Abstract
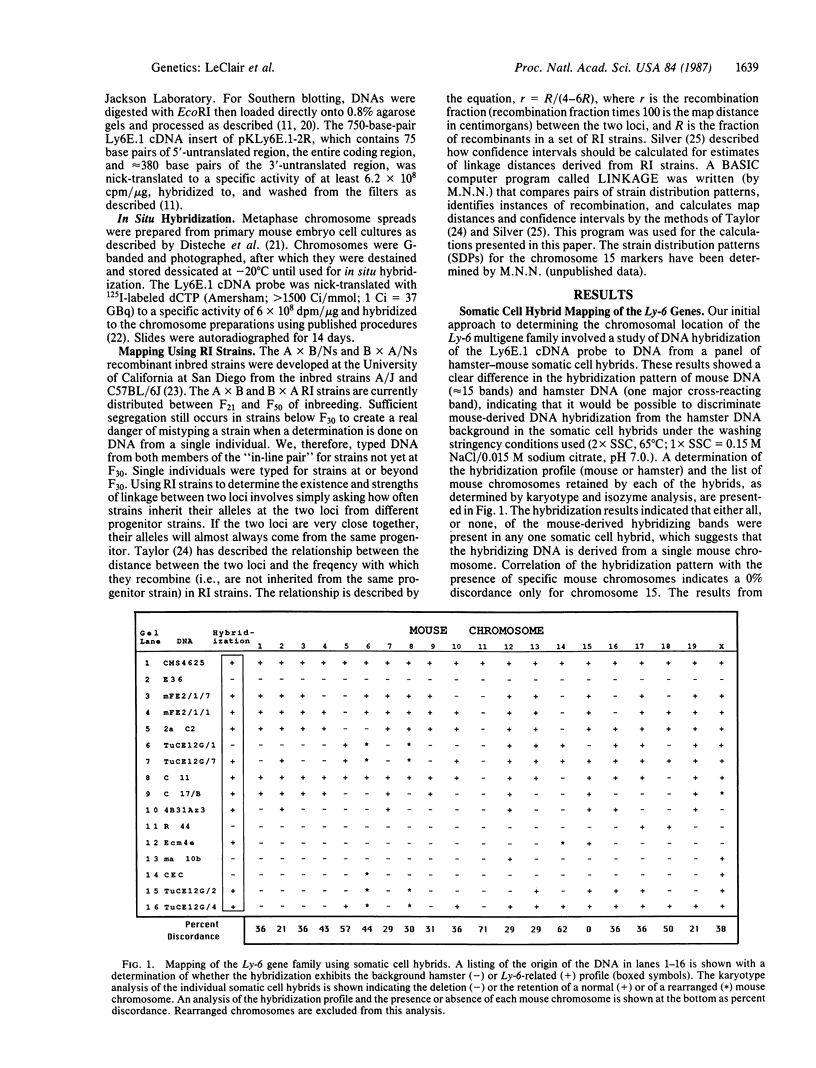
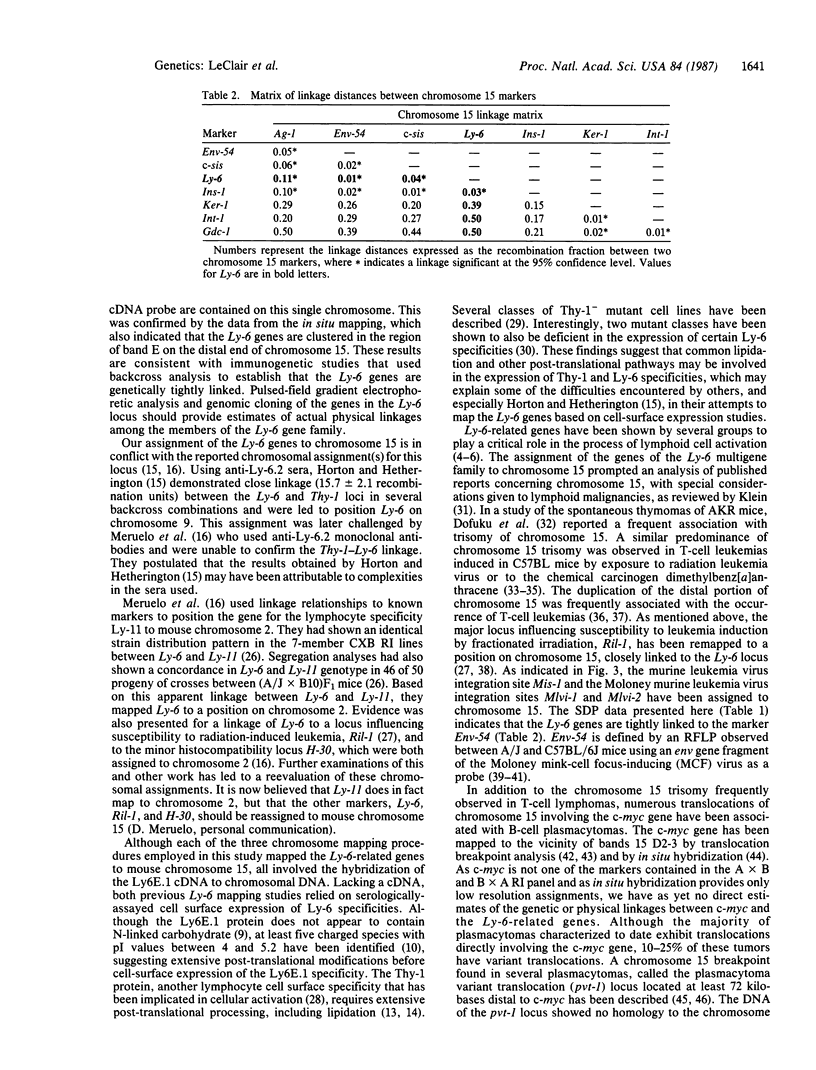
Murine Ly-6-encoded molecules play an important role in the antigen-independent activation of lymphocytes. We have described the cloning of a cDNA encoding the protein component of an Ly-6 molecule. Hybridization studies indicated that this cDNA identified multiple DNA fragments on Southern blots. The banding pattern exhibits a restriction fragment length polymorphism from mice bearing either the Ly-6a or the Ly-6b allele. We have employed three independent chromosomal mapping techniques, somatic cell hybrids, in situ hybridization, and strain distribution pattern analysis of the restriction fragment length polymorphism of DNA from recombinant inbred lines, to ascertain the chromosomal origins of these bands. We report that all members of the Ly-6 multigene family are tightly linked on chromosome 15 and have been regionalized by in situ hybridization analysis to band 15E on the distal portion of this chromosome. Linkage analysis has indicated that the Ly-6 genes are located within 1 map unit of Env-54 (a retroviral envelope restriction fragment length polymorphism probe), 3 map units from ins-1, (insulin-related gene), and 4 map units from the protooncogene c-sis. The possible involvement of the Ly-6 lymphocyte activation and differentiation antigen genes in chromosome 15-related lymphoid malignancies is discussed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banerjee M., Wiener F., Spira J., Babonits M., Nilsson M. G., Sumegi J., Klein G. Mapping of the c-myc, pvt-1 and immunoglobulin kappa genes in relation to the mouse plasmacytoma-associated variant (6;15) translocation breakpoint. EMBO J. 1985 Dec 1;4(12):3183–3188. doi: 10.1002/j.1460-2075.1985.tb04063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatt C., Mileham K., Haas M., Nesbitt M. N., Harper M. E., Simon M. I. Chromosomal mapping of the mink cell focus-inducing and xenotropic env gene family in the mouse. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6298–6302. doi: 10.1073/pnas.80.20.6298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosselman R. A., van Straaten F., Van Beveren C., Verma I. M., Vogt M. Analysis of the env gene of a molecularly cloned and biologically active Moloney mink cell focus-forming proviral DNA. J Virol. 1982 Oct;44(1):19–31. doi: 10.1128/jvi.44.1.19-31.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay S. K., Cloyd M. W., Linemeyer D. L., Lander M. R., Rands E., Lowy D. R. Cellular origin and role of mink cell focus-forming viruses in murine thymic lymphomas. Nature. 1982 Jan 7;295(5844):25–31. doi: 10.1038/295025a0. [DOI] [PubMed] [Google Scholar]
