Abstract
A polymicrobial infection comprising subgingival biofilms is the trigger for the chronic immunoinflammatory lesions of periodontitis. These microbial biofilms interface with host immune cells that increase with progressing disease and could result in HIV reactivation in HIV-1-infected patients. Previous reports have focused on the ability of monospecies challenge of macrophages and dendritic cells to detail molecular aspects of their detection and signalling pathways. This study provides a seminal description of the responses of macrophages and dendritic cells to a polybacterial challenge using various oral bacteria as prototype stimuli to examine these response characteristics. The investigation employed a model of HIV-promoter activation and reactivation of HIV viral replication. Oral Gram-negative bacteria elicited significantly greater levels of HIV promoter activation and viral replication from all cell types, compared with Gram-positive bacteria. Selected combinations of oral Gram-negative bacteria elicited synergistic HIV promoter activation and viral replication in macrophages and immature dendritic cells. In mature dendritic cells, there was no synergism in HIV promoter activation and viral replication. Gram-positive bacteria showed no synergism in any cell model. These findings support the importance of determining the characteristics and impact of polybacterial challenges on immune cells to clarify the potential immune recognition and antigen processing that can occur in the oral cavity.
Keywords: Gram-negative bacteria, HIV latency, HIV promoter activation, polybacterial infection
Introduction
Periodontitis is a polymicrobial disease that presents as a chronic immunoinflammatory lesion that undermines soft tissue integrity and progresses to resorption of alveolar bone.1,2 The lesion is a result of a complex host response comprising inflammatory cells, cytokines, chemokines and mediators produced by resident gingival cells and inflammatory cells that move by chemotaxis into the infected tissues. It is clear that a polymicrobial challenge derived from subgingival biofilms triggers this response in local host tissues.3,4 Microbiological features of periodontal health, reversible inflammatory gingivitis and irreversible periodontal diseases encompass nearly 700 bacterial taxa, phylotypes and species that can colonize the oral cavity of humans. It remains unclear how the bacteria in the oral microbiome compete, co-exist and synergize to initiate this chronic disease process.3 Nevertheless, numerous studies have focused on the microbial ecology of ‘pathogenic biofilms’ to identify specific bacteria or bacterial consortia that ‘hallmark’ progressing disease.5 Major complexes have been observed in subgingival plaque,6 particularly representative of the climax community in biofilms at progressing disease sites.7 It is now reasonably certain that within the biofilm during disease, there are distinguishing bacteria, and that novel complexes or consortia of species are a trait of this environment consistent with disease and may provide a synergistic pathogenicity. During a polybacterial infection, complementary and competing signals may be given to the host tissues. The local cellular mechanisms responsible for bacterial uptake, and processing and presentation of antigens to the adaptive immune system must therefore interact with the individual microbes in the context of a polybacterial challenge, and may be differentially affected in their engagement of individual species by the complexity of the challenge.
However, few data are available elucidating how host tissues and cells interact with, and respond to, a polybacterial challenge that would occur with the complex oral microbial ecology. The introduction of highly active antiretroviral therapy (HAART) regimens has significantly modified the course of HIV disease in HIV-infected individuals.8,9 However, complete eradication of HIV infection cannot be achieved with the currently available antiretroviral regimens because of the establishment of a pool of latently infected CD4+ T cells, macrophages and other host cells (e.g. dendritic cells, mast cells) during the earliest stages of acute HIV infection that persists with an extremely long half-life.10 Various exogenous stimuli have been shown to reactivate HIV-1 infections, including microbial co-infections that can result in activation of pro-inflammatory cytokines, with subsequent induction of HIV-1 production by CD4+ T cells and macrophages.10,11 Finally, it has been suggested that dendritic cells (DCs) are potential first target cells in HIV-1 infection.12 They are also considered to be central in the activation of naive T cells, which can become permissive for HIV-1 infection.13 Gram-negative bacterial infections and mucosal-associated bacterial translocation in HIV-1-infected individuals may therefore lead to the reactivation of HIV-1, resulting from direct bacterial interaction with latently infected cells and bystander CD4+ T cells and macrophages caused by pro-inflammatory cytokines released by stimulated DCs.
The oral cavity and other mucosal sites of the body, e.g. respiratory, gastrointestinal, genitourinary, represent the entry portal for a wide array of antigenic challenges, particularly via the substantial microbial colonization that exists at mucosal sites. As a result of the chronic microbial colonization of these sites, the contiguous host tissues often demonstrate some level of localized inflammation, reflecting the locally stressed environment. These infections also result in systemic inflammatory/immune responses that demonstrate the potential for these microbial challenges to alter systemic cell functions that could contribute to changes in HIV status in the infected host.14 The potential role of oral bacteria and oral infection in HIV reactivation in the oral cavity has not been adequately addressed (C. B. Huang, Y. Alimova, J. L. Ebersole, submitted).15 The study presented here evaluated the concept that oral co-infections in HIV-infected patients represent an important risk factor for HIV recrudescence, and that the polybacterial challenge occurring during periodontitis signals HIV reactivation.
