Abstract
Although age-related macular degeneration (AMD) is not a classic inflammatory disease like uveitis, inflammation has been found to have an important role in disease pathogenesis and progression. Innate immunity and autoimmune components, such as complement factors, chemokines, cytokines, macrophages, and ocular microglia, are believed to be heavily involved in AMD development. Targeting these specific inflammatory molecules has recently been explored in an attempt to better understand and treat AMD. Although antivascular endothelial growth factor therapy is the first line of defence against neovascular AMD, anti-inflammatory agents such as corticosteroids, nonsteroidal anti-inflammatory drugs (NSAIDs), immunosuppressive agents (eg, methotrexate and rapamycin), and biologics (eg, infliximab, daclizumab, and complement inhibitors) may provide an adjunct or alternative mechanism to suppress the inflammatory processes driving AMD progression. Further investigation is required to evaluate the long-term safety and efficacy of these drugs for both neovascular and non-neovascular AMD.
Keywords: age-related macular degeneration, inflammation, corticosteroid, nonsteoridal anti-inflammatory drug, immunosuppressant, biologics
Age-related macular degeneration (AMD) is a disease affecting the central regions of the retina and choroid that can lead to irreversible central vision loss. AMD is the leading cause of permanent vision impairment and blindness among the elderly, affecting ∼30–50 million individuals worldwide.1, 2 In the United States, advanced AMD affects over 1.75 million people and the number is expected to increase to 2.95 million by the year 2020.2
Two clinically recognized subtypes of AMD are non-neovascular geographic atrophic (‘dry') AMD and neovascular exudative (‘wet') AMD.3 Non-neovascular AMD is characterized by the accumulation of lipo-glyco-proteinaceous deposits (drusen) as well as the degeneration of the retinal pigment epithelium (RPE) and photoreceptors. Geographic atrophy (GA) accompanies the advanced form of non-neovascular AMD and results in RPE atrophy, degeneration of the outer retinal layer, and sclerosis of choriocapillaris. Choroidal neovascularization (CNV) is the hallmark of neovascular AMD, which affects the choroid/Bruch's membrane/RPE complex and leads to exudation and bleeding within the macula. Disciform scarring, characterized by predominantly fibrotic tissue, few remaining neovascular lumens, and neural tissue loss, is often present during the late stages of neovascular AMD. Although neovascular AMD accounts for only 10–20% of total AMD cases, it is responsible for the sudden and severe vision loss in the majority of AMD patients.4 In contrast, GA, the end-stage of non-neovascular AMD, usually results in gradual and less severe loss of vision.2
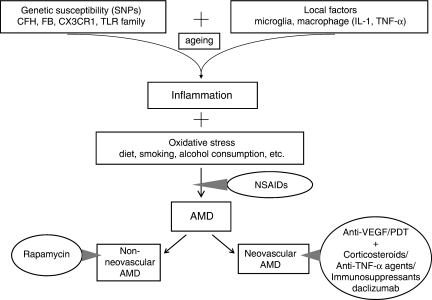
AMD is a highly complex disease that is affected by multiple factors, such as ageing, genetic predisposition, environmental elements, oxidative stress, and inflammatory effects (Figure 1).3, 5, 6 A number of important risk factors that are related to oxidative stress such as age, smoking, alcohol consumption, diet, and obesity have also been reported.5, 6 Several single-nucleotide polymorphisms (SNPs) that confer increased or decreased risk of inflammation have been identified. They include the well-recognized complement factor H (CFH), CX3CR1, Toll-like receptor 3 (TLR3), TLR4, and interleukin 8 (IL-8).7
Figure 1.
Intervention of anti-inflammatory agents in AMD pathway.
