralnik IJ, Fullen J, Franchini G. The p12I, p13II, and p30II proteins encoded by human T-cell leukemia/lymphotropic virus type I open reading frames I and II are localized in three different cellular compartments. J Virol. 1993;67:2360–2366. doi: 10.1128/jvi.67.4.2360-2366.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lal RB, Rudolph DL, Schmid DS, Lairmore MD. Concomitant augmentation of CD4+, CD29+ helper inducer and diminution of CD4+, CD45RA+ suppressor inducer subset in patients infected with human T-cell lymphotropic virus types I or II. Clin Exp Immunol. 1992;87:293–297. doi: 10.1111/j.1365-2249.1992.tb02990.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemasson I, Polakowski NJ, Laybourn PJ, Nyborg JK. Transcription factor binding and histone modifications on the integrated proviral promoter in human T-cell leukemia virus-I-infected T-cells. J Biol Chem. 2002;277:49459–49465. doi: 10.1074/jbc.M209566200. [DOI] [PubMed] [Google Scholar]
- Lewis RS. Calcium signaling mechanisms in T lymphocytes. Annu Rev Immunol. 2001;19:497–521. doi: 10.1146/annurev.immunol.19.1.497. [DOI] [PubMed] [Google Scholar]
- Li W, Llopis J, Whitney M, Zlokarnik G, Tsien RY. Cell-permeant caged InsP3 ester shows that Ca2+ spike frequency can optimize gene expression. Nature. 1998;392:936–941. doi: 10.1038/31965. [DOI] [PubMed] [Google Scholar]
- Liu C, Hermann TE. Characterization of ionomycin as a calcium ionophore. J Biol Chem. 1978;253:5892–5894. [PubMed] [Google Scholar]
- Ludlow JW, Skuse GR. Viral oncoprotein binding to pRB, p107, p130, and p300. Virus Res. 1995;35:113–121. doi: 10.1016/0168-1702(94)00094-s. [DOI] [PubMed] [Google Scholar]
- Manninen A, Saksela K. HIV-1 Nef interacts with inositol trisphosphate receptor to activate calcium signaling in T cells. J Exp Med. 2002;195:1023–1032. doi: 10.1084/jem.20012039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins ML, Soares BC, Ribas JG, Thorun GW, Johnson J, Kroon EG, Carneiro-Prioetti AB, Bonjardim CA. Frequency of p12K and p12R alleles of HTLV type 1 in HAM/TSP patients and in asymptomatic HTLV type 1 carriers. AIDS Res Hum Retroviruses. 2002;18:899–902. doi: 10.1089/088922202760265560. [DOI] [PubMed] [Google Scholar]
- Marzio G, Giacca M. Chromatin control of HIV-1 gene expression. Genetica. 1999;106:125–130. doi: 10.1023/a:1003797332379. [DOI] [PubMed] [Google Scholar]
- Michael B, Nair A, Lairmore MD. Role of accessory proteins of HTLV-1 in viral replication, T cell activation, and cellular gene expression. Front Biosci. 2004;9:2556–2576. doi: 10.2741/1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulloy JC, Crownley RW, Fullen J, Leonard WJ, Franchini G. The human T-cell leukemia/lymphotropic virus type 1 p12I proteins bind the interleukin-2 receptor beta and gamma chains and affects their expression on the cell surface. J Virol. 1996;70:3599–3605. doi: 10.1128/jvi.70.6.3599-3605.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair A, Michael B, Hiraragi H, Fernandez S, Feuer G, Boris-Lawrie K, Lairmore M. Human T lymphotropic virus type 1 accessory protein p12I modulates calcium-mediated cellular gene expression and enhances p300 expression in T lymphocytes. AIDS Res Hum Retroviruses. 2005;21:273–284. doi: 10.1089/aid.2005.21.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parekh AB, Penner R. Store depletion and calcium influx. Physiol Rev. 1997;77:901–930. doi: 10.1152/physrev.1997.77.4.901. [DOI] [PubMed] [Google Scholar]