Materials and methods
Cell lines and culture
The BF24 cell line used in this study is a subclone of the monocytic leukaemia cell line THP-1 which was obtained through the National Institutes of Health AIDS Research and Reference Program, Division AIDS, NIAID, NIH reagents program (Cat# 1296) (http://www.aidsreagent.org). These cells are transfected with the chloramphenicol acetyltransferase (CAT) reporter gene driven by HIV-1 long terminal repeat LTR promoter. These cells were cultured as previously described.15 The THP-1 cell line stably transfected and integrated with the HIV LTR was obtained from Dr Klebanoff (University of Washington, Seattle).16 The THP89GFP cells were a generous gift from Dr David Levy (New York University, New York, NY).17 These cells were infected with an HIV-1 strain that expresses the enhanced green fluorescent protein (EGFP) under the control of the HIV-1 promoter without removing any viral sequences. Fluorescence and virus production are tightly coupled in THP89GFP cells.17,18
Both THP-1 and THP89GFP were used to generate DCs using recombinant granulocyte–macrophage colony-stimulating factor and recombinant interleukin-4 for immature DCs (iDCs), followed by tumour necrosis factor-α to induce maturation to mature DCs (mDC) as described previously.15 These cells were characterized using various phenotypic markers, CD86, HLA-DR, CD83 and CD80, in FACS analyses, as we have described previously.15
Microorganisms
The bacterial strains used in this study were Aggregatibacter actinomycetemcomitans strain JP2, Porphyromonas gingivalis ATCC 33277, Prevotella intermedia ATCC 25611, Fusobacterium nucleatum ATCC 25586, Treponema denticola ATCC 35404, Streptococcus mutans ATCC 33535, Streptococcus gordonii ATCC 10558, and Streptococcus sanguinis ATCC 10556. These bacteria were grown under standard conditions as described previously.19 Bacterial extracts were obtained by pelleting, washing and resuspending in PBS with a complete EDTA-free protease inhibitor cocktail (Roche, Mannheim, Germany). The bacteria were sonicated using an ultrasonic disrupter (Branson Sonifier model 450; Branson Ultrasonics Corporation, Danbury, CT, USA), centrifuged at 13 000 g for 10 min at 4° and protein concentration of supernatants was determined by bicinchoninic acid assay (Pierce, Rockford, IL).
In vitro models
BF24 or THP89GFP were placed into 24-well plates at a cell density of 2.5 × 105 cells/well in 500 μl RPMI-1640 medium supplemented with 10% fetal bovine serum. The BF24 cells were treated medium supplemented with 10% fetal bovine serum in duplicate and incubated overnight (16 hr) for all of the comparisons. BF24 and THP89GFP cells were differentiated into DCs first, as described previously,20,21 and treated with various bacterial sonicates.
Assays of HIV promoter activation
In the BF24 cells, HIV-1 promoter activation was measured by quantifying CAT expression levels using a CAT-ELISA kit (Roche). The CAT is used as a reporter gene in the BF24 cells under the control of HIV-1 promoter.15 The level of CAT expression reflects the activity of the HIV-1 promoter following stimulation of these cells. Briefly, BF24 cells were harvested and washed twice with 1 × PBS at 3000 g for 15 min. The pellets were resuspended in lysis buffer for 30 min at room temperature. The extracts from lysed cells (210 μl) were added into 96-well plates and CAT detection was performed following manufacturer's instructions. The absorbance was measured using an MRX 4000 plate reader (Dynatech Laboratories , Chantilly, VA) at 405 nm.
Expression of luciferase activity driven by HIV-1 LTR promoter was measured in the DCs. Luciferase is used as a reporter or reference gene in DCs, which is under the control of HIV-1 promoter.15,16 The level of luciferase expression in DCs will reflect the activity of HIV-1 promoter and in turn it can measure the effect of oral bacteria on HIV promoter activation. Briefly, DCs were harvested and lysed in lysis buffer (Luciferase system; Promega, Madison, WI), 100 μl of the cell lysate was added in duplicate to a 96-well microtitre plate. Fifty microlitres luciferase substrate was added in duplicate to a 96-well microtitre plate. The photon/light intensities were measured with a photometer and expression of luciferase driven by HIV LTR was measured using a photometer reader (MicroLumat LB96V, EG&G Berthold, Germany).
Assays of HIV viral replication
The THP89GFP cells were subjected to different mono- and polybacterial treatments. Tumour necrosis factor-α (3 ng/ml) was used as a positive control. The EGFP serves as a reporter of viral reactivation in this cell model. These cells were infected with an HIV-1 strain that expresses the EGFP under the control of the HIV-1 promoter, in the presence of all viral sequences. Fluorescence levels and virus production are tightly coupled in the THP89GFP cells.17,18 The intensity of fluorescence reflects promoter activation and levels of HIV p24 protein document viral replication. THP89GFP-derived DCs treated with various amounts of bacterial sonicates were photographed in culture through a Nikon Eclipse TS100 Tokyo, Japan inverted microscope (Nikon Metrology, Inc. Brighton, MI) using 10 × magnification with a QICAM Fast1394 device camera (QImaging, Surrey, BC, Canada). To detect EGFP fluorescence the 495-nm green fluorescent protein filter set was used.