- Cory S., Graham M., Webb E., Corcoran L., Adams J. M. Variant (6;15) translocations in murine plasmacytomas involve a chromosome 15 locus at least 72 kb from the c-myc oncogene. EMBO J. 1985 Mar;4(3):675–681. doi: 10.1002/j.1460-2075.1985.tb03682.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Disteche C. M., Eicher E. M., Latt S. A. Late replication in an X-autosome translocation in the mouse: correlation with genetic inactivation and evidence for selective effects during embryogenesis. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5234–5238. doi: 10.1073/pnas.76.10.5234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dofuku R., Biedler J. L., Spengler B. A., Old L. J. Trisomy of chromosome 15 in spontaneous leukemia of AKR mice. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1515–1517. doi: 10.1073/pnas.72.4.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flood P. M., Murphy D. B., Horowitz M., LeClair K. P., Smith F. R., Stockert E., Palladino M. A., Jr, DeLeo A. B. A monoclonal antibody that recognizes an Ly-6-linked antigen inhibits the generation of functionally active T cell subsets. J Immunol. 1985 Jul;135(1):63–72. [PubMed] [Google Scholar]
- Graham M., Adams J. M., Cory S. Murine T lymphomas with retroviral inserts in the chromosomal 15 locus for plasmacytoma variant translocations. 1985 Apr 25-May 1Nature. 314(6013):740–743. doi: 10.1038/314740a0. [DOI] [PubMed] [Google Scholar]
- Gunter K. C., Malek T. R., Shevach E. M. T cell-activating properties of an anti-Thy-1 monoclonal antibody. Possible analogy to OKT3/Leu-4. J Exp Med. 1984 Mar 1;159(3):716–730. doi: 10.1084/jem.159.3.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton M. A., Hetherington C. M. Genetic linkage of Ly-6 and Thy-1 loci in the mouse. Immunogenetics. 1980;11(5):521–525. doi: 10.1007/BF01567820. [DOI] [PubMed] [Google Scholar]
- Horton M. A., Hyman R. Genetic basis for Ly-6- defect: complementation between Ly-6- and Thy-1- mutant cell lines. Immunogenetics. 1983;17(3):261–270. doi: 10.1007/BF00364410. [DOI] [PubMed] [Google Scholar]
- Houlden B. A., Hogarth P. M., McKenzie I. F. Interrelationships of the "Ly-6 complex" antigens. Immunogenetics. 1986;23(4):226–232. doi: 10.1007/BF00373017. [DOI] [PubMed] [Google Scholar]
