Abstract
Invariant natural killer T (iNKT) cells are a small subset of lymphocytes that recognize glycolipid antigens in the context of CD1d and consequently produce large quantities of pro-inflammatory and/or anti-inflammatory cytokines. Several transmembrane glycoproteins have been implicated in the co-stimulation of iNKT cell responses. However, whether glycosylphosphatidylinositol (GPI)-anchored proteins can function in this capacity is not known. Here, we demonstrate that antibody-mediated cross-linking of the prototype mouse GPI-anchored protein Thy-1 (CD90) on the surface of a double-negative (CD4−CD8−) iNKT cell line leads to cytokine production at both the mRNA and protein levels. In addition, Thy-1 triggering enhanced cytokine secretion by iNKT cells that were concomitantly stimulated with α-galactosylceramide (αGC), consistent with a co-stimulatory role for Thy-1 in iNKT cell activation. This was also evident when a CD4+ mouse iNKT cell line or primary hepatic NKT cells were stimulated with αGC and/or anti-Thy-1 antibody. Cross-linking Ly-6A/E, another GPI-anchored protein, could also boost cytokine secretion by αGC-stimulated iNKT cells, suggesting that the observed effects reflect a general property of GPI-anchored proteins. To extend these results from mouse to human cells, we focused on CD55, a GPI-anchored protein that, unlike Thy-1, is expressed on human iNKT cells. Cross-linking CD55 augmented αGC-induced iNKT cell responses as judged by more vigorous proliferation and higher CD69 expression. Collectively, these findings demonstrate for the first time that GPI-anchored proteins are able to co-stimulate CD1d-restricted, glycolipid-reactive iNKT cells in both mice and humans.
Keywords: α-galactosylceramide, CD55, glycosylphosphatidylinositol-anch-ored proteins, invariant natural killer T cell, Thy-1
Introduction
Invariant natural killer T (iNKT) cells are a rare, but extremely potent subpopulation of lymphocytes that link innate and adaptive immune mechanisms.1,2 They simultaneously express NK cell markers and a canonical T-cell receptor (TCR) whose α chain invariably exhibits a Vα14-Jα18 rearrangement in mice and a Vα24-Jα18 rearrangement in humans and pairs with a limited number of Vβ chains.3,4 Unlike conventional T cells that detect peptide–MHC complexes, the TCR of iNKT cells recognizes and responds to glycolipid molecules presented within the deep, hydrophobic pocket of CD1d, an MHC class I-like molecule expressed by a variety of cell types. Although the physiological ligands for iNKT cells remain ill-defined, a synthetic derivative (KRN7000) of marine sponge-derived glycolipid called α-galactosylceramide (αGC) has been widely used to study both mouse and human iNKT cell responses.5,6
Once activated by their cognate glycolipid antigens, iNKT cells swiftly release remarkably high amounts, on a per cell basis, of T helper type 1 (Th1) and/or Th2 cytokines, and transactivate several other immunocytes, including NK cells,7,8 B cells9 and conventional T cells.10 The unique property of iNKT cells to modulate a plethora of immune functions by virtue of the pro-inflammatory and/or anti-inflammatory cytokines they secrete has earned them the moniker ‘double-edged swords’ of the immune system,11 and led to intense investigations aimed at designing novel and effective iNKT cell-based immunotherapeutic modalities. For instance, αGC and ex vivo-expanded iNKT cells have been used in several clinical trials for various types of cancer and have shown some promise.12–14 KRN7000 was in fact discovered in a screen for novel anti-cancer agents.5
The role of iNKT cells in regulation of immune responses has come under scrutiny but it is equally important to understand how the responsiveness of iNKT cells themselves is modulated. Other than requisite interactions between αGC: CD1d complexes and the canonical TCR of iNKT cells, several cell surface molecules have been implicated in the regulation of iNKT cell activation in various in vitro and in vivo settings. These include CD28,15 CD40,15 inducible co-stimulator,16,17 glucocorticoid-induced tumour necrosis factor receptor,18 CD137 (4-1BB)19,20 and CD134 (OX40).21 These are membrane-spanning glycoproteins that function mainly in the capacity of co-stimulatory molecules to boost or optimize iNKT cell responses.
Glycosylphosphatidylinositol (GPI)-anchored proteins constitute a family of cell surface proteins that exhibit potent signalling activity in various cell types, including conventional T lymphocytes.22 As GPI-anchored proteins lack a cytoplasmic tail, their signalling capacity is probably the result of their association with lipid rafts, membrane microdomains rich in signalling intermediates.
The prototype GPI-anchored protein Thy-1 is a small, heavily glycosylated molecule found abundantly on the surface of mouse and human thymocytes as well as mouse peripheral T cells.23,24 Antibody-mediated cross-linking of Thy-1 is known to initiate T-cell proliferation and interleukin-2 (IL-2) production.25 We previously demonstrated that Thy-1 can provide a surrogate signal 1 to initiate T cell responses on its own or co-stimulate T cell activation in the presence of classical TCR triggering.26 We therefore proposed a dual signalling property for Thy-1 in the context of T cell activation. While the role of Thy-1 and other GPI-anchored proteins in conventional T cell responses has been studied extensively, it is not known whether these molecules participate in or modulate the responses elicited by glycolipid-reactive iNKT cells. This is an important question given the prominent immunomodulatory roles played by these lymphocytes in health and disease.
In this study, we investigated the expression and/or function of mouse Thy-1 and Ly-6A/E as well as human CD55 in iNKT cells. CD55, also called decay-accelerating factor, is a GPI-anchored protein that, unlike Thy-1, is expressed by human peripheral T cells27 and is able to co-stimulate conventional T cells.28
We found that Thy-1 cross-linking alone leads to cytokine secretion by mouse iNKT cells. Furthermore, when combined with glycolipid-induced TCR signalling, Thy-1 ligation augmented iNKT cell activation, which is consistent with a co-stimulatory function for this molecule in the context of iNKT cell responses. A similar effect was observed when mouse Ly-6A/E was ligated. We also demonstrated that human iNKT cells can be co-stimulated through CD55. Together, these findings highlight an important and novel role for GPI-anchored proteins in iNKT cell responses.
Materials and methods
Mice
Female and male C57BL/6 mice, 6–10 weeks of age, were purchased from Charles River Canada Inc. (St Constant, QC, Canada), housed under specific pathogen-free conditions in our animal care facility at The University of Western Ontario, and cared for in accordance with institutional regulations and the guidelines established by the Canadian Council for Animal Care. Jα18−/− mice that are iNKT cell deficient29 were obtained from Dr Masaru Taniguchi (RIKEN Research Center for Allergy and Immunology, Yokohama, Japan) and bred in the same facility.
Cell lines
DN32.D3, a Vα14+ CD4−CD8− mouse iNKT cell line,4 was provided by Dr Albert Bendelac (The University of Chicago, Chicago, IL). The Vα14+CD4+CD8− mouse iNKT cell line N38-2C1230 and the non-invariant mouse NKT cell line N37-1A1231 were obtained from Dr Kyoko Hayakawa (Fox Chase Cancer Center, Philadelphia, PA). The CD4+ mouse T cell line C6E1 with specificity for pigeon cytochrome c32 was kindly provided by Dr Joaqúin Madrenas (The University of Western Ontario, London, ON, Canada). Cells were maintained in RPMI-1640 medium supplemented with 10% heat-inactivated fetal bovine serum, 0·1 mm minimal essential medium non-essential amino acids, 2 mm l-glutamine, 1 mm sodium pyruvate, 100 U/ml penicillin, 100 μg/ml streptomycin and 50 μm 2-mercaptoethanol, which will, hereafter, be referred to as complete medium.
Cytofluorimetric analysis and sorting
Mice were killed by carbon dioxide asphyxiation. Spleen cell suspensions were prepared in ice-cold PBS (pH 7·2–7·4) using a glass tissue homogenizer. Cell preparations were depleted of erythrocytes by incubation in ACK lysis buffer for 5 min at room temperature with occasional, gentle shaking. To obtain hepatic lymphoid mononuclear cells, livers were removed, perfused by injecting 10 ml PBS into the portal vein, cut into small pieces and pressed through a 200-μm metallic mesh. After washing in cold PBS, the liver homogenate was resuspended in a 33·75% Percoll™ PLUS solution (GE Healthcare Bio-Sciences AB, Uppsala, Sweden) and centrifuged at 700 g for 12 min at room temperature. The pelleted cells were then washed and treated with ACK lysis buffer to deplete erythrocytes as described above. The percentage of splenic and hepatic iNKT cells was then determined by CD1d tetramer staining. Cells were first incubated on ice with anti-CD16/CD32 monoclonal antibody (mAb) (clone 2.4G2, Fc Block) followed by concomitant staining with FITC-conjugated anti-TCR-β mAb (clone H57-597) purchased from eBioscience (San Diego, CA) and allophycocyanin (APC)-conjugated PBS-57-loaded mouse CD1d tetramers. Unloaded mouse CD1d tetramers conjugated with APC were used in parallel as a negative control. Both PBS-57-loaded and -unloaded tetramer reagents were generously provided by the NIH Tetramer Core Facility (Atlanta, GA). To determine the expression of Fcγ receptors II/III and Thy-1 by mouse iNKT cells, phycoerythrin (PE)-conjugated anti-CD16/32 mAb (clone 2.4G2) and PE-conjugated anti-Thy-1.2 mAb (clone 30-H12) were purchased from BD Pharmingen (San Jose, CA) and eBioscience, respectively, and used in our cell staining protocols. Cells were washed and fixed in 1% paraformaldehyde before a BD FACSCalibur or BD FACSCanto II flow cytometer was used for data acquisition. Data analysis was carried out using FlowJo software (Tree Star, Ashland, OR).