The complement system is a major contributor to innate immunity. There are several complement components (C3, C5, C5b-9 membrane attack complex (MAC), and CD46) found in drusen.8 These findings indicate that the complement components and regulators may contribute to the formation of drusen. Several SNPs of complement components and regulators associated with age-related diseases have been identified. Varying CFH Y402H genotypes exhibited significant differences in the frequency of early AMD, thereby implicating the CFH gene as one of the most important determinants of AMD predisposition.9 This high-risk variant of CFH increases the risk of AMD by 5- to 7-fold in Caucasians. Additional complement components, such as complement factor B (FB), C2, and C3, have also been reported to affect the risk of developing AMD.8 C3 activation is believed to contribute to AMD progression independent of CFH polymorphism.10
Although AMD is not a classic inflammatory disease, inflammatory cells have an important role in AMD pathogenesis and progression (Figure 1).3, 11, 12 Macrophages and giant cells have been reported to localize near drusen, at the breakdown of Bruch's membrane, and in the CNV membrane. Furthermore, macrophage-derived cytokines, such as tumour necrosis factor-α (TNF-α) and IL-1, have been shown to promote the expression of intercellular adhesion molecule-1 (ICAM-1) in the RPE and vascular endothelial cells, thereby inducing additional inflammatory cellular infiltration. Macrophages can also induce proliferation and migration of vascular endothelial cells by cytokines, which accelerates angiogenesis and CNV formation.13
In addition to macrophages, microglial cells are also involved in the pathogenesis of AMD. As the resident phagocytes of the innate immune system, the stellate-shaped microglia enter the inner retina during embryological development.14 Upon activation by retinal injury and degeneration, microglial cells transform to an amoeboid-like appearance and migrate to the injured outer retina. The activated microglia facilitate the phagocytosis of debris as well as produce various proinflammatory cytokines and chemokines that create a neurotoxic milieu leading to disease progression. The activated microglia are also found in the outer retina and the subretinal regions of eyes with AMD.15
Autoimmunity has also been suggested to have a role in drusen formation and AMD pathogenesis. The presence of a number of antiretinal autoantibodies, such as anti-carboxyethylpyrrole and anti-astrocyte antibodies, has been suggested as early features of AMD pathogenesis.16, 17 Recently, Morohoshi et al18 demonstrated that 94% of patients with early-stage AMD and 83% of patients with late-stage AMD had elevated levels of serum retinal autoantibodies, compared with only 9% of normal controls. Numerous other antiretinal autoantibodies (such as glial fibrillary acid protein, α-Crystallin, α-Enolase, and Annexin II) have also been identified as potential mediators in the pathogenesis of AMD.
Evidence has also suggested that some infectious agents are associated with AMD. Although some studies challenged the role of Chlamydia pneumoniae in AMD pathogenesis,19 several studies have shown that C. pneumoniae infection is related to the increased risk of AMD.20, 21, 22 Fujimoto et al23 showed that C. pneumoniae can trigger inflammatory responses in the eye and promote experimental CNV in a TLR2-dependent manner. Baird et al24 also demonstrated the existence of a gene–environment interaction between pathogenic load of C. pneumoniae and the CFH in the aetiology of AMD. Moreover, cytomegalovirus (CMV) infection is reported to be highly associated with the progression from non-neovascular to neovascular AMD. CMV could infect monocytes, neutrophils, and choriocapillaris endothelium, which could contribute to the initiation of CNV.25
Para-inflammation is a tissue adaptive response to noxious stress or malfunction and is regarded as an intermediate to the basal and inflammatory states.26 Normal para-inflammatory responses are beneficial for repairing damage and restoring tissue functionality. Studies suggest that innate immunity pathways are involved in para-inflammation in the retina during ageing, and para-inflammation-related tissue repairing is disrupted in AMD. Although the understanding of the molecular pathways of para-inflammation is very limited, further study on the influence of para-inflammation on AMD pathogenesis could provide crucial information on developing effective treatments.
Because of the substantial amount of evidence suggesting the underlying role of inflammation in AMD, it is logical to target the specific molecules involved in inflammatory pathways. By increasing our knowledge of the complex immunological and inflammatory processes at play, we have the opportunity to develop a more comprehensive understanding of AMD and improve current therapies for this important disease. This review focuses on the therapeutic use of anti-inflammatory agents for AMD.
Corticosteroids
Corticosteroids are well known for their anti-inflammatory, anti-angiogenic, anti-fibrotic, and anti-permeability properties. They have been widely used to treat ocular disorders involving macular oedema and angiogenesis.