- Hyman R. Cell-surface-antigen mutants of haematopoietic cells. Tools to study differentiation, biosynthesis and function. Biochem J. 1985 Jan 1;225(1):27–40. doi: 10.1042/bj2250027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura S., Tada N., Liu-Lam Y., Hämmerling U. Studies of the mouse Ly-6 alloantigen system. II. Complexities of the Ly-6 region. Immunogenetics. 1984;20(1):47–56. doi: 10.1007/BF00373446. [DOI] [PubMed] [Google Scholar]
- Klein G. Lymphoma development in mice and humans: diversity of initiation is followed by convergent cytogenetic evolution. Proc Natl Acad Sci U S A. 1979 May;76(5):2442–2446. doi: 10.1073/pnas.76.5.2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein G. The role of gene dosage and genetic transpositions in carcinogenesis. Nature. 1981 Nov 26;294(5839):313–318. doi: 10.1038/294313a0. [DOI] [PubMed] [Google Scholar]
- LeClair K. P., Palfree R. G., Flood P. M., Hammerling U., Bothwell A. Isolation of a murine Ly-6 cDNA reveals a new multigene family. EMBO J. 1986 Dec 1;5(12):3227–3234. doi: 10.1002/j.1460-2075.1986.tb04633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low M. G., Kincade P. W. Phosphatidylinositol is the membrane-anchoring domain of the Thy-1 glycoprotein. Nature. 1985 Nov 7;318(6041):62–64. doi: 10.1038/318062a0. [DOI] [PubMed] [Google Scholar]
- Malek T. R., Ortega G., Chan C., Kroczek R. A., Shevach E. M. Role of Ly-6 in lymphocyte activation. II. Induction of T cell activation by monoclonal anti-Ly-6 antibodies. J Exp Med. 1986 Sep 1;164(3):709–722. doi: 10.1084/jem.164.3.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meruelo D., Offer M., Flieger N. Genetics of susceptibility for radiation-induced leukemia. Mapping of genes involved to chromosomes 1, 2, and 4, and implications for a viral etiology in the disease. J Exp Med. 1981 Oct 1;154(4):1201–1211. doi: 10.1084/jem.154.4.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meruelo D., Offer M., Rossomando A. Evidence for a major cluster of lymphocyte differentiation antigens on murine chromosome 2. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7460–7464. doi: 10.1073/pnas.79.23.7460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meruelo D., Offer M., Rossomando A. Induction of leukemia by both fractionated x-irradiation and radiation leukemia virus involves loci in the chromosome 2 segment H-30-A. Proc Natl Acad Sci U S A. 1983 Jan;80(2):462–466. doi: 10.1073/pnas.80.2.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meruelo D., Paolino A., Flieger N., Offer M. Definition of a new T lymphocyte cell surface antigen, Ly 11.2. J Immunol. 1980 Dec;125(6):2713–2718. [PubMed] [Google Scholar]