To obtain highly purified liver NKT cells from mice, hepatic mononuclear cells were incubated on ice with Fc Block for 20 min and subsequently stained with FITC-conjugated anti-TCR-β mAb and PE-conjugated anti-NK1.1 mAb (clone PK136) purchased from BD Pharmingen. In a limited number of control experiments, these cells were additionally stained with APC-conjugated PBS-57-loaded or -unloaded mouse CD1d tetramers. To prevent clumping, cells were washed and incubated with PBS containing 0·1% BSA and 10 mm EDTA for 10 min. Cells were washed again, filtered through a 40-μm strainer, and resuspended at 3 × 107 to 4 × 107 cells/ml in Hanks’ balanced salt solution supplemented with 0·1% BSA. The TCR-β+ NK1.1+ cells were then sorted using a BD FACSVantage Cell Sorter and bd FACSDiva software. The purity of sorted NKT cells was typically > 99%.
For the staining of human iNKT cells, the cells were stained first with APC-conjugated PBS-57-loaded or -unloaded human CD1d tetramers at room temperature for 30 min followed by a single wash and subsequent staining with additional fluorochrome-labelled reagents for 30 min on ice. The FITC-conjugated anti-CD3 mAb (clone OKT3), PE-conjugated anti-CD69 mAb (clone FN50), PE-conjugated anti-Thy-1 mAb (clone 5E10) and APC-eFluor780-conjugated anti-CD3 mAb (clone UCHT1) were all purchased from eBioscience. The PE-conjugated anti-CD55 mAb (clone BRIC216) was obtained from The International Blood Group Reference Laboratory (Bristol, UK). Human CD1d tetramer reagents were provided by the NIH Tetramer Core Facility.
All isotype control reagents used in this study were purchased from eBioscience with the sole exception of rat IgG2c, which was obtained from Medical & Biological Laboratories Co., Ltd (Nagoya, Japan).
Generation of mouse bone marrow-derived dendritic cells
Dendritic cells (DCs) were generated from bone marrow precursors as previously described.33 In brief, bone marrow cells were flushed out of femurs and tibias using cold PBS and filtered through a 70-μm strainer. Erythrocytes were removed by treatment with ACK lysis buffer, and the remaining marrow cells were resuspended at 4 × 106 cells/ml in complete medium containing 4 ng/ml granuloctye–macrophage colony-stimulating factor (GM-CSF) and 1000 U/ml IL-4 (PeproTech Inc., Rocky Hill, NJ). Twenty million cells were seeded into each well of a six-well polystyrene plate. On days 2 and 4, floating cells were removed and fresh medium containing GM-CSF and IL-4 was added to the wells. On day 6, when DCs detach, all floating cells were harvested, washed and used as accessory cells in our experimental protocols.
Mouse NKT and T cell activation
Between 20 000 and 100 000 NKT cells were seeded in a total volume of 200 μl/well in 96-well polystyrene microtitre plates. When N38-2C12 iNKT cells or sorted hepatic NKT cells were assayed, bone marrow-derived DCs were also added to the wells at an NKT : DC ratio of 5 : 1. The NKT cells were stimulated with synthetic αGC (KRN7000) obtained from Kirin Brewery (Gunma, Japan) and/or anti-Thy-1 mAb (clone G7) purchased from eBioscience. Control cultures received polysorbate vehicle and/or a rat IgG2c isotype control as indicated. In some experiments, FcγRs II/III were blocked using 5 μg/ml Fc block for 20 min before adding anti-Thy-1 mAb to cultures.
To activate DN32.D3 iNKT cells with αGC and G7, there was no requirement for the presence of accessory cells in culture.34 In contrast, bone marrow-derived DCs were present in culture when DN32.D3 cells were stimulated with a combination of αGC and anti-Ly-6A/E mAb (clone D7) purchased from e-Bioscience.
In a limited number of experiments, DN32.D3 iNKT cells, N37-1A12 non-invariant NKT cells and C6E1 conventional T cells were stimulated with plate-coated anti-CD3 mAb (Cedarlane Laboratories, Burlington, ON) in the presence or absence of soluble G7.
For in vivo iNKT cell activation, mice were injected intraperitoneally with 2 μg αGC (or vehicle). After 72 hr, a time-point at which iNKT cells are known to reach their peak frequency following αGC-mediated expansion,35 mice were killed and splenic and hepatic mononuclear cells were isolated as described above.
Cytokine quantification by ELISA
Culture supernatants were harvested from wells containing iNKT cell lines or primary hepatic NKT cells at 24 hr or 48 hr after stimulation, respectively. The cytokine content of samples was quantified by ELISA using Ready-Set-Go kits for mouse IL-2, IL-4 and interferon-γ (IFN-γ) (eBioscience). Plates were read at a dual wavelength of 450/570 nm using a Benchmark Microplate Reader (Bio-Rad Laboratories, Hercules, CA).
Reverse transcriptase quantitative PCR
Total RNA was extracted using TRIzol Reagent (Invitrogen, Burlington, ON). RNA concentration and purity were determined using a NanoDrop 1000 Spectrophotometer (Thermo Scientific, Waltham, MA). One microgram of RNA was reverse transcribed into cDNA using oligo-dT(18) primers and Advantage RT-for-PCR Kit (Clontech Laboratories Inc., Mountain View, CA). For quantitative PCR, the resulting cDNA was diluted 1 : 100 in diethylpyrocarbonate-treated water (Invitrogen) for glyceraldehyde 3-phosphate dehydrogenase amplification and 1 : 5 for IL-2 and IL-4 amplification, or left undiluted for IFN-γ amplification. Briefly, 2·5 μl of cDNA was added to 10 μl QuantiTect SYBR Green (Qiagen Inc., Mississauga, ON), 2·4 μl of 1·25 μm forward and reverse primers, and 5·1 μl diethylpyrocarbonate-treated water for a total volume of 20 μl per tube. Primer sequences used for cDNA amplification were as follows: glyceraldehyde 3-phosphate dehydrogenase (forward: 5′-CGT CCC GTA GAC AAA ATG GT-3′; reverse: 5′-TTG ATG GCA ACA ATC TCC AC-3′); IL-2 (forward: 5′-AAC TCC CCA GGA TGC TCA C -3′; reverse: 5′-CGC AGA GGT CCA AGT TCA TC-3′); IL-4 (forward: 5′-TGA ACG AGG TCA CAG GAG AA-3′; reverse: 5′-CGA GCT CAC TCT CTG TGG TG-3′); IFN-γ (forward: 5′-ACA GCA AGG CGA AAA AGG AT-3′; reverse: 5′-TGA GCT CAT TGA ATG CTT GG-3′).
The amplification protocol for all the above genes was the following: Taq DNA polymerase was activated for 15 min at 95° followed by 40 cycles of denaturation for 15 seconds at 95° and annealing for 30 seconds at 60°. A melt analysis was always performed post-cDNA amplification to ensure that only one PCR product was being amplified. Data were acquired on a Rotor-Gene 3000 (Corbett Research, Mortlake, Australia) and analysed using the ΔΔCt method.36 This method was applicable because all primers had 100% efficiency as judged by linear regression analysis of a standard cDNA dilution series. Messenger RNA levels were expressed relative to untreated cells, which were assigned an arbitrary expression ratio of 1.
Human peripheral blood mononuclear cell isolation
All human work was performed in accordance with a protocol approved by The University of Western Ontario Research Ethics Board for Health Sciences Research Involving Human Subjects. Peripheral blood was collected from healthy volunteers (men and women, ranging in age from 23 to 44 years) and two patients (one man and one woman) with paroxysmal nocturnal haemoglobinuria into heparin-containing vacutainer tubes and subsequently diluted with an equal volume of PBS. The diluted blood was overlaid onto a Ficoll-Paque gradient (GE Healthcare) and spun at 800 g for 30 min. Peripheral blood mononuclear cells (PBMCs) forming the buffy coat layer were collected, washed and spun three times in PBS, twice at 456 g and once at 233 g to remove platelets, before being resuspended in complete medium.
Human iNKT cell proliferation
Human PBMCs were incubated with 5 μm carboxyfluorescein succinimidyl ester (CFSE; Molecular Probes, Eugene, OR) for 15 min at 37°. Cells were subsequently washed and incubated in complete medium. The CFSE-stained cells were seeded at 3 × 106 cells/well in a 24-well plate. Some wells were previously coated overnight with 10 μg/ml anti-CD55 mAb diluted in PBS. In addition to plate-bound anti-CD55 mAb, αGC was added to some cultures. On day 6, cells were harvested and iNKT cells were identified through staining with APC-conjugated human CD1d tetramer. In some experiments, iNKT cells were co-stained with both tetramer and APC-eFluor780-conjugated anti-CD3 mAb. After data acquisition on a BD FACSCanto II, CD1d tetramer+iNKT cells were gated upon and their CFSE dilution was assessed as a measure of cellular proliferation.
Statistical analysis
Data are shown as mean ± SD in triplicate wells of independent experiments yielding similar results. Statistical analyses were conducted with the aid of GraphPad prism software. Statistical comparisons were performed using Student's t-test and differences were considered statistically significant at P-values < 0·05, where *, ** and *** denote P<0·05, P <0·01 and P<0·001, respectively. Experiments were repeated three times unless otherwise stated.