Anti-inflammatory mechanism of corticosteroids
Corticosteroids were among the first anti-inflammatory drugs evaluated for treating CNV in AMD patients. Although the anti-inflammatory mechanism of corticosteroids is not fully understood, several characteristics of these drugs have been elucidated: (1) corticosteroids induce lipocortin synthesis, which directly inhibits phospholipase A2 activity and release of arachidonic acid, ultimately decreasing the formation of prostaglandins (PGs) and leukotrienes via cyclooxygenase (COX) and lipoxygenase (LPO) pathways accordingly;27 (2) corticosteroids inhibit release of proinflammatory cytokines (IL-1, IL-3, and TNF-α) on endothelial and other inflammatory cells; (3) corticosteroids inhibit migration and activation of inflammatory cells including macrophages, monocytes, and leukocytes; (4) corticosteroids inhibit accumulation of endothelial leukocyte adhesion molecule-1 mRNA for endotoxin and IL-1-stimulated cells;28 (5) corticosteroids decrease the number and size of microglial cells;12 and (6) corticosteroids downregulate the cytokine-induced expression of ICAM-1, MHC-I, and MHC-II on endothelial cells, which further inhibits adhesion and migration of inflammatory cells.12, 27
In addition to the anti-inflammatory effects, corticosteroids can directly and indirectly reduce the permeability of choroidal endothelial cells and the outer blood retina barrier, inhibit the activation of matrix metalloproteinase, and suppress vascular endothelial growth factor (VEGF) expression.29 Because VEGF and inflammatory cells closely interact with each other, inhibition of VEGF might strengthen the anti-inflammatory activity in neovascular AMD. The downregulation of inflammatory agents and inhibition of blood vessel permeability are regarded as the main goals of AMD treatment.
Dexamethasone
Dexamethasone is regarded as one of the most potent corticosteroid agents. Several reports have shown that dexamethasone can be combined with verteporfin photodynamic therapy (PDT) and anti-VEGF agents to treat CNV lesions from AMD. The use of these three combinations is known as triple therapy, which can decrease the number of required anti-VEGF injections and stabilize visual acuity in neovascular AMD patients.30, 31, 32 In a prospective and noncomparative case study, 104 patients with CNV due to AMD received the triple therapy of PDT, intravitreal dexamethasone (800 μg), and bevacizumab (1.5 mg).31 Significant and sustained improvements in visual acuity were observed after only one cycle of treatment. A total of 18 patients received an additional bevacizumab injection and only five patients required a second cycle of triple therapy. After 40 weeks, mean visual acuity improved from 20/126 to 20/85 and mean retinal thickness decreased from 463.5 to 281 μm.
Two retrospective studies confirmed the effectiveness of triple therapy using various doses and follow-up times. Ehmann and Garcia32 reviewed 32 neovascular AMD eyes treated with PDT followed by an intravitreal dexamethasone (800 μg) and an intravitreal bevacizumab (1.25 mg) injection 1 and 7 weeks later. The mean number of treatment cycles of bevacizumab injections was 1.4 and 2.8, respectively. After 12 months, visual acuity improved from 20/100 to 20/70 and foveal thickness decreased from 328 to 216 μm. No rise in intraocular pressure (IOP) was recorded in any of the patients. Bakri et al30 tested lower doses of triple therapy by treating 31 patients, 18 of whom had previously received anti-VEGF injections, with intravitreal dexamethasone (200 μg). Mean visual acuity of the 31 patients improved from 20/80 to 20/60 and mean central macular thickness decreased from 293 to 245 μm at the final examination (mean 13.7 months). Although both studies illustrated the importance of dexamethasone in AMD treatment, neither set of data were statistically significant because of the small sample size. It is important to note that patients previously treated with anti-VEGF agents did not respond as well to triple therapy as treatment-naive patients. In addition to dexamethasone's effectiveness at improving vision and reducing macular oedema, these three studies also reported no elevation of IOP or other side effects in their patients. This could be attributed to the rapid action, short duration, and fast clearance of dexamethasone in the vitreous.33 Because of dexamethasone's efficacy and safety, intravitreal use of dexamethasone should be considered in combination with PDT and anti-VEGF agents to treat neovascular AMD.
Triamcinolone acetonide (TA)
TA has been used widely in the treatment of macular oedema and uveitis. Although a single dose of TA has only a fifth of dexamethasone's corticosteroid power, it has a much longer duration of action in the vitreous because of its large particle size.34 Several clinical studies have evaluated periocular or intravitreal TA in the treatment of neovascular AMD. Gillies et al35 conducted a double-masked, placebo-controlled, randomized clinical trial of intravitreal TA (4 mg) injection in 151 patients with classic CNV and 1-year follow-up. The results showed no effect on the risk of vision loss with a single dose of intravitreal TA in their patients. Moreover, there were no differences in size of CNV membranes between the TA and control groups after 1 year, even though CNV membranes were smaller after 3 months in the TA group.