In addition, after different polybacterial challenges, THP89GFP cells were harvested and washed once in PBS at 1000 g for 5 min. Pellets were resuspended in 150 μl lysis buffer (50 mm Tris–HCl, 150 mm NaCl, 1% Nonidet 200 g) for 20 min on ice. Cell lysates were then centrifuged at 10 000 × g for 5 min at 4° and 100 μl supernatant was added into *Optilux* 96-well Clear-Bottom Plates (BD Falcon, Franklyn Lakes, NJ) for fluorescence analysis using a Microplate Fluorescence Reader FLx800 (BIO-TEK Instruments, Inc., Winooski, VT). The wavelengths used for excitation and emission were 485 and 528 nm, respectively.
Statistical analyses
Statistical analyses were performed using a Mann–Whitney U-test or Kruskal–Wallis analysis of variance on ranks (SigmaStat 3.5; Point Richmond, San Jose, CA). An α value of P < 0.05 was accepted as statistically significant when comparing the test conditions with untreated cells.
Results
Polybacterial effects on HIV promoter activation
We hypothesized that a polymicrobial challenge of the BF24 macrophage cell model of HIV latency would provide altered outcomes of HIV reactivation when compared with challenge of these cells with the individual bacteria. Figure 1 demonstrates a significant difference in HIV reactivation in the BF24, in the presence of Gram-negative compared with Gram-positive bacteria. Extension of these findings showed that A. actinomycetemcomitans would synergize with P. ginigivalis for HIV promoter activation, although A. actinomycetemcomitans at the highest dose was significantly greater than the other bacteria in this capacity (Fig. 2). In contrast, the Gram-positive bacteria demonstrated no synergy.
Figure 1.

Macrophages (BF24) were stimulated with various concentrations of oral Gram-positive and Gram-negative bacterial sonicates (1, 5, 10 μg/ml) and cells were harvested for chloramphenicol acetyltransferase (CAT) analysis of HIV promoter reactivation. The bars denote the mean of at least triplicate determinations and the vertical bracket denotes 1 SD. The symbols *P<0·05, †P < 0·01, ‡P < 0·001 denote significantly greater than controls.
Figure 2.

Macrophages (BF24) were stimulated with polybacterial combinations of oral Gram-positive and Gram-negative bacterial sonicates at various concentrations (1, 5, 10 μg/ml) and cells were harvested for chloramphenicol acetyltransferase (CAT) analysis of HIV promoter reactivation. The bars denote the means of at least triplicate determinations for each stimulant or combination of bacterial sonicates and the vertical brackets enclose 1 SD. Aa, Aggregatibacter actinomycetemcomitans; Pg, Porphyromonas gingivalis; Pi, Prevotella intermedia; Fn, Fusobacterium nucleatum; Td, Treponema denticola; Sm, Streptococcus mutans; Sg, Streptococcus gordonii; Ss, Streptococcus sanguinis. The designations in the graphs identify the micro-organism and concentration used [i.e. Aa(1) = Aa at 1 μg/ml or Sm(5) –Sm at 5 μg/ml]. The symbols (*, †) P<0·05 denotes significantly greater than additive levels for the pairs of bacteria.
These studies also used an in vitro model of iDCs and mDCs to examine bacterial activation of the HIV promoter related to a polymicrobial challenge. The results in Fig. 3 show that as was noted with the macrophage cell studies, only the Gram-negative bacteria demonstrated a synergistic stimulation of the HIV-promoter in the iDCs. This was particularly noted with the A. actinomycetemcomitans and P. gingivalis challenge and the P. gingivalis and F. nucleatum challenge. In contrast, no synergism was observed with the P. gingivalis/Pr. intermedia combination. Extension of this observation (Fig. 4) noted that higher dosages of these two species actually appeared to suppress the reactivation, suggesting that not only do combinations of different species have the capacity to interact differently with various host cells for inducing biological responses, but also that some levels of challenge may actually suppress the host responses. However, the experimental results show a minimal synergism for the pairs of bacteria, beyond their expected additive effects in reactivating the HIV promoter in the mDCs (data not shown).
Figure 3.

THP-derived immature dendritic cells (iDCs) were challenged with 2 or 5 μg/ml combinations of Gram-negative or Gram-positive bacteria. Aa, Aggregatibacter actinomycetemcomitans; Pg, Porphyromonas gingivalis; Pi, Prevotella intermedia; Fn, Fusobacterium nucleatum; Td, Treponema denticola; Sm, Streptococcus mutans; Sg, Streptococcus gordonii; Ss, Streptococcus sanguinis. Pg + Aa denotes addition of the values from the two individual challenge conditions and Pg/Aa denotes an experimental challenge with the combination of the bacteria. The bars denote the mean of at least triplicate determinations and the vertical bracket enclose 1 SD. The symbols (*, †) P<0·05 denote significantly greater than additive levels for the pairs of bacteria.