Results
Thy-1 is strongly expressed on both resting and activated mouse iNKT cells
Following its discovery almost half a century ago, Thy-1 was adopted as a marker for thymus-derived, peptide: MHC-responsive mouse lymphocytes.37 Whether glycolipid-reactive, CD1d-restricted NKT cells, which are profoundly different from conventional T cells, express functional Thy-1 is not known. To begin to address this question, we used DN32.D3 mouse iNKT cells that are known to express ample CD1d34 and the characteristic Vα14-Jα18 invariant TCR.4 The αGC-induced activation of DN32.D3 cells does not require the co-presence of accessory cells in culture, which provides us with a clean system in which to study the expression and function of Thy-1 in iNKT cells.
DN32.D3 cells were left untreated or were stimulated with 100 ng/ml αGC for 24 hr. The optimal αGC dose and time point for DN32.D3 cell stimulation were determined in previous experiments based on their ability to secrete IL-2, which is the main readout for mouse iNKT cell lines.34 Both unstimulated and αGC-stimulated DN32.D3 cells were subsequently stained with a fluorochrome-labelled anti-Thy-1.2 mAb and exhibited high expression levels of Thy-1 (Fig. 1a).
Figure 1.
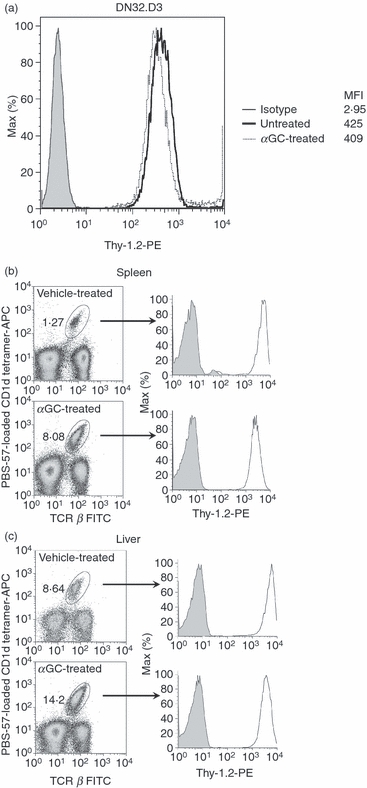
Thy-1 is expressed on a mouse invariant natural killer (iNKT) cell line and primary iNKT cells. DN32.D3 mouse iNKT cells were left untreated or stimulated with α-galactosylceramide (αGC; 100 ng/ml) for 24 hr. Cells were harvested and stained with phycoerythrin (PE)-conjugated anti-Thy-1.2 monoclonal antibodies (mAb) (open histograms) or isotype control (filled histogram). The expression of Thy-1 was analysed by flow cytometry and the mean fluorescence intensity was calculated (a). C57BL/6 mice were injected intraperitoneally with 2 μg αGC or vehicle. Splenocytes (b) and hepatic lymphoid mononuclear cells (c) were prepared 72 hr after injection. Cells were stained with a FITC-conjugated anti-T-cell receptor-β mAb, allophycocyanin-conjugated CD1d tetramer, and either PE-conjugated anti-Thy-1.2 mAb (open histogram) or isotype control (filled histogram). T-cell receptor-β+ CD1d tetramer+iNKT cells were gated on and Thy-1 expression was analysed.
Next, we examined the expression of Thy-1 on primary iNKT cells. Mice received a single αGC dose of 2 μg or its corresponding vehicle intraperitoneally. Three days later, a time-point at which iNKT cell frequency is known to reach its peak following αGC injection,35 mice were killed and their spleen and liver were isolated. Consistent with previous reports,35,38αGC treatment was able to vigorously expand splenic and hepatic iNKT cell populations that exhibit a TCR β+ CD1d tetramer+ phenotype (Fig. 1b,c). Thy-1 was found to be strongly expressed on both splenic and hepatic iNKT cells examined in their steady and activated states. As predicted, conventional T cells (TCR β+ CD1d tetramer−) were also high Thy-1 expressors, whereas non-T cells (TCR β−) did not show marked expression of Thy-1 (data not shown). Control Jα18−/− mice that are devoid of iNKT cells29 did not exhibit any staining with the mouse CD1d tetramer reagent used in these experiments as predicted (data not shown). Collectively, these data establish that both a mouse iNKT cell line and primary iNKT cells abundantly express Thy-1 regardless of their activation status.
Thy-1 cross-linking on iNKT cells results in robust cytokine secretion
Although Thy-1 is known to play a role in non-specific polyclonal responses of conventional T cells,26,39 whether its triggering can bypass the requirement for recognition of glycolipid: CD1d complexes by iNKT cells remains essentially unexplored. Given that the physiological ligand for T cell-expressed Thy-1, if it exists, is still unknown, we incubated DN32.D3 cells with increasing doses of an anti-Thy-1 mAb (clone G7), which is known to cross-link this tiny GPI-anchored protein in soluble form and without any requirement for a secondary antibody.25 Stimulation of DN32.D3 cells with G7, but not with a rat IgG2c isotype control, led to robust IL-2 production that occurred in a dose-dependent fashion and reached a plateau at a mAb dose of 40 μg/ml (Fig. 2a).
Figure 2.
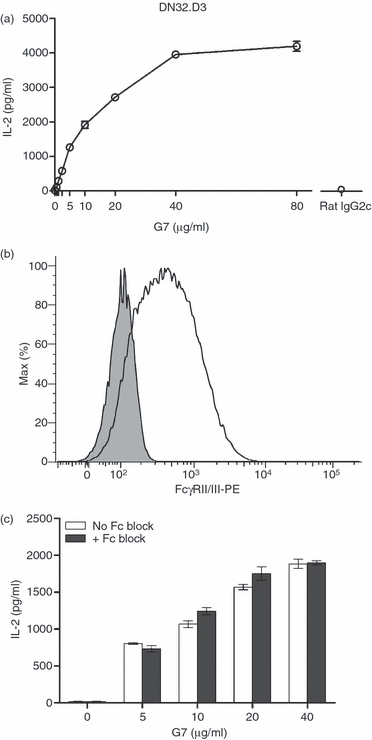
Thy-1 cross-linking leads to mouse invariant natural killer (iNKT) cell activation in an Fcγ receptor (FcγR)-independent fashion. DN32.D3 cells were incubated with increasing doses of a soluble anti-Thy-1 monoclonal antibody (mAb; clone G7) or an isotype control (rat IgG2c). After 24 hr, culture supernatants were harvested and interleukin-2 (IL-2) levels were measured by ELISA (a). DN32.D3 cells were stained with a phycoerythrin-conjugated anti-FcγRII/III mAb (open histogram) or isotype control (filled histogram). FcγRII/III expression on cells was analysed by flow cytometry (b). FcγRs expressed by DN32.D3 cells were blocked by treatment with an anti-FcγRII/III mAb (Fc Block). After 20 min, indicated doses of soluble G7 were added into the cultures. Culture supernatants were harvested after 24 hr and the IL-2 content of the samples was quantified by ELISA (c). Data are representative of two independent experiments yielding similar results.
It is noteworthy that iNKT cells bear surface Fcγ receptors (FcγRs),40 and we confirmed that DN32.D3 cells express substantial levels of FcγRII/III (Fig. 2b). To ensure that iNKT cell activation by G7 was a consequence of Thy-1 triggering and not the result of potential binding of G7 to FcγRs, DN32.D3 cells were incubated with an anti-FcγRII/III mAb (Fc Block) before stimulation with increasing concentrations of G7. The blockade of FcγRs failed to prevent or attenuate G7-induced cytokine production (Fig. 2c), indicating that iNKT cell activation by G7 was indeed caused by Thy-1 cross-linking. This is consistent with our previous report that the presence of FcγR-bearing cells is not required for G7-mediated activation of conventional T cells,26 which occurs probably because of the cross-linking of a large number of Thy-1 molecules on T or iNKT cells (Fig. 1), or may simply be a reflection of a potential self-aggregating characteristic of the G7 anti-Thy-1 mAb per se.
Thy-1 cross-linking augments iNKT cell responses to αGC
Invariant natural killer T cells express a number of co-stimulatory molecules shared by conventional T cells. However, one cannot always assume similar functional outcomes arising from the engagement of these molecules in iNKT and T cells. Resting iNKT cells have a pre-activated or ‘memory-like’ phenotype, which is manifest even in germ-free animals41 and in human cord blood.42 This suggests that iNKT cells may have a lower threshold for activation, co-stimulation and elicitation of effector responses in comparison with naive conventional T cells. Furthermore, although several transmembrane proteins have been reported to co-stimulate iNKT cells, whether cross-linking of GPI-anchored proteins, typified by Thy-1, augments iNKT cell responses to glycolipid antigens is not known.
We addressed this question by simultaneously stimulating DN32.D3 cells with αGC and anti-Thy-1 mAb (G7). A mAb concentration of 5 μg/ml was adopted as the low dose G7 because it induced slightly less than the half maximal IL-2 response by DN32.D3 cells, and a G7 dose of 40 μg/ml was selected as the high dose at which the IL-2 response began to plateau (Fig. 2a). When DN32.D3 cells were stimulated concomitantly with αGC and either a low or high dose of G7, they secreted significantly greater amounts of IL-2 in comparison with treatment with αGC or G7 alone (Fig. 3a). This clearly demonstrates that Thy-1 cross-linking enhances αGC-mediated iNKT cell activation.
Figure 3.