In contrast, Jonas et al36 treated 115 patients with intravitreal TA (25 mg) and recruited 72 patients as non-treatment controls in a prospective, comparative, nonrandomized clinical interventional study. They found significantly increased visual acuities in the TA-treated group at 1 and 3 months when compared with the nontreated group. In an earlier study, these authors reported that intravitreal TA (20 mg) alone improved visual acuity temporarily in 20 patients with bilateral neovascular AMD.37 They concluded that visual improvement was negatively correlated with visual acuity before the injection. Maximal gain in vision was significantly larger in eyes with RPE detachment than in eyes with minimally classic subfoveal neovascularization. This finding may explain the poor outcome in the study by Gillies et al35, which studied patients with baseline vision of 20/200 or better.
The beneficial effect of TA is only temporary. There are a few small-scale studies of intravitreal TA (4 and 25 mg) with a short-term effect of improving or maintaining visual acuity lasting only 1–6 months.38, 39 A randomized clinical trial of 67 patients with neovascular AMD showed no reduction of fluorescein leakage 3 months after a single periocular injection of TA and PDT (n=34) when compared with PDT alone (n=33).40 Lee et al41 reported no beneficial effects after intravitreal injection of TA (20–25 mg) in 39 eyes with minimally classic or occult CNV. Nicolo et al42 confirmed this by showing that TA injection to the vitreous of two patients with serous pigment epithelial detachment (PED) and occult CNV produced no effect. Recently, an intravitreal injection of bevacizumab (1.5 mg) and TA (20 mg) were studied in a comparative, nonrandomized clinical study of 305 patients with progressive AMD.43 The results showed that bevacizumab was far superior to TA as it produced a higher improvement in visual acuity and lower IOP within 2 months of injection.
Agurto-Rivera et al44 compared transpupillary thermotherapy (TTT) (n=12) with TA and TTT alone (n=14) for all types of subfoveal CNV in AMD patients. They found that TTT combined with TA for new subfoveal CNV had a tendency towards better functional results, although there was no statistical significance because of the small sample size of this study. However, Weber45 did not observe the same beneficial effects of TTT and TA in the advanced stage of exudative AMD.
One prospective randomized clinical trial involved 30 eyes of AMD patients with occult or minimally classic CNV. These patients were treated with PDT alone or TA (12 mg) plus PDT treatment.46 Active lesions were treated every 3 months over a 1-year period. Mean visual acuity remained stable in the PDT plus TA group, whereas visual acuity declined significantly in the PDT alone group. The mean number of treatments was 1.13 in the PDT plus TA group and 3.6 in the PDT alone group. This study demonstrated effective stabilization of visual acuity and reduced treatment frequency after 12 months with a combination of PDT plus intravitreal TA. However, larger randomized trials are required to determine the efficacy and risks of PDT with intravitreal TA injection.46 Another study compared PDT plus intravitreal TA (n=66) treatment against PDT-only (n=73) treatment.47 Results show that patients treated with PDT plus TA therapy experienced greater improvements of visual acuity compared with patients treated only with PDT. Choroidal hypofluorescence was evaluated for PDT alone (n=92), PDT plus posterior sub-Tenon TA (n=90), and PDT plus intravitreal bevacizumab (n=60) treatments for subfoveal CNV in 242 AMD patients.48 The degree of choroidal hypofluorescence at 3 months after PDT in the TA and bevacizumab groups was higher than that of PDT-alone group. This study suggests that both sub-Tenon TA and intravitreal bevacizumab could sustain choroidal hypofluorescence after PDT. Additional clinical studies have also suggested that PDT with TA (4 and 25 mg) therapy is able to stabilize vision for up to 2 years.49, 50 Other benefits of PDT plus TA therapy include longer remission time and reduced vascular leakage. Matri et al51 reported that combined therapy with intravitreal TA (4 mg) and bevacizumab (1.25 mg) was an effective and safe method of treating CNV-associated PED in AMD.