- Nesbitt M. N., Francke U. A system of nomenclature for band patterns of mouse chromosomes. Chromosoma. 1973;41(2):145–158. doi: 10.1007/BF00319691. [DOI] [PubMed] [Google Scholar]
- Nesbitt M. N., Skamene E. Recombinant inbred mouse strains derived from A/J and C57BL/6J: a tool for the study of genetic mechanisms in host resistance to infection and malignancy. J Leukoc Biol. 1984 Sep;36(3):357–364. doi: 10.1002/jlb.36.3.357. [DOI] [PubMed] [Google Scholar]
- Nichols E. A., Ruddle F. H. A review of enzyme polymorphism, linkage and electrophoretic conditions for mouse and somatic cell hybrids in starch gels. J Histochem Cytochem. 1973 Dec;21(12):1066–1081. doi: 10.1177/21.12.1066. [DOI] [PubMed] [Google Scholar]
- Ohno S., Migita S., Wiener F., Babonits M., Klein G., Mushinski J. F., Potter M. Chromosomal translocations activating myc sequences and transduction of v-abl are critical events in the rapid induction of plasmacytomas by pristane and abelson virus. J Exp Med. 1984 Jun 1;159(6):1762–1777. doi: 10.1084/jem.159.6.1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palfree R. G., Dumont F. J., Hammerling U. Ly-6A.2 and Ly-6E.1 molecules are antithetical and identical to MALA-1. Immunogenetics. 1986;23(3):197–207. doi: 10.1007/BF00373821. [DOI] [PubMed] [Google Scholar]
- Palfree R. G., Hämmerling U. Biochemical characterization of the murine activated lymphocyte alloantigen Ly-6E.1 controlled by the Ly-6 locus. J Immunol. 1986 Jan;136(2):594–600. [PubMed] [Google Scholar]
- Rabin M., Watson M., Barker P. E., Ryan J., Breg W. R., Ruddle F. H. NRAS transforming gene maps to region p11----p13 on chromosome 1 by in situ hybridization. Cytogenet Cell Genet. 1984;38(1):70–72. doi: 10.1159/000132032. [DOI] [PubMed] [Google Scholar]
- Reiser H., Oettgen H., Yeh E. T., Terhorst C., Low M. G., Benacerraf B., Rock K. L. Structural characterization of the TAP molecule: a phosphatidylinositol-linked glycoprotein distinct from the T cell receptor/T3 complex and Thy-1. Cell. 1986 Nov 7;47(3):365–370. doi: 10.1016/0092-8674(86)90593-3. [DOI] [PubMed] [Google Scholar]
- Reiser H., Yeh E. T., Gramm C. F., Benacerraf B., Rock K. L. Gene encoding T-cell-activating protein TAP maps to the Ly-6 locus. Proc Natl Acad Sci U S A. 1986 May;83(9):2954–2958. doi: 10.1073/pnas.83.9.2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock K. L., Yeh E. T., Gramm C. F., Haber S. I., Reiser H., Benacerraf B. TAP, a novel T cell-activating protein involved in the stimulation of MHC-restricted T lymphocytes. J Exp Med. 1986 Feb 1;163(2):315–333. doi: 10.1084/jem.163.