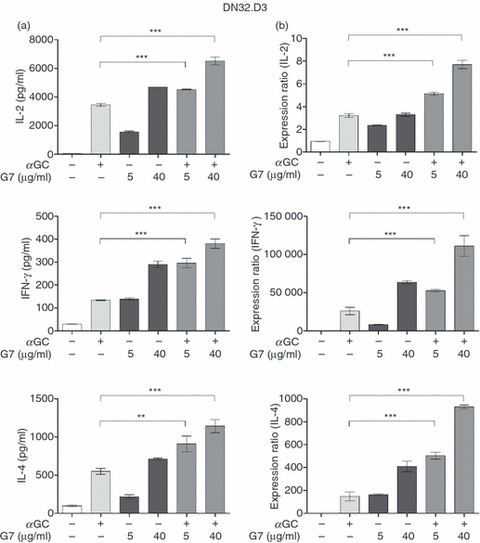
Thy-1 cross-linking enhances α-galactosylceramide (αGC)-mediated cytokine production by mouse CD4−CD8− invariant natural killer (iNKT) cells. DN32.D3 cells were incubated with αGC alone (100 ng/ml), with a low (5 μg/ml) or high (40 μg/ml) dose of G7, or with a combination of αGC and G7. After 24 hr, culture supernatants were harvested and interleukin-2 (IL-2), interferon-γ (IFN-γ) and IL-4 levels were measured by ELISA (a). After 24 hr, cells were harvested and total RNA was extracted. RNA was reverse transcribed into cDNA, which was then amplified by quantitative PCR using primers specific for IL-2, IFN-γ and IL-4. Cytokine mRNA levels were normalized to glyceraldehyde 3-phosphate dehydrogenase and the normalized values were graphed as expression ratios. Expression ratios were relative to untreated cells, which were assigned a value of 1 (b). Statistical significance is denoted by asterisks, where ** and *** represent P < 0·01 and P < 0·001, respectively.
We also quantified the levels of IFN-γ and IL-4, prototype Th1- and Th2-type cytokines released by iNKT cells, respectively, in culture supernatants. The Thy-1 cross-linking by low- and high-dose G7 alone induced IFN-γ and IL-4 secretion by DN32.D3 cells (Fig. 3a). Furthermore, G7 was able to augment glycolipid-mediated IFN-γ and IL-4 production in a similar manner to that seen with IL-2. Similar results were obtained when we used the CD4+iNKT cell line N38-2C12 (Fig. 4), suggesting that enhanced TCR-mediated cytokine production after Thy-1 cross-linking was not influenced by CD4 expression and that our findings are applicable to both double-negative (CD4−CD8−) and CD4+iNKT cells.
Figure 4.
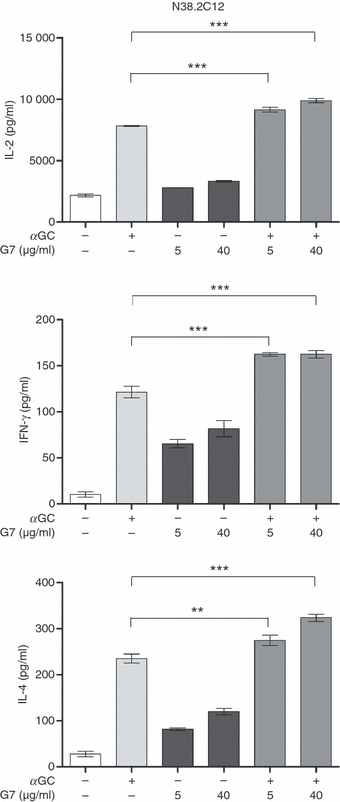
Thy-1 cross-linking augments α-galactosylceramide (αGC)-mediated cytokine secretion by mouse CD4+ invariant natural killer (iNKT) cells. N38-2C12 cells were co-incubated with bone marrow-derived dendritic cells and stimulated with αGC alone (100 ng/ml), with a low (5 μg/ml) or high (40 μg/ml) dose of G7, or with a combination of αGC and G7. After 24 hr, culture supernatants were harvested and interleukin-2 (IL-2), interferon-γ (IFN-γ) and IL-4 levels were measured by ELISA. ** and *** represent P < 0·01 and P < 0·001, respectively. Adding G7 to bone marrow-derived dendritic cells alone did not result in cytokine secretion (not shown).
While considered a reliable measure of cellular activation, the cytokine content of culture supernatants does not always necessarily reflect de novo cytokine synthesis. This is particularly important in the case of iNKT cells because they uniquely contain pre-formed mRNA for pro-inflammatory and anti-inflammatory cytokines, which explains the rapidity with which they secrete these cytokines.43,44 Importantly, the initial burst of cytokines from iNKT cells may be independent of certain co-stimulatory molecules such as CD40 ligand.44 Therefore, it was of interest to determine whether the observed co-stimulatory function of Thy-1 correlated exclusively with enhanced cytokine secretion or reflected increased cytokine production at both the mRNA and protein levels. We therefore quantified mRNA transcripts for IL-2, IFN-γ and IL-4 in DN32.D3 cells stimulated with αGC and/or G7. These cells exhibited substantial levels of mRNA for IL-2, but little to no IFN-γ or IL-4 in their steady state. Nevertheless, the expression ratios of all these cytokines were significantly greater in cultures receiving a combination of αGC and G7 compared with cultures receiving either treatment alone (Fig. 3b), which is consistent with what was observed at the secreted protein level (Fig. 3a).
To translate our findings from mouse cell lines to primary iNKT cells, we examined the consequences of Thy-1 cross-linking on hepatic NKT cells. Cultures containing iNKT cells that were sorted based on their binding to CD1d tetramer contained high background cytokine levels (data not shown), which is consistent with their partial activation by CD1d tetramer reagents leading to spontaneous cytokine secretion.38 Therefore, we stained and isolated hepatic NKT cells based on their concomitant expression of TCR β and NK1.1 and according to standard protocols. In our hands, the vast majority of these TCR β+ NK1.1+ cells are iNKT cells as evidenced by their reactivity with glycolipid-loaded CD1d tetramer (Fig. 5a). Thy-1 cross-linking by G7 alone induced marked IFN-γ and IL-4 production by freshly isolated hepatic NKT cells (Fig. 5b,c). Furthermore, co-stimulation with G7 boosted cytokine production in response to αGC.
Figure 5.
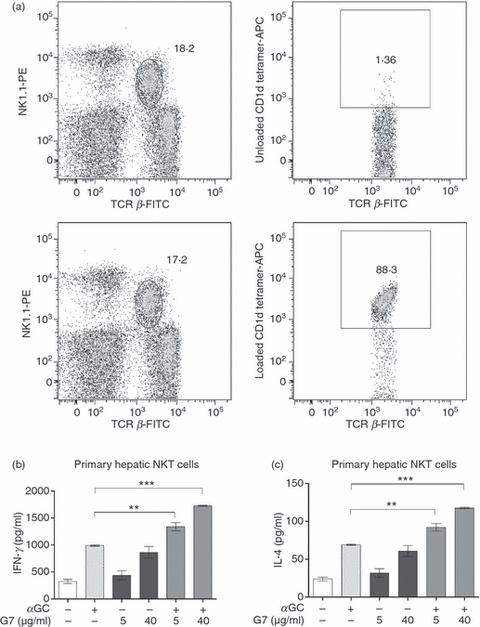
Thy-1 cross-linking boosts α-galactosylceramide (αGC)-mediated cytokine secretion by primary mouse natural killer (NKT) cells. Hepatic lymphoid mononuclear cells were stained with a FITC-conjugated anti-T-cell receptor-β (TCR β) mAb, a phycoerythrin-conjugated anti-NK1.1 monoclonal antibody and either PBS-57-loaded or -unloaded CD1d tetramer (labelled with allophycocyanin). TCR-β+ NK1.1+ cells were gated on (a; left panels) and assessed for their reactivity with CD1d tetramer reagents (a; right panels). Highly pure TCR β+ NK1.1+ hepatic NKT cells were sorted and co-cultured with bone marrow-derived dendritic cells at a ratio of five iNKT cells to one bone marrow-derived dendritic cell. Cells were incubated with αGC alone (100 ng/ml), a low (5 μg/ml) or high (40 μg/ml) dose of G7, or a combination αGC and G7. After 48 hr, culture supernatants were harvested and interferon-γ (IFN-γ) levels (b) and interleukin-4 (IL-4) levels (c) were measured by ELISA. Statistical significance is denoted by asterisks, where ** and *** represent P < 0·01 and P < 0·001, respectively.
Classical co-stimulatory molecules such as CD28 and CD40 ligand have been reported to contribute differentially to the regulation of Th1 and Th2 functions of iNKT cells.15 We therefore determined whether Thy-1 cross-linking creates a pronounced bias towards either a Th1 or Th2 phenotype in NKT cells. We calculated the ratios of IFN-γ : IL-4 production by hepatic NKT cells in different groups. These ratios were then compared with αGC-treated cells, which were assigned an arbitrary value of 1. We did not find any difference between αGC-activated NKT cells and cells activated with a combination of αGC and G7 in this regard (data not shown). Collectively, our findings demonstrate that Thy-1 triggering can enhance classical TCR-mediated activation of NKT cells without any cytokine-biased Th response.
TCR-mediated activation of non-invariant NKT and conventional T cells is augmented by concomitant Thy-1 triggering
We previously reported that Thy-1 cross-linking enhances anti-CD3-induced activation of primary mouse T cells.26 In the present study, we compared the cytokine response of DN32.D3 iNKT cells with that of N37-1A12 cells (a non-invariant NKT cell line) and C6E1 cells (an MHC-restricted conventional T cell line) upon co-stimulation through Thy-1. In this experiment, we chose plate-coated anti-CD3 mAb as the source of signal 1 for two reasons. First, N37-1A12 and C6E1 cells are not responsive to αGC. Second, this approach enabled us to properly control for the intensity of signal 1. Therefore, using anti-CD3 mAb to trigger the TCR of iNKT and non-iNKT cell lines allowed for a true head-to-head comparison of these cell types. We found that Thy-1 cross-linking augments anti-CD3-induced IL-2 secretion by DN32.D3, N37-1A12 and C6E1 cells alike (Fig. 6). This strongly suggests that GPI-anchored proteins fulfil a similar co-stimulatory role in both NKT and conventional T cells.