- Petrij F, Giles RH, Dauwerse HG, Saris JJ, Hennekam RC, Masuno M, Tommerup N, van Ommen GJ, Goodman RH, Peters DJ. Rubinstein–Taybi syndrome caused by mutations in the transcriptional co-activator CBP. Nature. 1995;376:348–351. doi: 10.1038/376348a0. see comments. [DOI] [PubMed] [Google Scholar]
- Plemper RK, Hammond AL, Cattaneo R. Measles virus envelope glycoproteins hetero-oligomerize in the endoplasmic reticulum. J Biol Chem. 2001;276:44239–44246. doi: 10.1074/jbc.M105967200. [DOI] [PubMed] [Google Scholar]
- Putney JW., Jr Capacitative calcium entry revisited. Cell Calcium. 1990;11:611–624. doi: 10.1016/0143-4160(90)90016-n. [DOI] [PubMed] [Google Scholar]
- Quintana A, Griesemer D, Schwarz EC, Hoth M. Calcium-dependent activation of T-lymphocytes. Pflugers Arch. 2005;450:1–12. doi: 10.1007/s00424-004-1364-4. [DOI] [PubMed] [Google Scholar]
- Rao A, Luo C, Hogan PG. Transcription factors of the NFAT family: regulation and function. Annu Rev Immunol. 1997;15:707–747. doi: 10.1146/annurev.immunol.15.1.707. [DOI] [PubMed] [Google Scholar]
- Ruiz MC, Cohen J, Michelangeli F. Role of Ca2+ in the replication and pathogenesis of rotavirus and other viral infections. Cell Calcium. 2000;28:137–149. doi: 10.1054/ceca.2000.0142. [DOI] [PubMed] [Google Scholar]
- Tian P, Estes MK, Hu Y, Ball JM, Zeng CQ, Schilling WP. The rotavirus nonstructural glycoprotein NSP4 mobilizes Ca2+ from the endoplasmic reticulum. J Virol. 1995;69:5763–5772. doi: 10.1128/jvi.69.9.5763-5772.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokumitsu H, Enslen H, Soderling TR. Characterization of a Ca2+/calmodulin-dependent protein kinase cascade. Molecular cloning and expression of calcium/calmodulin-dependent protein kinase kinase. J Biol Chem. 1995;270:19320–19324. doi: 10.1074/jbc.270.33.19320. [DOI] [PubMed] [Google Scholar]
- van Kuppeveld FJ, Hoenderop JG, Smeets RL, Willems PH, Dijkman HB, Galama JM, Melchers WJ. Coxsackievirus protein 2B modifies endoplasmic reticulum membrane and plasma membrane permeability and facilitates virus release. EMBO J. 1997;16:3519–3532. doi: 10.1093/emboj/16.12.3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vo N, Goodman RH. CREB-binding protein and p300 in transcriptional regulation. J Biol Chem. 2001;276:13505–13508. doi: 10.1074/jbc.R000025200. [DOI] [PubMed] [Google Scholar]
- Yang XJ, Ogryzko VV, Nishikawa J, Howard BH, Nakatani Y. A p300/CBP-associated factor that competes with the adenoviral oncoprotein E1A. Nature. 1996;382:319–324. doi: 10.1038/382319a0. [DOI] [PubMed] [Google Scholar]
- Yoshie O, Fujisawa R, Nakayama T, Harasawa H, Tago H, Izawa D, Hieshima K, Tatsumi Y, Matsushima K, Hasegawa H, Kanamaru A, Kamihira S, Yamada Y. Frequent expression of CCR4 in adult T-cell leukemia and human T-cell leukemia virus type 1-transformed T cells. Blood. 2002;99:1505–1511. doi: 10.1182/blood.v99.5.1505. [DOI] [PubMed] [Google Scholar]
- Younis I, Green PL. The human T-cell leukemia virus Rex protein. Front Biosci. 2005;10:431–445. doi: 10.2741/1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Nisbet JW, Bartoe JT, Ding W, Lairmore MD. Human T-lymphotropic virus type 1 p30(II) functions as a transcription factor and differentially modulates CREB-responsive promoters. J Virol. 2000;74:11270–11277. doi: 10.1128/jvi.74.23.11270-11277.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Nisbet JW, Albrecht B, Ding W, Kashanchi F, Bartoe JT, Lairmore MD. Human T-lymphotropic virus type 1 p30(II) regulates gene transcription by binding CREB binding protein/p300. J Virol. 2001;75:9885–9895. doi: 10.1128/JVI.75.20.9885-9895.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]