Figure 4.
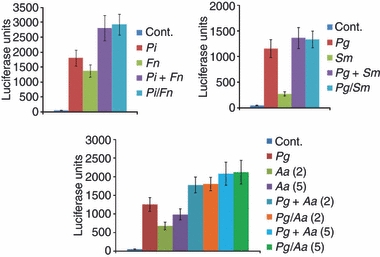
THP-derived mature dendritic cells (mDCs) were challenged with 5 μg/ml of individual bacteria or combinations of bacterial sonicates. Aa, Aggregatibacter actinomycetemcomitans; Pg, Porphyromonas gingivalis; Pi, Prevotella intermedia; Fn, Fusobacterium nucleatum; Td, Treponema denticola; Sm, Streptococcus mutans; Sg, Streptococcus gordonii; Ss, Streptococcus sanguinis. As example, Pi + Fn denotes addition of the values from the two individual challenge conditions and Pi/Fn denotes an experimental challenge with the combination of the bacteria. The mDCs were challenged with 5 μg/ml of Pg or Aa sonicates [i.e. Aa(2) or Aa(5)] individually, or combinations of Pg with Aa. Pg + Aa denotes addition of the values from the two individual challenge conditions and Pg/Aa denotes an experimental challenge with the combination of the bacteria. The bars denote the mean of at least triplicate determinations and the vertical brackets enclose 1 SD.
Polybacterial effects on HIV reactivation
In the THP89GFP-derived iDCs, as expected, some of the polybacterial pairs showed synergism in stimulation leading to an increase of HIV-EGFP production (Fig. 5). Although P. gingivalis paired with both Pr. intermedia and T. denticola actually appeared to suppress the response of the individual bacteria, similar suppressive interactions were observed with a number of the bacterial pairs in challenging the macrophage model system. Only a combination of Pr. intermedia/T. denticola demonstrated any synergism.
Figure 5.

THP89GFP macrophages and THP89GFP-derived dendritic cells (DCs) were treated with 5 μg/ml of individual bacteria or combinations of bacterial sonicates and mean cumulative fluorescence (MCF) was determined. Aa, Aggregatibacter actinomycetemcomitans; Pg, Porphyromonas gingivalis; Pi, Prevotella intermedia; Fn, Fusobacterium nucleatum; Td, Treponema denticola; Sm, Streptococcus mutans; Sg, Streptococcus gordonii; Ss, Streptococcus sanguinis. As example, Aa + Pg denotes addition of the values from the two individual challenge conditions and Aa/Pg denotes an experimental challenge with the combination of the bacteria. The bars denote the mean of at least triplicate determinations and the vertical brackets enclose 1 SD. *Significantly different at least at P<0·05 than additive levels for the pairs of bacteria.
Polymicrobial challenge of THP89GFP macrophages and iDCs showed some additive/cumulative effects on viral replication in these model cells (Fig. 6). Similar additive effects in reactivating HIV replication in the THP89GFP-derived mDCs were observed (data not shown). A more quantitative evaluation of these polymicrobial challenge effects on the HIV latently infected cells is shown in Fig. 7. In Fig. 7(a) only three pairs of Gram-negative bacteria (Pr. intermedia/P. gingivalis, Pr. intermedia/T. denticola, P. gingivalis/T. denticola) demonstrated a synergistic response for viral production (i.e p24 synthesis) in macrophages. In contrast, nearly all pairs of the Gram-negative bacteria when used in combination suppressed the iDC viral reactivation. A similar outcome was obtained when the macrophages or iDCs were challenged with combinations of three Gram-negative oral bacteria. As shown in Fig. 7(b), nearly all of the combinations significantly suppressed the viral reactivation in both macrophages and iDCs, compared with the stimulatory effect of each of the bacteria individually.
Figure 6.

(a) Fluorescence micrographs of macrophages THP89GFP which were stimulated with a combination of oral Gram-negative bacterial sonicates, Td and Pi. (b) Fluorescence microscopy of iDCs derived from THP89GFP which were challenged with various combination of Gram-negative bacteria, Td and Pi.
Figure 7.

Production of p24 in THP89GFP and THP89GFP-HIV-derived dendritic cells (DCs) induced by oral bacteria. The figures are representative of two independent experiments. Data are expressed as means ± standard deviations. The bars denote the mean of at least triplicate determinations and the vertical brackets enclose 1 SD. *Significantly different at least at P<0·05 than additive levels for the pairs of bacteria. (a) The p24 production of cells which was stimulated with combinations of two oral Gram-negative bacterial sonicates. (b) The p24 production of cells, which was stimulated with combinations of three oral Gram-negative bacterial sonicates. Aa, Aggregatibacter actinomycetemcomitans; Pg, Porphyromonas gingivalis; Pi, Prevotella intermedia; Fn, Fusobacterium nucleatum; Td, Treponema denticola.