Figure 6.
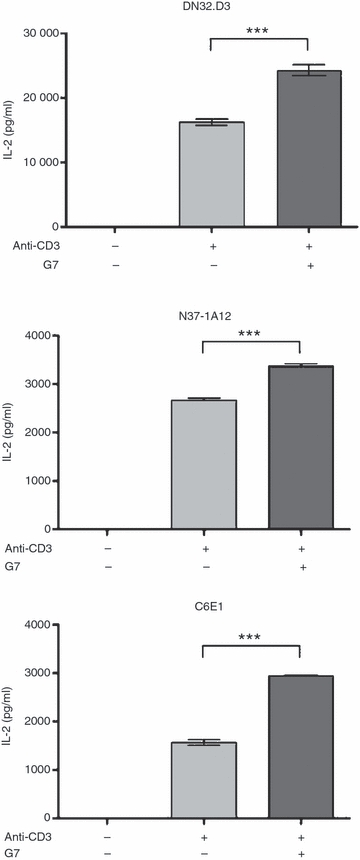
Thy-1 cross-linking augments the interleukin-2 (IL-2) response of invariant natural killer (iNKT) cell, non-invariant NKT cell and conventional T cell lines. DN32.D3, N37-1A12 and C6E1 cells were stimulated with plate-coated anti-CD3 (10 μg/ml for overnight coating at 4°) in the absence or presence of soluble G7 (40 μg/ml). Culture supernatants were harvested 24 hr later and assayed for IL-2. *** denotes a statistically significant difference with a P-value < 0·001.
Cross-linking of Ly-6A/E co-stimulates iNKT cells
To examine whether the observed enhancing effect of G7 merely reflects a co-stimulatory function for Thy-1 in the context of iNKT cell activation, or represents a general property of GPI-anchored proteins, we extended our studies to Ly-6A/E, another GPI-anchored protein expressed by mouse T cells. DN32.D3 cells were incubated with αGC in the presence or absence of an anti-Ly-6A/E mAb (clone D7) and the IL-2 content of culture supernatants was quantified. Unlike G7, which can cross-link Thy-1 on its own, stimulation with D7 requires the presence of accessory cells (e.g. bone marrow-derived DCs) in culture.45 Stimulation with D7 alone failed to induce IL-2 production (Fig. 7). However, Ly-6A/E cross-linking enhanced the IL-2 response of αGC-stimulated iNKT cells. Similar results were obtained when IFN-γ and IL-4 were assayed (data not shown). Therefore, iNKT cells can apparently be co-stimulated by GPI-anchored proteins other than Thy-1, strongly suggesting a general co-stimulatory function for this class of cell surface proteins in regulation of iNKT cell responsiveness.
Figure 7.
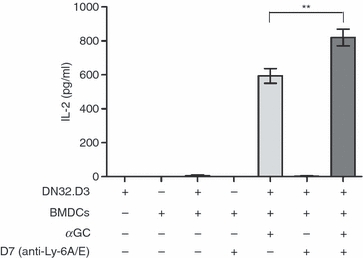
Ly-6A/E cross-linking augments α-galactosylceramide (αGC)-induced cytokine production by mouse invariant natural killer (iNKT) cells. DN32.D3 cells were co-incubated with bone marrow-derived dendritic cells and stimulated with αGC alone (100 ng/ml), anti-Ly-6A/E mAb (D7) alone (5 μg/ml), or a combination αGC and D7. After 24 hr, culture supernatants were harvested and IL-2 levels were measured by ELISA. ** denotes a statistically significant difference with a P-value < 0·01.
CD55 cross-linking enhances TCR-mediated human iNKT cell activation
In humans, Thy-1 is expressed on a small subpopulation of cortical thymocytes, but its expression is lost on peripheral conventional T lymphocytes.46 To extend our findings from mice to humans and in an effort to explore the potential co-stimulatory function of GPI-anchored proteins in human iNKT cells, we asked whether Thy-1 is present on human iNKT cells. Human PBMCs obtained from healthy donors were tested for human CD1d tetramer reactivity and iNKT cells were identified as CD3+ CD1d-tetramer+ cells. Within this small subpopulation of lymphocytes, no Thy-1 expression was observed (Fig. 8a). This was not because of the possible, but unlikely, inability of the mAb employed to stain the cells because the same mAb showed strong reactivity with human mesenchymal stem cells that were used as positive controls because of their known expression of Thy-147 (data not shown).
Figure 8.
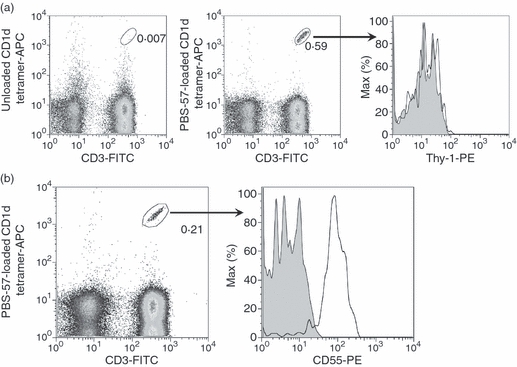
CD55, but not Thy-1, is expressed on human peripheral blood invariant natural killer (iNKT) cells. Peripheral blood was collected from healthy donors and mononuclear cells were isolated using a Ficoll-Paque gradient. Cells were stained with a FITC-conjugated anti-CD3 monoclonal antibody (mAb), allophycocyanin-conjugated human PBS-57-unloaded (control) or -loaded CD1d tetramer, and a phycoerythrin-conjugated anti-Thy-1 mAb (open histogram) or isotype control (filled histogram). CD3+ CD1d tetramer+iNKT cells were gated on and Thy-1 expression was analysed by flow cytometry (a). Cells were stained with a FITC-conjugated anti-CD3 mAb, allophycocyanin-conjugated human CD1d tetramer, and a phycoerythrin-conjugated anti-CD55 mAb (open histogram) or isotype control (filled histogram). CD3+ CD1d tetramer+iNKT cells were gated on and CD55 expression was analysed by flow cytometry (b). Data are representative of at least two independent experiments yielding similar results.
As Thy-1 is absent from the human iNKT cell membrane, to explore the potential co-stimulatory capacity of human GPI-anchored proteins, we shifted our focus from Thy-1 to CD55. Also known as decay-accelerating factor, CD55 is a GPI-anchored protein with wide expression among human PBMCs, and is best known for its regulatory function in the complement activation cascade.27 However, CD55 has also been implicated in co-stimulation of conventional human T cells because co-engagement of CD55 and CD3 on CD4+ T cells results in enhanced T cell proliferation, CD25 and CD69 activation marker expression, and IL-10 and GM-CSF secretion.28 Therefore, CD55 was an excellent candidate GPI-anchored protein for our studies in human iNKT cells. Unlike Thy-1, CD55 was strongly expressed on human peripheral blood iNKT cells (Fig. 8b), enabling us to investigate whether CD55 plays a role in co-stimulation of human iNKT cells. Hence, CFSE-stained human PBMCs were incubated with plate-bound anti-CD55 mAb alone, αGC, or a combination of both. The iNKT cell proliferation, as judged by CFSE dye dilution, was measured 6 days later as an indicator of cellular activation. CD55 cross-linking alone did not provoke proliferation by iNKT cells, but enhanced iNKT cell proliferation in response to αGC (Fig. 9a). This was a consistent observation for PBMCs obtained from several donors (Fig. 9b). In control experiments, a plate-bound mouse IgG1 mAb (the isotype control for anti-CD55) failed to augment the proliferative response of αGC-stimulated iNKT cells (data not shown). In addition to proliferation, the expression of the early activation marker CD69 by iNKT cells was heightened following simultaneous αGC stimulation and CD55 cross-linking when compared with levels seen in cells treated with αGC alone (Fig. 9c,d). Taken together, these results clearly indicate that when coupled with a classical TCR-mediated activation signal, CD55 is capable of co-stimulating human iNKT cell responses.
Figure 9.
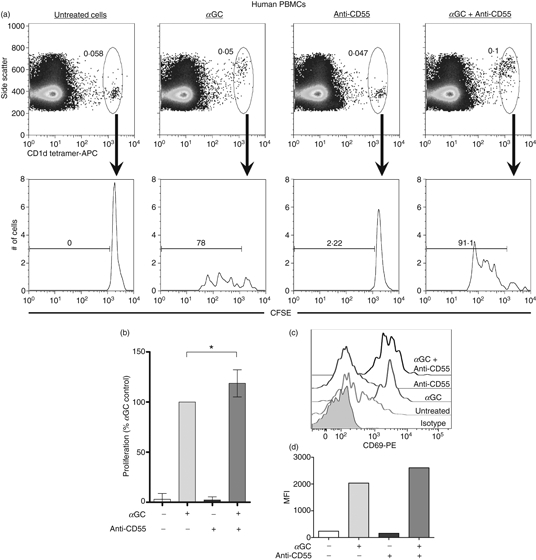
CD55 cross-linking enhances T-cell receptor-mediated human invariant natural killer (iNKT) cell activation. Human peripheral blood mononuclear cells (PBMCs) were labelled with 5 μm carboxyfluorescein succinimidyl ester (CFSE) and seeded into a 24-well plate. Some wells contained a plate-bound anti-CD55 monoclonal antibody (mAb) and some wells received 100 ng/ml α-galactosylceramide (αGC). After 6 days of culture, cells were harvested and stained with allophycocyanin (APC)-conjugated PBS-57-loaded human CD1d tetramer. Using flow cytometry, CD1d tetramer+iNKT cells were gated upon and CFSE dilution was evaluated as a measure of iNKT cell proliferation. Data are representative of four independent experiments yielding similar results (a). The proliferative responses of human iNKT cells from four healthy donors were averaged and graphed. For each donor, treatment with αGC alone served as the response control and was assigned a value of 100% for comparison purposes. The proliferation values resulting from all other treatment groups were expressed as a percentage of the control (b). Human PBMCs were stimulated with αGC and/or a plate-bound anti-CD55 mAb for 6 days, harvested and stained with an APC-eFluor780-conjugated anti-CD3 mAb, APC-conjugated human CD1d tetramer, and a phycoerythrin-conjugated anti-CD69 mAb (open histograms) or isotype control (filled histogram). CD3+ CD1d tetramer+iNKT cells were gated upon and CD69 expression was evaluated as an indicator of iNKT cell activation (c). Mean fluorescence intensity (MFI) values for each treatment group were graphed for one donor (d). Similar results were obtained for three donors.