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver J. Confidence limits for estimates of gene linkage based on analysis of recombinant inbred strains. J Hered. 1985 Nov-Dec;76(6):436–440. doi: 10.1093/oxfordjournals.jhered.a110140. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Spira J., Babonits M., Wiener F., Ohno S., Wirschubski Z., Haran-Ghera N., Klein G. Nonrandom chromosomal changes in thy-1-positive and thy-1-negative lymphomas induced by 7,12-dimethylbenzanthracene in SJL mice. Cancer Res. 1980 Jul;40(7):2609–2616. [PubMed] [Google Scholar]
- Tse A. G., Barclay A. N., Watts A., Williams A. F. A glycophospholipid tail at the carboxyl terminus of the Thy-1 glycoprotein of neurons and thymocytes. Science. 1985 Nov 29;230(4729):1003–1008. doi: 10.1126/science.2865810. [DOI] [PubMed] [Google Scholar]
- Villeneuve L., Rassart E., Jolicoeur P., Graham M., Adams J. M. Proviral integration site Mis-1 in rat thymomas corresponds to the pvt-1 translocation breakpoint in murine plasmacytomas. Mol Cell Biol. 1986 May;6(5):1834–1837. doi: 10.1128/mcb.6.5.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H. C., Fedoroff S. Banding in human chromosomes treated with trypsin. Nat New Biol. 1972 Jan 12;235(54):52–54. doi: 10.1038/newbio235052a0. [DOI] [PubMed] [Google Scholar]
- Wiener F., Babonits M., Bregula U., Klein G., Léonard A., Wax J. S., Potter M. High resolution banding analysis of the involvement of strain BALB/c- and AKR-derived chromosomes No. 15 in plasmacytoma-specific translocations. J Exp Med. 1984 Jan 1;159(1):276–291. doi: 10.1084/jem.159.1.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiener F., Ohno S., Spira J., Haran-Ghera N., Klein G. Chromosome changes (trisomies #15 and 17) associated with tumor progression in leukemias induced by radiation leukemia virus. J Natl Cancer Inst. 1978 Jul;61(1):227–237. doi: 10.1093/jnci/61.1.227. [DOI] [PubMed] [Google Scholar]
- Wiener F., Ohno S., Spira J., Haran-Ghera N., Klein G. Cytogenetic mapping of the trisomic segment of chromosome 15 in murine T-cell leukaemia. Nature. 1978 Oct 19;275(5681):658–660. doi: 10.1038/275658a0. [DOI] [PubMed] [Google Scholar]
- Wiener F., Spira J., Ohno S., Haran-Ghera N., Klein G. Chromosome changes (trisomy 15) in murine T-cell leukemia induced by 1,12-dimethylbenz(a)anthracene (DMBA). Int J Cancer. 1978 Oct 15;22(4):447–453. doi: 10.1002/ijc.2910220413. [DOI] [PubMed] [Google Scholar]
- Yeh E. T., Reiser H., Benacerraf B., Rock K. L. The expression, function, and ontogeny of a novel T cell-activating protein, TAP, in the thymus. J Immunol. 1986 Aug 15;137(4):1232–1238. [PubMed] [Google Scholar]