Discussion
This investigation hypothesized that the responses of macrophages and DCs to a polybacterial challenge would be different from the responses to the individual components of the microbial mixture. These differences focused on the capacity of the polybacterial challenge to reactivate HIV in these cellular reservoirs, manifest by activation of the HIV promoter in cell model systems. As proposed, we observed that selected polybacterial pairs demonstrated a synergism in the stimulation of increases in HIV promoter activation and HIV replication in both macrophages and DCs. It was also noted that these differences varied among the Gram-negative bacteria, as well as showing some specificity in effects related to the type of host cell that was challenged. While this is the first study to examine polybacterial effects on HIV reactivation using these in vitro models, the biological relevance of the magnitude of differences in HIV reactivation remains to be determined. It is clear from the lack of existing literature that translating these cell biology studies to in situ levels of viral recrudescence leading to immunosuppression and disease expression in patients is minimal. Albeit, this type of quantitative correlation for in vitro–in vivo relationships is also generally absent in studies of most human pathogens. Because the oral cavity provides a readily accessible human model to determine mucosal infection, disease and inflammatory responses, as well as the connection with systemic responses, a future goal would be to explore how best to use an oral model to address this question.
We have noted previously that different oral bacteria vary in their capacity to stimulate the HIV promoter in both macrophages and DCs.15,19 The microbiome of the human oral cavity has been estimated to contain nearly 700 species of bacteria,22,23 with estimates of 75–100 species in the mouth of each person. The species range across the spectrum of morphotypes, structural characteristics, motility and metabolic strategies. Moreover, substantial evidence has demonstrated cognate interactions that occur among species that enable a sequential accumulation of species within the organized oral biofilms, as well as nutritional interdependency of microbes in the ecology and the potential for virulence synergism in pathogenic biofilms.24 In the present investigation we selected a range of bacterial species that reflect the variety that can occur in these biofilms and included Gram-negative species, A. actinomycetemcomitans, P. gingivalis, Pr. intermedia and F. nucleatum, and the major oral spirochaete, T. denticola, all of which have been closely linked to periodontal disease.6,25 Additionally, we used the Gram-positive species, S. mutans, S. gordonii and S. sanguinis, which are generally considered pioneer bacteria in colonizing the oral cavity and have primarily been implicated in dental caries, but not considered as aetiological for periodontal disease.26,27 The experiments were not specifically designed to attempt to document unique characteristics and specific functions of individual microorganisms. The focus was to evaluate various combinations of these different types of bacteria to estimate polybacterial effects on host cell functions. As data were generated from individual experiments, we modified the microbial challenges to attempt to provide an overview of the various polybacterial interactions that might occur. These cells are decorated with a range of receptors to recognize various microbial structural patterns, e.g. Toll-like receptors, so it would be expected that these different bacterial components appear to have a predilection for combinations of receptors that would trigger a range of intracellular signalling pathways.28 As importantly, because the HIV promoter contains motifs for various transcription factors (e.g. nuclear factor-κB, activating protein-1, Sp/KLF factor 1, controlled amino acid therapy/enhancer binding protein),29 receptor signalling by the complex of bacterial components altering the levels of a range of these factors, would be predicted to stimulate the promoter activation differently. This is specifically noted with the Gram-negative pairs demonstrating synergism for HIV promoter stimulation, suggesting that either increased levels of a particular transcription factor or, more likely, the combination of the bacteria triggered multiple transcription factors that engaged the HIV promoter more effectively. The demonstration of these molecular changes following a polybacterial challenge remains to be documented.
Variations in the magnitude of macrophage responses supported unique features of the response profile to a polymicrobial challenge. It was observed, particularly with the macrophages, that the synergism was most notable when lower amounts of the microbial stimuli were used. One interpretation of these data is that synergistic effects may only be demonstrated when the receptors for specific microbial ligands on the macrophages remain unsaturated. Most studies of pathogen-related receptor recognition have been limited to studies with individual bacteria, or products of the bacteria, and have generally targeted approaches to document the specificity for individual receptors, e.g. Toll-like receptors.30–32 Moreover, these studies are extended by determining the characteristics of intracellular signalling pathways and transcription factor specificity resulting in innate immune responses by various cell types when challenged with a single bacterial species.33,34 In contrast, a clear understanding of cellular dynamics and characteristics of responses following a polybacterial challenge of host cells remains lacking. Negligible information is available delineating variations in intracellular signalling and kinetics/distribution/quantity of activation of the range of transcription factors that are involved in the innate immune responses that occur after a polybacterial stimulation of cells.34
As noted previously, differences in the magnitude of responses by iDCs to oral bacterial challenge suggested that different receptors and intracellular circuits were engaged.15 It appeared that generally Gram-negative oral bacterial pairs synergized for HIV promoter activation, although the combinations of Gram-positive micro-organisms and Gram-negative/Gram-positive pairs did not exhibit this polybacterial impact on the iDCs. The synergism of Gram-negative bacteria was not universally observed, e.g. a combination of P. gingivalis and Pr. intermedia decreased the promoter activation. This finding supported the hypothesis that bacteria related to periodontal infections can trigger latently HIV-infected iDCs, and that a polymicrobial challenge elicits a different pattern of responses compared with the individual species. However, the most notable outcome of these experiments was obtained using a model of viral reactivation in macrophages and DCs. In this system, numerous pairs of Gram-negative bacteria, and combinations of three species uniformly suppressed the viral reactivation in iDCs. This contrasted with the macrophage studies, which demonstrated some synergism with selected pairs of bacteria. The molecular basis for these variations remains to be delineated, with regards to engagement of cell surface receptors and intracellular signalling pathways that would lead to HIV reactivation.