Discussion
In this study, we explored a role for GPI-anchored proteins in modulation of iNKT cell responses. We demonstrate that cross-linking of mouse Thy-1, mouse Ly-6A/E and human CD55 results in enhanced iNKT cell responses to αGC.
Co-stimulation is a critical requirement for priming naive conventional T cells because triggering their TCR by peptide: MHC complexes in the absence of a co-stimulatory signal may lead to anergy rather than activation.48 In contrast, iNKT cells occur in a ‘pre-activated’ state and may have a less stringent requirement for co-stimulation. Nevertheless, several membrane-spanning cell surface molecules with documented co-stimulatory functions modulate iNKT cell responses. Here, we demonstrated that the GPI-anchored protein Thy-1 can serve in the capacity of co-stimulatory molecule for both iNKT cell lines and primary mouse NKT cells without skewing their responsiveness towards a Th1- or Th2-type cytokine secretion profile. This is unlike certain other co-stimulatory molecules, such as CD28 and CD40 ligand, whose ligation may result in biased cytokine secretion by iNKT cells.15
The vast majority of hepatic NKT cells sorted based on their co-expression of TCR β and NK1.1 express the invariant TCR. Furthermore, αGC used as a signal-1 stimulus can only activate iNKT cells present among sorted cells. Therefore, the observed effects on primary hepatic NKT cells are highly likely to represent the responsiveness of iNKT cells.
In this study, we used mouse hepatic NKT cells because of their abundance in this organ. It is important to note that functional differences exist between NKT cells residing in different tissues. For instance, splenic and hepatic NKT cells exhibit distinct functions in regulation of anti-tumour immunity.49 In addition, although liver double-negative (CD4−CD8−) iNKT cells participate in tumour rejection, other subsets, such as thymus-derived iNKT cells and liver-derived CD4+iNKT cells, are far less potent in this capacity.49 In our experiments, CD4− CD8− and CD4+iNKT cell lines behaved similarly in response to stimulation with αGC ± anti-Thy-1 mAb. However, as far as primary iNKT cells are concerned, it will be both important and interesting to explore whether and how cross-linking of GPI-anchored proteins such as Thy-1 may affect the activation threshold, overall responsiveness and cytokine secretion profile of various iNKT cell subsets in different tissues.
To provide iNKT cells with a classical TCR-based signal 1, we typically stimulated the cells with αGC, the prototype iNKT cell agonist that can induce both pro-inflammatory and anti-inflammatory cytokines. Several synthetic analogues of αGC are capable of skewing iNKT cell responses towards either a Th1- or Th2-type secretion phenotype. For instance, OCH, an αGC analogue with a substantially shorter sphingosine chain,50 and C20:2, an αGC analogue that contains an 11,14-cis-diunsaturated C20 fatty acid,51 promote Th2 responses, whereas a C-glycoside analogue of αGC favours Th1 responses.52 Whether cross-linking of GPI-anchored proteins affects the cytokine profile of iNKT cells concomitantly receiving a Th1- or Th2-polarizing signal 1 warrants further investigation.
Unlike typical co-stimulatory molecules, Thy-1 cross-linking, on its own, was also able to induce the activation of both iNKT cell lines and primary NKT cells, albeit to a lesser extent when compared with simultaneous stimulation through TCR and Thy-1. This is consistent with a dual signalling property for Thy-1, which we previously proposed in the context of conventional T cell activation.26 Accordingly, in the absence of αGC-mediated classical TCR signalling, Thy-1 triggering can supply iNKT cells with a ‘surrogate signal 1’. However, under the circumstances when the canonical TCR of iNKT cells provides signal 1, Thy-1 may function in the capacity of a co-stimulatory molecule.
Several proteins have been suggested to function as a ligand or counter-receptor for Thy-1. Mouse, rat and human Thy-1 contain a conserved RGD-like sequence that serves as a binding motif for integrins αVβ3 and αMβ2, thereby enabling Thy-1 expressed by EL-4 thymoma cells to interact with integrin β3 on astrocytes.53 In addition, Thy-1 on PMA-stimulated human dermal microvascular endothelial cells can interact with integron αMβ2 on leucocytes, hence mediating leucocyte firm adhesion to activated endothelium and promoting leucocyte transendothelial migration.54 However, a ligand for T cell-associated Thy-1 remains to be discovered. Therefore, we took advantage of a stimulatory anti-Thy-1 mAb (clone G7) to cross-link Thy-1 in our study. Thy-1 triggering by this frequently used antibody is presumed to mimic Thy-1 cross-linking by its putative physiological ligand.26 Although using an anti-Thy-1 mAb may not completely represent the function of putative Thy-1 ligand, it is reassuring that, in cases where the ligands for GPI-anchored proteins are known, cognate ligation of these proteins has yielded similar results to those obtained using cross-linking mAbs.55
Our understanding of Thy-1-coupled signal transduction pathway(s) is far from clear. Using a panel of pharmacological inhibitors of intracellular signalling intermediates, we previously demonstrated that Thy-1- and TCR/CD3-associated pathways are not identical.56 At the cell membrane level, the possibility that T and iNKT cell activation by Thy-1 cross-linking alone may be merely the result of Thy-1 localization within lipid rafts by virtue of its GPI anchor does not seem likely. This is because cross-linking some other GPI-anchored proteins (e.g. mouse Ly-6A/E and human CD55 in this study) alone fails to induce a similar result. Thy-1 is one of the most heavily expressed proteins of the T cell membrane. This is in contrast with CD55, for example, that is only present at approximately 9000 copies per cell.27 We found that Thy-1 is constitutively strongly expressed by mouse iNKT cells at mean fluorescence intensity values that are much higher than those seen for CD55 on human iNKT cells (our unpublished data). It is therefore possible that the anti-mouse Thy-1 mAb G7 can, by itself, cross-link a high number of Thy-1 molecules on the surface of iNKT cells, which is sufficient to generate an activating signal.
Our findings that mouse iNKT cells can be co-stimulated through Thy-1 or Ly-6A/E support our hypothesis that GPI-anchored proteins should be generally regarded as co-stimulators of mouse iNKT cell functions. By focusing on CD55, which, unlike Thy-1, is expressed by human T and iNKT cells, we extended our findings from mouse to human cells. Triggering CD55 led to enhanced human iNKT cell activation in response to αGC as judged by their increased proliferation and CD69 expression. Although these experiments were conducted on unfractionated PBMCs, it is important to note that PBMCS incubated with plate-coated anti-CD55 mAb alone do not show any signs of activation, and iNKT cells are the only αGC-responsive population among human PBMCs. Therefore, we can claim with confidence that our findings reflect the functional aspects of human iNKT cells. Nevertheless, it will be interesting to repeat these experiments with highly pure human iNKT cell lines or clones such as those that can be maintained and expanded by non-specific stimuli such as phytohaemagglutinin, although such cells may not be a true representative of intact human iNKT cells.
Although some members of the co-stimulatory machinery of the immune system may have redundant functions, it will be interesting to examine the presence and functionality of iNKT cells in Thy-1-deficient mice. Endogenous GPI anchor can bind CD1d and be presented to iNKT cells,57 and the ability of pathogen-derived GPI to activate iNKT cells remains controversial.58,59 Nevertheless, whether NKT cell development can be affected by the absence of GPI is unknown. Deficiency of GPI-anchored proteins, including but not restricted to CD55, is encountered in a rare, acquired disorder of the haematopoietic system called paroxysmal nocturnal haemoglobinuria (PNH), which results from mutations in the phosphatidylinositol glycan complementation class A (PIG-A) gene.60 We had the opportunity to evaluate PBMCs obtained from two patients with PNH (one woman and one man, both in their 20s) for the presence of CD3+ CD1d-tetramer+iNKT cells. These patients had relatively increased, rather than decreased peripheral iNKT cell frequencies (0·28% and 0·095% of total PBMCs, respectively) when compared with a pool of 22 healthy donors with a median iNKT cell frequency of 0·029. This suggests that endogenous GPI may not be required for iNKT cell development in humans, although caution needs to be exercised not only because of the small patient sample size, but also because of the clonal nature of PNH. In many PNH patients, unlike the presence of large numbers of GPI−/− myeloid cells, GPI−/− lymphocytes tend to be present in low frequencies. Therefore, the impact of GPI deficiency on lymphocyte functions cannot be definitively determined in PNH. To overcome this obstacle in a mouse model, Bessler et al.61 used PIG-A−/− embryonic stem cells to reconstitute the immune system of Rag−/− mice. Although normal lymphocyte function was altered in these mice, the proportion of CD4+ and CD8+ T lymphocytes was similar to those in control animals. It will be informative to use a similar approach for examination of iNKT cell development and responsiveness in the complete absence of GPI-anchored proteins.