To prove the possible tachyphylactic response to bevacizumab and identify ways to decrease biological response, Schaal et al52 investigated whether the repetitive injection of TA and/or bevacizumab resulted in a decrease of the biological response. They recorded standardized volumetric change index from optical coherence tomography scans of 43 patients with exudative AMD and observed a biphasic biological response to TA characterized by a rapid increase in efficacy with the second injection, peaking at the third injection and gradually decreasing afterward. However, the decrease in biological response could partially be alleviated with the combined therapy of TA.
Recently, additional triple therapy clinical studies have been conducted to evaluate the efficiency and safety of these drugs on AMD patients. Yip et al53 evaluated the efficacy and safety of triple therapy on 36 eyes with neovascular AMD. At 6 months, 61.1% (22/36) of patients showed stable or better vision and 27.8% (10/36) gained ⩾3 lines of vision. In all, 28 eyes (77.8%) achieved CNV resolution after a single session of triple therapy. Thus, the triple therapy may become a promising option for neovascular AMD based on its low retreatment rates, lasting CNV eradication, and visual improvement.
The adverse effects of corticosteroids, particularly elevation of IOP and cataract formation, have been of great concern. Although the efficacy of TA and dexamethasone is comparable, TA produces more adverse effects than dexamethasone, such as increased IOP and cataract, because of its longer duration in the eye. Elevation of IOP has been reported in 21–41% of AMD patients who were treated with intravitreal TA (4 and 20 mg) and in 21% patients who were treated with periocular TA.35, 40, 54 Gillies et al55 reported that 28.6% of their patients underwent cataract surgery 12 months after intravitreal injection of 4 mg TA alone. In another prospective randomized trial, Chaudhary et al46 reported cataract progression in four of the seven phakic eyes that were treated with PDT and intravitreal TA. Regular monitoring of IOP and incorporation of antiglaucoma therapy can usually control IOP within the normal range.56
Periocular or intravitreal TA is not recommended as monotherapy for AMD treatment. However, a combination of anti-inflammatory and antiangiogenic agents may produce synergistic effects, such as inhibiting and minimizing the pathologic processes of CNV with inflammatory components. The combined therapy also requires fewer treatment cycles of PDT and/or anti-VEGF injections, thereby lowering the cost and discomfort of intravitreal injections. The general consensus in the literature is that TA should be used as an adjunct agent combined with PDT and/or anti-VEGF agents for treatment of neovascular AMD.
Nonsteroidal anti-inflammatory drugs (NSAIDs)
NSAIDs are a group of chemically heterogeneous compounds that are widely used based on their anti-inflammatory, analgesic, and antipyretic properties. Topical NSAIDs are effectively used to relieve postoperative pain by inhibiting inflammation, countering allergic conjunctivitis and keratitis, inhibiting miosis during cataract surgery, and decreasing cystoid macular oedema.57 The development of new NSAID formulations with better potency, efficacy, and penetration have resulted in the application of these drugs to the treatment of inflammatory and neovascular diseases in the retina and choroid.
Anti-inflammatory mechanism of NSAIDs
NSAIDs are potent inhibitors of the COX pathway, one of the arachidonic acid metabolic pathways that are involved in the production of PGs.58 COX is an important enzyme in eicosanoid metabolism and converts free arachidonic acid to inflammatory and protective PGs. Three isoforms of COX have been identified as COX-1, COX-2, and COX-3. COX-1 is a constitutive enzyme and is expressed in normal tissue. It catalyses the production of PGs (such as PGI2 and PGE2) that mediate normal physiological functions and has a ‘housekeeping' role that maintains homeostasis.59 COX-3 is an acetaminophen-sensitive alternatively spliced variant of COX-1 and has not been well defined.60 In comparison, COX-2 is an inducible enzyme that is expressed in specific cells. This enzyme catalyses the production of inflammatory PGs (such as PGE2 and PGF2α) and is believed to have a role in inflammatory response.59 COX-2 and its catalysed PGs can cause undesirable ocular effects such as increased vascular permeability and disruption of the blood-aqueous barrier.57 COX-2 has been detected in the RPE and vascular endothelial cells in human CNV membranes.61 Its expression is dramatically increased by macrophages and proinflammatory cytokines. In addition to its proinflammatory properties, COX-2 has also been shown to modulate the expression of VEGF and its receptors, which are involved with CNV formation in AMD.57