Our previous findings also demonstrated differences in the response of iDCs and mDCs to individual oral bacteria.15 This is consistent with differences in the functions of these cell types, as well as substantial variations in the cell surface receptors that are designed to detect microbial patterns/antigens (i.e iDCs) and in the expression of co-stimulatory molecules for presenting antigens (i.e mDCs).35,36 Interestingly, no bacterial synergism was observed with HIV reactivation in the mDCs. Although the overall HIV reactivation levels were greater in mDCs than iDCs, these findings suggested that it is likely that different cell surface receptors on mDCs interacted with the bacteria than on iDCs. This might be expected because of the significant decrease in Toll-like receptors during maturation from iDC to mDC.37 Once the cells have matured into antigen-presenting mDCs, their function transitions to one of interacting with host cells via specific cell surface receptor molecules,38,39 and is no longer targeted towards detection and engagement of foreign antigens. However, when they are activated towards maturation, this process may actually prime the mDCs and sensitize them for subsequent bacterial triggering of the HIV-promoter. Whether this occurs through non-Toll-like receptors that can recognize microbial-associated molecular patterns, or other non-bacterial receptors expressed on the mDC surface remains to be determined.
The extent of the existing literature has almost exclusively examined cellular responses following challenge with a single micro-organism, including bacteria, viruses, fungi and protozoa. All of the available data on signalling via pathogen-related receptors have examined how one specific micro-organism or isolated structures of the micro-organism activate host cell responses. Hence, the behaviour of various types of host cells, including macrophages and DCs, engaging a polymicrobial challenge remains undetermined. It is clear from the range of responses, and selected co-operative stimuli among selected bacterial consortia that the net response of the host cells is not simply a summation of the capabilities of the individual stimuli. The results suggest the potential for variations in the ‘strength’ of signalling by individual bacteria that varies with monobacterial versus polybacterial infections, as well as selective engagement of various host receptors in a milieu of a complex bacterial consortium. Consequently, the engagement of surface pathogen-related receptors and the resulting signalling pathways are not simply ‘on–off’ switches, but interface dynamically with the cells’ microenvironment, and would be predicted to have a role in the ability of host cells to discriminate between pathogens and commensal bacteria at mucosal surfaces. As importantly, our studies have focused on the characteristics of the ‘innate immune responses’ of these cells to a polybacterial challenge leading to cytokine production or HIV-reactivation.40 Remaining to be addressed is the likelihood that these polybacterial effects would also be manifest in altering the adaptive immune capabilities of the DCs with regards to effective antigen presentation.
Acknowledgments
This work was supported by U.S.P.H.S. grant P20RR020145 from the National Center for Research Resources of the National Institutes of Health.
Disclosures
The author declares no conflict of interest.
References
- 1.Tatakis DN, Kumar PS. Etiology and pathogenesis of periodontal diseases. Dent Clin North Am. 2005;49:491–516. doi: 10.1016/j.cden.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 2.Paster BJ, Boches SK, Galvin JL, Ericson RE, Lau CN, Levanos VA, Sahasrabudhe A, Dewhirst FE. Bacterial diversity in human subgingival plaque. J Bacteriol. 2001;183:3770–83. doi: 10.1128/JB.183.12.3770-3783.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paster BJ, Olsen I, Aas JA, Dewhirst FE. The breadth of bacterial diversity in the human periodontal pocket and other oral sites. Periodontol 2000. 2006;42:80–7. doi: 10.1111/j.1600-0757.2006.00174.x. [DOI] [PubMed] [Google Scholar]
- 4.Kesavalu L, Sathishkumar S, Bakthavatchalu V, Matthews C, Dawson D, Steffen M, Ebersole JL. Rat model of polymicrobial infection, immunity, and alveolar bone resorption in periodontal disease. Infect Immun. 2007;75:1704–12. doi: 10.1128/IAI.00733-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ximénez-Fyvie LA, Haffajee AD, Socransky SS. Comparison of the microbiota of supra- and subgingival plaque in health and periodontitis. J Clin Periodontol. 2000;27:648–57. doi: 10.1034/j.1600-051x.2000.027009648.x. [DOI] [PubMed] [Google Scholar]