Taken together, our results demonstrate for the first time that triggering GPI-anchored proteins, best exemplified by mouse Thy-1, mouse Ly-6A/E and human CD55, can augment iNKT cell responses. Human and mouse iNKT cells are functionally homologous to the extent that human iNKT cells recognize mouse CD1d and vice versa62 In fact, αGC has been employed not only in experimental studies in mouse models, but also in several clinical trials for cancer12–14 and viral diseases.63,64 Our finding that GPI-anchored proteins enhance iNKT cell responses to this glycolipid may therefore open new avenues of investigation for iNKT cell-based immunotherapeutic manipulation in a wide range of diseases, including cancer and infectious diseases.
Acknowledgments
S.M.M.H. is Canada Research Chair in Viral Immunity and Pathogenesis. This work was supported by grants from The Cancer Research Society Inc. and the Canadian Institutes of Health Research to S.M.M.H. L.A.M. was a recipient of a Canada Graduate Scholarship from the Natural Sciences and Engineering Research Council of Canada. We are grateful to Delfina Mazzuca Siroen, Jin Hayatsu, Dr Marianne van den Heuvel and Dr Mantej Bharhani for their expert technical assistance.
Disclosures
The authors declare no conflict of interest.
References
- 1.Kronenberg M. Toward an understanding of NKT cell biology: progress and paradoxes. Annu Rev Immunol. 2005;23:877–900. doi: 10.1146/annurev.immunol.23.021704.115742. [DOI] [PubMed] [Google Scholar]
- 2.Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu Rev Immunol. 2007;25:297–336. doi: 10.1146/annurev.immunol.25.022106.141711. [DOI] [PubMed] [Google Scholar]
- 3.Fowlkes BJ, Kruisbeek AM, Ton-That H, et al. A novel population of T-cell receptor αβ-bearing thymocytes which predominantly expresses a single Vβ gene family. Nature. 1987;329:251–4. doi: 10.1038/329251a0. [DOI] [PubMed] [Google Scholar]
- 4.Lantz O, Bendelac A. An invariant T cell receptor α chain is used by a unique subset of major histocompatibility complex class I-specific CD4+ and CD4–8– T cells in mice and humans. J Exp Med. 1994;180:1097–106. doi: 10.1084/jem.180.3.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morita M, Motoki K, Akimoto K, et al. Structure-activity relationship of α-galactosylceramides against B16-bearing mice. J Med Chem. 1995;38:2176–87. doi: 10.1021/jm00012a018. [DOI] [PubMed] [Google Scholar]
- 6.Kawano T, Cui J, Koezuka Y, et al. CD1d-restricted and TCR-mediated activation of vα14 NKT cells by glycosylceramides. Science. 1997;278:1626–9. doi: 10.1126/science.278.5343.1626. [DOI] [PubMed] [Google Scholar]
- 7.Carnaud C, Lee D, Donnars O, et al. Cutting edge: cross-talk between cells of the innate immune system: NKT cells rapidly activate NK cells. J Immunol. 1999;163:4647–50. [PubMed] [Google Scholar]
- 8.Eberl G, MacDonald HR. Selective induction of NK cell proliferation and cytotoxicity by activated NKT cells. Eur J Immunol. 2000;30:985–92. doi: 10.1002/(SICI)1521-4141(200004)30:4<985::AID-IMMU985>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 9.Galli G, Nuti S, Tavarini S, et al. Innate immune responses support adaptive immunity: NKT cells induce B cell activation. Vaccine. 2003;21(Suppl. 2):S48–54. doi: 10.1016/s0264-410x(03)00200-7. [DOI] [PubMed] [Google Scholar]
- 10.Stober D, Jomantaite I, Schirmbeck R, Reimann J. NKT cells provide help for dendritic cell-dependent priming of MHC class I-restricted CD8+ T cells in vivo. J Immunol. 2003;170:2540–8. doi: 10.4049/jimmunol.170.5.2540. [DOI] [PubMed] [Google Scholar]
- 11.Smyth MJ, Godfrey DI. NKT cells and tumor immunity – a double-edged sword. Nat Immunol. 2000;1:459–60. doi: 10.1038/82698. [DOI] [PubMed] [Google Scholar]
- 12.Haeryfar SM. Invariant natural killer T cells in immune surveillance and tumor immunotherapy: perspectives and potentials. Arch Iran Med. 2008;11:186–95. [PubMed] [Google Scholar]
- 13.Terabe M, Berzofsky JA. The role of NKT cells in tumor immunity. Adv Cancer Res. 2008;101:277–348. doi: 10.1016/S0065-230X(08)00408-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hong C, Park SH. Application of natural killer T cells in antitumor immunotherapy. Crit Rev Immunol. 2007;27:511–25. doi: 10.1615/critrevimmunol.v27.i6.20. [DOI] [PubMed] [Google Scholar]
- 15.Hayakawa Y, Takeda K, Yagita H, Van KL, Saiki I, Okumura K. Differential regulation of Th1 and Th2 functions of NKT cells by CD28 and CD40 costimulatory pathways. J Immunol. 2001;166:6012–8. doi: 10.4049/jimmunol.166.10.6012. [DOI] [PubMed] [Google Scholar]
- 16.Kaneda H, Takeda K, Ota T, et al. ICOS costimulates invariant NKT cell activation. Biochem Biophys Res Commun. 2005;327:201–7. doi: 10.1016/j.bbrc.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 17.Akbari O, Stock P, Meyer EH, et al. ICOS/ICOSL interaction is required for CD4+ invariant NKT cell function and homeostatic survival. J Immunol. 2008;180:5448–56. doi: 10.4049/jimmunol.180.8.5448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim HJ, Kim HY, Kim BK, Kim S, Chung DH. Engagement of glucocorticoid-induced TNF receptor costimulates NKT cell activation in vitro and in vivo. J Immunol. 2006;176:3507–15. doi: 10.4049/jimmunol.176.6.3507. [DOI] [PubMed] [Google Scholar]
- 19.Vinay DS, Choi BK, Bae JS, Kim WY, Gebhardt BM, Kwon BS. CD137-deficient mice have reduced NK/NKT cell numbers and function, are resistant to lipopolysaccharide-induced shock syndromes, and have lower IL-4 responses. J Immunol. 2004;173:4218–29. doi: 10.4049/jimmunol.173.6.4218. [DOI] [PubMed] [Google Scholar]
- 20.Kim DH, Chang WS, Lee YS, et al. 4-1BB engagement costimulates NKT cell activation and exacerbates NKT cell ligand-induced airway hyperresponsiveness and inflammation. J Immunol. 2008;180:2062–8. doi: 10.4049/jimmunol.180.4.2062. [DOI] [PubMed] [Google Scholar]
- 21.Zaini J, Andarini S, Tahara M, et al. OX40 ligand expressed by DCs costimulates NKT and CD4+ Th cell antitumor immunity in mice. J Clin Invest. 2007;117:3330–8. doi: 10.1172/JCI32693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ilangumaran S, He HT, Hoessli DC. Microdomains in lymphocyte signalling: beyond GPI-anchored proteins. Immunol Today. 2000;21:2–7. doi: 10.1016/s0167-5699(99)01494-2. [DOI] [PubMed] [Google Scholar]
- 23.Haeryfar SM, Hoskin DW. Thy-1: more than a mouse pan-T cell marker. J Immunol. 2004;173:3581–8. doi: 10.4049/jimmunol.173.6.3581. [DOI] [PubMed] [Google Scholar]
- 24.Bradley JE, Ramirez G, Hagood JS. Roles and regulation of Thy-1, a context-dependent modulator of cell phenotype. Biofactors. 2009;35:258–65. doi: 10.1002/biof.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gunter KC, Malek TR, Shevach EM. T cell-activating properties of an anti-Thy-1 monoclonal antibody. Possible analogy to OKT3/Leu-4. J Exp Med. 1984;159:716–30. doi: 10.1084/jem.159.3.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haeryfar SM, Al-Alwan MM, Mader JS, Rowden G, West KA, Hoskin DW. Thy-1 signaling in the context of costimulation provided by dendritic cells provides signal 1 for T cell proliferation and cytotoxic effector molecule expression, but fails to trigger delivery of the lethal hit. J Immunol. 2003;171:69–77. doi: 10.4049/jimmunol.171.1.69. [DOI] [PubMed] [Google Scholar]
- 27.Kinoshita T, Medof ME, Silber R, Nussenzweig V. Distribution of decay-accelerating factor in the peripheral blood of normal individuals and patients with paroxysmal nocturnal hemoglobinuria. J Exp Med. 1985;162:75–92. doi: 10.1084/jem.162.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Capasso M, Durrant LG, Stacey M, Gordon S, Ramage J, Spendlove I. Costimulation via CD55 on human CD4+ T cells mediated by CD97. J Immunol. 2006;177:1070–7. doi: 10.4049/jimmunol.177.2.1070. [DOI] [PubMed] [Google Scholar]
- 29.Cui J, Shin T, Kawano T, et al. Requirement for Vα14 NKT cells in IL-12-mediated rejection of tumors. Science. 1997;278:1623–6. doi: 10.1126/science.278.5343.1623. [DOI] [PubMed] [Google Scholar]
- 30.Gui M, Li J, Wen LJ, Hardy RR, Hayakawa K. TCR β chain influences but does not solely control autoreactivity of V α 14J281T cells. J Immunol. 2001;167:6239–46. doi: 10.4049/jimmunol.167.11.6239. [DOI] [PubMed] [Google Scholar]