Topical NSAIDs currently available exhibit varying degrees of COX inhibition. For example, bromfenac has been reported to be a more potent inhibitor of COX-2 than COX-1. Therefore, each NSAID elicits a unique anti-inflammatory response. Nepafenac also exhibits better corneal penetration, longer inhibition of PG synthesis, and increased vascular permeability.62 These drugs have been shown to reduce inflammation, but could also have adverse effects in the gastrointestinal tract when they are absorbed through the mucosal membrane.63 Because of the proinflammatory effects of COX-2 and its metabolic products of PGs, NSAIDs that specifically inhibit COX-2 have been developed. These COX-2-specific NSAIDs, such as celecoxib, have the potential to relieve inflammation without the adverse effects associated with COX-1. However, these NSAIDs have been reported to display cardiovascular toxicity, thereby limiting their application.57 Additionally, diclofenac appears to have a dual effect as it also reduces the products of the LPO pathway, another metabolic pathway for arachidonic acid and the intracellular level of free arachidonic acid.64
In a large-scale randomized trial, 19 716 female health professionals were treated with aspirin (100 mg, every other day), whereas another 19 705 female health professionals were treated with placebo.65 After an average of 10 years of treatment, 111 patients using aspirin developed AMD, whereas 134 patients on placebo (hazard ratio, 0.82) developed AMD. There was an 18% reduction in risk for visually significant AMD and 10% reduction in risk for advanced AMD, neither of which was statistically significant. The study concluded that although low-dose aspirin had no significant contribution to the risk of developing AMD, the potential beneficial effects of aspirin on AMD progression could not be ruled out. The differences in AMD incidence may have been small because of the low dose of aspirin used as well as the large number of patients <65 years. Future studies that examine an older cohort of patients or higher doses of aspirin may produce more definitive results. McGeer et al66 reported a statistically significant decrease of AMD incidence in patients with rheumatoid arthritis (RA) compared with patients without RA. They hypothesized that RA patients were spared from AMD because of long-term treatment with NSAIDs and more powerful anti-inflammatory agents, such as prednisone. This study suggests that a higher dose of aspirin or a more potent anti-inflammatory agent might be more effective at decreasing the prevalence of AMD.
Several small clinical observations have evaluated the effects of topical NSAIDs in the treatment of neovascular AMD. In a case study, Libondi and Jonas67 observed that the application of topical nepafenac (0.1%, 2 to 3 times daily, for 8 weeks) caused regression of macular oedema and a reduction of fluorescein extravasation in one patient with neovascular AMD. However, two other clinical studies failed to show the beneficial effects of topical NSAIDs in patients with neovascular AMD. Zweifel et al68 reviewed 21 neovascular AMD patients (22 eyes) with persistent subretinal and/or intraretinal fluid after anti-VEGF therapy. Topical bromfenac (0.09%, twice daily, for 2 months) was administered in combination with intravitreal bevacizumab (1.25 or 2.5 mg) or ranibizumab (0.5 mg). The results showed no improvement in visual acuity, central retinal thickness, or PED at 1 and 2 months. In another randomized, prospective, and double-masked clinical trial of 61 patients with neovascular AMD, topical diclofenac sodium (0.1%, 4 times daily, for 12 weeks) combined with verteporfin therapy had visual outcomes similar to PDT alone after 12 weeks.69 There is an ongoing phase II trial using a combination of intravitreal ranibizumab injection and topical bromfenac in neovascular AMD patients.70
Some preclinical studies have demonstrated the safety of single intravitreal injections of both diclofenac and ketorolac in healthy rabbits.57 Baranano et al71 showed that intravitreal injection of diclofenac and ketorolac has potent anti-inflammatory effects on a rabbit model of lipopolysaccharide-induced ocular inflammation. Based on these results, Soheilian et al72 conducted a pilot study of 10 patients with refractory macular oedema of various aetiologies, two of whom had neovascular AMD. At 8 weeks after the intravitreal injection of diclofenac (0.5 mg), increased visual acuity was noted in both neovascular AMD patients. Ocular examination and electroretinography (ERG) showed no toxicity in the eyes after 8 weeks. However, this study did not show a significant decrease in central macular thickness, which may be related to the refractory macular oedema in many of these patients.