- 6.Socransky SS, Haffajee AD. Periodontal microbial ecology. Periodontol 2000. 2005;38:135–87. doi: 10.1111/j.1600-0757.2005.00107.x. [DOI] [PubMed] [Google Scholar]
- 7.Kigure T, Saito A, Seida K, Yamada S, Ishihara K, Okuda K. Distribution of Porphyromonas gingivalis and Treponema denticola in human subgingival plaque at different periodontal pocket depths examined by immunohistochemical methods. J Periodontal Res. 1995;30:332–41. doi: 10.1111/j.1600-0765.1995.tb01284.x. [DOI] [PubMed] [Google Scholar]
- 8.Chen R, Westfall A, Cloud G, et al. Chicago, IL: 2001. Long-term survival after initiation of antiretroviral therapy. 8th Conference on Retroviruses and Opportunistic Infections Abstract 341. [Google Scholar]
- 9.Kaplan J, Hanson DL, Karon J, et al. Durban, South Africa: 2000. Early initiation of combination antiretroviral therapy (ART): does it affect clinical outcome? 13th International AIDS Conference Abstract LbPeB7051. [Google Scholar]
- 10.Moriuchi H, Moriuchi M, Mizell SB, Ehler LA, Fauci AS. In vitro reactivation of human immunodeficiency virus 1 from latently infected, resting CD4+ T cells after bacterial stimulation. Infect Dis. 2000;181:2041–4. doi: 10.1086/315496. [DOI] [PubMed] [Google Scholar]
- 11.Chun TW, Engel D, Mizell SB, Ehler LA, Fauci AS. Induction of HIV-1 replication in latently infected CD4 T cells using a combination of cytokines. J Exp Med. 1998;188:83–91. doi: 10.1084/jem.188.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simonitsch I, Geusau A, Chott A, Jurecka W. Cutaneous dendritic cells are main targets in acute HIV-1-infection. Mod Pathol. 2000;13:1232–7. doi: 10.1038/modpathol.3880227. [DOI] [PubMed] [Google Scholar]
- 13.Frank I, Kacani L, Stoiber H, Stössel H, Spruth M, Steindl F, Romani N, Dierich MP. Human immunodeficiency virus type 1 derived from cocultures of immature dendritic cells with autologous T cells carries T-cell-specific molecules on its surface and is highly infectious. J Virol. 1999;73:3449–54. doi: 10.1128/jvi.73.4.3449-3454.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.González OA, Ebersole JL, Huang CB. Oral infectious diseases: a potential risk factor for HIV recrudescence? Oral Dis. 2009;15:313–27. doi: 10.1111/j.1601-0825.2009.01533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang CB, Emerson KA, González OA, Ebersole JL. Differential activation of HIV-1 promoter in T cells, macrophages, and dendritic cells by oral bacteria. Oral Microbiol Immunol. 2009;24:401–7. doi: 10.1111/j.1399-302X.2009.00533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klebanoff SJ, Kazazi F, Van Voorhis WC, Schlechte KG. Activation of the human immunodeficiency virus long terminal repeat in THP-1 cells by a staphylococcal extracellular product. Proc Natl Acad Sci U S A. 1994;91:10615–9. doi: 10.1073/pnas.91.22.10615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kutsch O, Benveniste EN, Shaw GM, Levy DN. Direct and quantitative single-cell analysis of human immunodeficiency virus type 1 reactivation from latency. J Virol. 2002;76:8776–86. doi: 10.1128/JVI.76.17.8776-8786.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.González OA, Li M, Ebersole JL, Huang CB. HIV-1 reactivation induced by periodontal pathogens Fusobacterium nucleatum and Porphyromonas gingivalis involves TLR2 and TLR9 activation in monocytes/macrophages. Clin Vaccine Immunol. 2010;17(9):1417–1427. doi: 10.1128/CVI.00009-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ebersole JL, Holt SC, Hansard R, Novak MJ. Microbiologic and immunologic characteristics of periodontal disease in Hispanic Americans with type 2 diabetes. J Periodontol. 2008;79:637–46. doi: 10.1902/jop.2008.070455. [DOI] [PubMed] [Google Scholar]
- 20.Kalinski P, Schuitemaker JH, Hilkens CM, Wierenga EA, Kapsenberg ML. Final maturation of dendritic cells is associated with impaired responsiveness to IFN-γ and to bacterial IL-12 inducers: decreased ability of mature dendritic cells to produce IL-12 during the interaction with Th cells. J Immunol. 1999;162:3231–6. [PubMed] [Google Scholar]
- 21.Ebner S, Ratzinger G, Krosbacher B, et al. Production of IL-12 by human monocyte-derived dendritic cells is optimal when the stimulus is given at the onset of maturation, and is further enhanced by IL-4. J Immunol. 2001;166:633–41. doi: 10.4049/jimmunol.166.1.633. [DOI] [PubMed] [Google Scholar]