- 31.Burdin N, Brossay L, Koezuka Y, et al. Selective ability of mouse CD1 to present glycolipids: α-galactosylceramide specifically stimulates V α 14+ NK T lymphocytes. J Immunol. 1998;161:3271–81. [PubMed] [Google Scholar]
- 32.Racioppi L, Ronchese F, Matis LA, Germain RN. Peptide–major histocompatibility complex class II complexes with mixed agonist/antagonist properties provide evidence for ligand-related differences in T cell receptor-dependent intracellular signaling. J Exp Med. 1993;177:1047–60. doi: 10.1084/jem.177.4.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lutz MB, Kukutsch N, Ogilvie AL, et al. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods. 1999;223:77–92. doi: 10.1016/s0022-1759(98)00204-x. [DOI] [PubMed] [Google Scholar]
- 34.Stuart JK, Bisch SP, Leon-Ponte M, et al. Negative modulation of invariant natural killer T cell responses to glycolipid antigens by p38 MAP kinase. Int Immunopharmacol. 2010;10:1068–76. doi: 10.1016/j.intimp.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 35.Parekh VV, Singh AK, Wilson MT, et al. Quantitative and qualitative differences in the in vivo response of NKT cells to distinct α- and β-anomeric glycolipids. J Immunol. 2004;173:3693–706. doi: 10.4049/jimmunol.173.6.3693. [DOI] [PubMed] [Google Scholar]
- 36.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Raff MC. Surface antigenic markers for distinguishing T and B lymphocytes in mice. Transplant Rev. 1971;6:52–80. doi: 10.1111/j.1600-065x.1971.tb00459.x. [DOI] [PubMed] [Google Scholar]
- 38.Berzins SP, Smyth MJ, Godfrey DI. Working with NKT cells – pitfalls and practicalities. Curr Opin Immunol. 2005;17:448–54. doi: 10.1016/j.coi.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 39.Haeryfar SM, Conrad DM, Musgrave B, Hoskin DW. Antibody blockade of Thy-1 (CD90) impairs mouse cytotoxic T lymphocyte induction by anti-CD3 monoclonal antibody. Immunol Cell Biol. 2005;83:352–63. doi: 10.1111/j.1440-1711.2005.01342.x. [DOI] [PubMed] [Google Scholar]
- 40.Kim HY, Kim S, Chung DH. FcγRIII engagement provides activating signals to NKT cells in antibody-induced joint inflammation. J Clin Invest. 2006;116:2484–92. doi: 10.1172/JCI27219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Park SH, Benlagha K, Lee D, Balish E, Bendelac A. Unaltered phenotype, tissue distribution and function of Vα14+ NKT cells in germ-free mice. Eur J Immunol. 2000;30:620–5. doi: 10.1002/1521-4141(200002)30:2<620::AID-IMMU620>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 42.D’Andrea A, Goux D, De Lalla C, et al. Neonatal invariant Vα24+ NKT lymphocytes are activated memory cells. Eur J Immunol. 2000;30:1544–50. doi: 10.1002/1521-4141(200006)30:6<1544::AID-IMMU1544>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 43.Stetson DB, Mohrs M, Reinhardt RL, et al. Constitutive cytokine mRNAs mark natural killer (NK) and NK T cells poised for rapid effector function. J Exp Med. 2003;198:1069–76. doi: 10.1084/jem.20030630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Matsuda JL, Gapin L, Baron JL, et al. Mouse V α 14i natural killer T cells are resistant to cytokine polarization in vivo. Proc Natl Acad Sci USA. 2003;100:8395–400. doi: 10.1073/pnas.1332805100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Malek TR, Ortega G, Chan C, Kroczek RA, Shevach EM. Role of Ly-6 in lymphocyte activation. II. Induction of T cell activation by monoclonal anti-Ly-6 antibodies. J Exp Med. 1986;164:709–22. doi: 10.1084/jem.164.3.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McKenzie JL, Fabre JW. Human thy-1: unusual localization and possible functional significance in lymphoid tissues. J Immunol. 1981;126:843–50. [PubMed] [Google Scholar]
- 47.Jo CH, Kim OS, Park EY, et al. Fetal mesenchymal stem cells derived from human umbilical cord sustain primitive characteristics during extensive expansion. Cell Tissue Res. 2008;334:423–33. doi: 10.1007/s00441-008-0696-3. [DOI] [PubMed] [Google Scholar]
- 48.Schwartz RH. A cell culture model for T lymphocyte clonal anergy. Science. 1990;248:1349–56. doi: 10.1126/science.2113314. [DOI] [PubMed] [Google Scholar]
- 49.Crowe NY, Coquet JM, Berzins SP, et al. Differential antitumor immunity mediated by NKT cell subsets in vivo. J Exp Med. 2005;202:1279–88. doi: 10.1084/jem.20050953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Miyamoto K, Miyake S, Yamamura T. A synthetic glycolipid prevents autoimmune encephalomyelitis by inducing TH2 bias of natural killer T cells. Nature. 2001;413:531–4. doi: 10.1038/35097097. [DOI] [PubMed] [Google Scholar]
- 51.Yu KO, Im JS, Molano A, et al. Modulation of CD1d-restricted NKT cell responses by using N-acyl variants of α-galactosylceramides. Proc Natl Acad Sci USA. 2005;102:3383–8. doi: 10.1073/pnas.0407488102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schmieg J, Yang G, Franck RW, Tsuji M. Superior protection against malaria and melanoma metastases by a C-glycoside analogue of the natural killer T cell ligand α-galactosylceramide. J Exp Med. 2003;198:1631–41. doi: 10.1084/jem.20031192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Leyton L, Schneider P, Labra CV, et al. Thy-1 binds to integrin β3 on astrocytes and triggers formation of focal contact sites. Curr Biol. 2001;11:1028–38. doi: 10.1016/s0960-9822(01)00262-7. [DOI] [PubMed] [Google Scholar]
- 54.Wetzel A, Chavakis T, Preissner KT, et al. Human Thy-1 (CD90) on activated endothelial cells is a counterreceptor for the leukocyte integrin Mac-1 (CD11b/CD18) J Immunol. 2004;172:3850–9. doi: 10.4049/jimmunol.172.6.3850. [DOI] [PubMed] [Google Scholar]
- 55.Moran M, Miceli MC. Engagement of GPI-linked CD48 contributes to TCR signals and cytoskeletal reorganization: a role for lipid rafts in T cell activation. Immunity. 1998;9:787–96. doi: 10.1016/s1074-7613(00)80644-5. [DOI] [PubMed] [Google Scholar]
- 56.Haeryfar SM, Hoskin DW. Selective pharmacological inhibitors reveal differences between Thy-1- and T cell receptor-mediated signal transduction in mouse T lymphocytes. Int Immunopharmacol. 2001;1:689–98. doi: 10.1016/s1567-5769(01)00002-9. [DOI] [PubMed] [Google Scholar]
- 57.Joyce S, Woods AS, Yewdell JW, et al. Natural ligand of mouse CD1d1: cellular glycosylphosphatidylinositol. Science. 1998;279:1541–4. doi: 10.1126/science.279.5356.1541. [DOI] [PubMed] [Google Scholar]
- 58.Schofield L, McConville MJ, Hansen D, et al. CD1d-restricted immunoglobulin G formation to GPI-anchored antigens mediated by NKT cells. Science. 1999;283:225–9. doi: 10.1126/science.283.5399.225. [DOI] [PubMed] [Google Scholar]
- 59.Molano A, Park SH, Chiu YH, Nosseir S, Bendelac A, Tsuji M. Cutting edge: the IgG response to the circumsporozoite protein is MHC class II-dependent and CD1d-independent: exploring the role of GPIs in NK T cell activation and antimalarial responses. J Immunol. 2000;164:5005–9. doi: 10.4049/jimmunol.164.10.5005. [DOI] [PubMed] [Google Scholar]
- 60.Takeda J, Miyata T, Kawagoe K, et al. Deficiency of the GPI anchor caused by a somatic mutation of the PIG-A gene in paroxysmal nocturnal hemoglobinuria. Cell. 1993;73:703–11. doi: 10.1016/0092-8674(93)90250-t. [DOI] [PubMed] [Google Scholar]
- 61.Bessler M, Rosti V, Peng Y, et al. Glycosylphosphatidylinositol-linked proteins are required for maintenance of a normal peripheral lymphoid compartment but not for lymphocyte development. Eur J Immunol. 2002;32:2607–16. doi: 10.1002/1521-4141(200209)32:9<2607::AID-IMMU2607>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 62.Brossay L, Chioda M, Burdin N, et al. CD1d-mediated recognition of an α-galactosylceramide by natural killer T cells is highly conserved through mammalian evolution. J Exp Med. 1998;188:1521–8. doi: 10.1084/jem.188.8.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Veldt BJ, van der Vliet HJ, von Blomberg BM, et al. Randomized placebo controlled phase I/II trial of α-galactosylceramide for the treatment of chronic hepatitis C. J Hepatol. 2007;47:356–65. doi: 10.1016/j.jhep.2007.04.018. [DOI] [PubMed] [Google Scholar]
- 64.Woltman AM, Ter Borg MJ, Binda RS, et al. α-Galactosylceramide in chronic hepatitis B infection: results from a randomized placebo-controlled Phase I/II trial. Antivir Ther. 2009;14:809–18. doi: 10.3851/IMP1295. [DOI] [PubMed] [Google Scholar]