In comparison with corticosteroids, which inhibit both LPO and COX, NSAIDs show fewer anti-inflammatory effects but do not have corticosteroid-induced IOP elevation.73 The adverse effects of topical NSAIDs often relate to allergy, hypersensitivity, and corneal toxicity.57 Komarowska et al74 observed retinal toxicity following multiple intravitreal ketorolac injections in rabbit eyes. As COX-2 and its catalysed PGs ameliorate the inflammatory process of neovascular AMD, topical and/or intravitreal use of NSAIDs might become a novel adjunct preventive and therapeutic approach with anti-VEGF agents in the treatment of neovascular AMD. However, the long-term efficacy and studies of both topical and intravitreal NSAIDs are needed.
Immunosuppressants
Methotrexate (MTX)
MTX is known to inhibit dihydrofolate reductase and was initially used in high doses to treat malignancies. Primary intraocular lymphoma is treated with intravitreal MTX.75 In 1985, it was demonstrated that low-dose MTX can treat RA without affecting humoral or cellular immunity.76 Furthermore, low doses of MTX have also been shown to be a potent anti-inflammatory agent and are used systemically as a corticosteroid-sparing agent for the treatment of noninfectious uveitis.
There are several proposed mechanisms for the anti-inflammatory effects of low-dose MTX. MTX inhibits cellular proliferation, such as T lymphocytes, by reducing the de novo synthesis of purine and pyrimidine. Additionally, MTX undergoes polyglutamation in cells and inhibits 5-aminoimidazole-4-carboxamide ribonucleotide (AICAR) transformylase, leading to the promotion of intracellular AICAR and the accumulation of extracellular adenosine. Subsequently, adenosine can then interact with the A2A receptor on stimulated inflammatory cells to inhibit cytokine production and diminish inflammation.77 MTX can also reduce intracellular glutathione concentrations, leading to the inhibition of macrophage and lymphocyte function.78 Finally, MTX inhibits the synthesis of potentially toxic compounds (the transmethylation products spermine and spermidine) that accumulate in inflamed tissues.79
Kurup et al80 reported that two neovascular AMD patients were unresponsive to anti-VEGF treatment but showed some improvement when treated with intravitreal MTX injection (400 μg). One patient showed improved visual acuity, whereas both patients showed decreased subretinal fluid accumulation and decreased perifoveal leakage 2 weeks after injection. Although these case reports provide limited data, they may provide substantiation for the role of MTX as an adjunct treatment for neovascular AMD, especially in patients who are resistant to the traditional anti-VEGF therapy. Large-scale and long-term clinical trials are needed to evaluate the effects of intravitreal MTX.
Rapamycin (sirolimus)
Rapamycin, also known as sirolimus, is a macrolytic lactone produced by Streptomyces hygroscopicus. Rapamycin has immunosuppressive, antimicrobial, anti-inflammatory, anti-proliferative, and anti-angiogenic properties. Rapamycin binds to cytosolic FK506 binding protein 12; this complex acts upon the protein kinase mammalian target of rapamycin (mTOR), an important regulator of cell growth.81 Rapamycin downregulates transcription factor E2F-1 and blocks G1-to-S-phase transition in a variety of cell types and species.82, 83 By inhibiting mTOR, rapamycin reduces proliferation and activation of T and B lymphocytes.83 As an anti-inflammatory agent, rapamycin inhibits COX-2 mRNA expression, decreases COX-2-mediated PGE2 production and accumulation, and inhibits inducible nitric oxide synthase expression.84 It also decreases expression of endothelial monocyte-activating polypeptide-II, which reduces inflammatory responses.82
Rapamycin was approved by the FDA for prevention of transplant rejection in 1999 and is used as an effective and potent immunosuppressive treatment in patients with noninfectious uveitis.85 Recently, Nussenblatt et al86 reported the use of rapamycin in three patients with neovascular AMD for 6 months. Although no definitive beneficial effects were found, rapamycin apparently decreased the need for anti-VEGF therapy. An ongoing randomized phase I and II clinical study is evaluating the safety and vision-preserving effects of rapamycin on GA of AMD when administered by subconjunctival injection.87 Another phase II trial is comparing the effectiveness of oral rapamycin, infliximab, and daclizumab in preventing new blood vessel growth in patients with neovascular AMD.88
Biologics
Anti-TNF-α agents