- 22.Aas JA, Paster BJ, Stokes LN, Olsen I, Dewhirst FE. Defining the normal bacterial flora of the oral cavity. J Clin Microbiol. 2005;43:5721–32. doi: 10.1128/JCM.43.11.5721-5732.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dewhirst FE, Chen T, Izard J, Paster BJ, Tanner AC, Yu WH, Lakshmanan A, Wade WG. The human oral microbiome. J Bacteriol. 2010;192:5002–17. doi: 10.1128/JB.00542-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kolenbrander PE, Andersen RN, Blehert DS, Egland PG, Foster JS, Palmer RJ., Jr Communication among oral bacteria. Microbiol Mol Biol Rev. 2002;66:486–505. doi: 10.1128/MMBR.66.3.486-505.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Socransky SS, Haffajee AD, Cugini MA, Smith C, Kent RL., Jr Microbial complexes in subgingival plaque. J Clin Periodontol. 1998;25:134–44. doi: 10.1111/j.1600-051x.1998.tb02419.x. [DOI] [PubMed] [Google Scholar]
- 26.Loesche WJ. Role of Streptococcus mutans in human dental decay. Microbiol Rev. 1986;50:353–80. doi: 10.1128/mr.50.4.353-380.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Becker MR, Paster BJ, Leys EJ, et al. Molecular analysis of bacterial species associated with childhood caries. J Clin Microbiol. 2002;40:1001–9. doi: 10.1128/JCM.40.3.1001-1009.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Warger T, Osterloh P, Rechtsteiner G, et al. Synergistic activation of dendritic cells by combined Toll-like receptor ligation induces superior CTL responses in vivo. Blood. 2006;108:544–50. doi: 10.1182/blood-2005-10-4015. [DOI] [PubMed] [Google Scholar]
- 29.Jeeninga RE, Hoogenkamp M, Armand-Ugon M, de Baar M, Verhoef K, Berkhout B. Functional differences between the long terminal repeat transcriptional promoters of human immunodeficiency virus type 1 subtypes A through G. J Virol. 2000;74:3740–51. doi: 10.1128/jvi.74.8.3740-3751.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang PL, Ohura K. Porphyromonas gingivalis lipopolysaccharide signaling in gingival fibroblasts-CD14 and Toll-like receptors. Crit Rev Oral Biol Med. 2002;13:132–42. doi: 10.1177/154411130201300204. [DOI] [PubMed] [Google Scholar]
- 31.Nussbaum G, Ben-Adi S, Genzler T, Sela M, Rosen G. Involvement of Toll-like receptors 2 and 4 in the innate immune response to Treponema denticola and its outer sheath components. Infect Immun. 2009;77:3939–47. doi: 10.1128/IAI.00488-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Asai Y, Makimura Y, Ogawa T. Toll-like receptor 2-mediated dendritic cell activation by a Porphyromonas gingivalis synthetic lipopeptide. J Med Microbiol. 2007;56:459–65. doi: 10.1099/jmm.0.46991-0. [DOI] [PubMed] [Google Scholar]
- 33.Hajishengallis G, Sojar H, Genco RJ, DeNardin E. Intracellular signaling and cytokine induction upon interactions of Porphyromonas gingivalis fimbriae with pattern-recognition receptors. Immunol Invest. 2004;33:157–72. doi: 10.1081/imm-120030917. [DOI] [PubMed] [Google Scholar]
- 34.Lee J, Hayashi M, Lo JF, Fearns C, Chu WM, Luo Y, Xiang R, Chuang TH. Nuclear factor κB (NF-κB) activation primes cells to a pro-inflammatory polarized response to a Toll-like receptor 7 (TLR7) agonist. Biochem J. 2009;421:301–10. doi: 10.1042/BJ20090013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Le Naour F, Hohenkirk L, Grolleau A, Misek DE, Lescure P, Geiger JD, Hanash S, Beretta L. Profiling changes in gene expression during differentiation and maturation of monocyte-derived dendritic cells using both oligonucleotide microarrays and proteomics. J Biol Chem. 2001;276:17920–31. doi: 10.1074/jbc.M100156200. [DOI] [PubMed] [Google Scholar]
- 36.Korthals M, Safaian N, Kronenwett R, et al. Monocyte derived dendritic cells generated by IFN-α acquire mature dendritic and natural killer cell properties as shown by gene expression analysis. J Transl Med. 2007;5:46. doi: 10.1186/1479-5876-5-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Visintin A, Mazzoni A, Spitzer JH, Wyllie DH, Dower SK, Segal DM. Regulation of Toll-like receptors in human monocytes and dendritic cells. J Immunol. 2001;166:249–55. doi: 10.4049/jimmunol.166.1.249. [DOI] [PubMed] [Google Scholar]
- 38.Cella M, Sallusto F, Lanzavecchia A. Origin, maturation and antigen presenting function of dendritic cells. Curr Opin Immunol. 1997;9:10–6. doi: 10.1016/s0952-7915(97)80153-7. [DOI] [PubMed] [Google Scholar]
- 39.Lanzavecchia A, Sallusto F. Regulation of T cell immunity by dendritic cells. Cell. 2001;106:263–6. doi: 10.1016/s0092-8674(01)00455-x. [DOI] [PubMed] [Google Scholar]
- 40.Huang CB, Altimova Y, Strange S, Ebersole JL. Polybacterial challenge effects on cytokine/chemokine production by macrophages and dendritic cells. Inflamm Res. 2010 doi: 10.1007/s00011-010-0242-0. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]