The clinical effects of anti-TNF-α agents have been proved to treat systemic autoimmune disorders such as RA and systemic lupus erythematosus. Recently, several kinds of TNF antagonists are used in the treatment of uveitis and have been demonstrated to be effective. Anti-TNF-α agents can directly neutralize the circulating TNF-α and prevent the binding of TNF to its receptors, p55 and p75. TNF-α antagonists also bind to transmembrane TNF-α, thereby inducing complement-dependent cytotoxicity and antibody-dependent cytotoxicity. The binding of anti-TNF-α agents with transmembrane TNF-α initiates outside-to-inside signal transduction, resulting in apoptosis, suppression of cytokine production, and cell growth arrest in proinflammatory cells. Consequently, the number of activated cells is significantly reduced at the inflamed site.89
Two kinds of anti-TNF-α agents are currently being evaluated for the treatment of neovascular AMD. Infliximab is a chimeric immunoglobin IgG1 monoclonal antibody composed of a constant human Fc region and a variable mouse Fab region. Markomichelakis et al90 studied the effects of intravenous infliximab for RA therapy on three patients with both neovascular AMD and inflammatory arthritic disorders. One treatment-naive patient and two PDT-resistant patients showed increased visual acuity and regression of CNV membrane at 3 months; a sustained infliximab-induced effect was demonstrated in the treatment-naive patient at 12 and 18 months of continuing infliximab therapy. The two PDT-resistant patients also experienced an increase in visual acuity and regression of the subretinal membrane after 3 months. Another report analysed the effects of intravitreal infliximab (1 to 2 mg) injection in three anti-VEGF-resistant neovascular AMD patients.91 One patient showed temporal regression of intraretinal fluid at 6 months, but displayed recurrence after 7 months (5 months after second injection). The other two patients showed improved visual acuity and complete resolution of diffuse intraretinal fluid 2 months after the infliximab injection. Giganti et al92 gave intravitreal injections of infliximab (0.5 mg) to two neovascular AMD patients who were resistant to multiple anti-VEGF injections. Although visual acuity improved in one patient at 12 weeks after injection, both patients had persistent macular oedema. The results of infliximab in neovascular AMD treatment may be inconsistent because of the limited clinical data and small sample size.
Current clinical trials are designed to evaluate the efficacy and safety of inflixibmab in neovascular AMD. The effects of intravenous injection of infliximab in neovascular AMD are being assessed in a phase II clinical trial.88 The other, an ongoing phase I pilot study, is designed to determine the safety and tolerability of intravitreal infliximab in neovascular AMD.93
Adalimumab is another anti-TNF-α agent currently used in clinical trials for neovascular AMD.94 Adalimumab is a fully humanized recombinant anti-TNF-α monoclonal IgG1 antibody and has properties similar to infliximab. An ongoing phase II study assesses the safety and efficacy of intravitreal adalimumab in neovascular AMD patients who are resistant to the conventional treatment of intravitreal ranibizumab.
The adverse effects of anti-TNF-α agents include increased risk of lymphoproliferative malignancy, cell-mediated infection (especially tuberculosis), and demyelinating disease.95 The development of human antichimeric antibodies (HACAs) is another concern for anti-TNF-α agents; adalimumab has a lower risk of causing HACA than infliximab because it is a fully humanized antibody.96 There are also reports of ocular inflammation and decreased response to ERG stimuli.92 Although anti-TNF-α agents can effectively inhibit TNF-α and treat neovascular AMD, more comparative studies are required to better determine efficacy and safety of systemic and intravitreal applications.
Daclizumab
Daclizumab is a fully humanized IgG1 monoclonal antibody directed against the α-chain (Tac/CD25) of the IL-2 receptor (IL-2R). The high-affinity IL-2R is only expressed on activated T cells and is required for clonal expansion and continued viability of T cells. Blockage of IL-2R results in decreased activation and proliferation of T-cell clones. An in vitro study shows that daclizumab directly and specifically interferes with IL-2 signalling at the receptor level by inhibiting the association and subsequent phosphorylation of the IL-2R β- and γ-chains induced by ligand binding.97 Daclizumab also inhibits IFN-γ expression in both CD4+ and CD8+ T cells.98 As an effective agent traditionally used in renal and bone marrow transplantation, daclizumab is now used to treat refractory uveitis based on its anti-T-cell property.99
A pilot prospective study evaluated the use of systemic daclizumab in four patients with neovascular AMD for 6 months.86 The results showed that daclizumab appeared to decrease the need for anti-VEGF intravitreal injections by approximately half. The effects of intravenous injection of daclizumab in neovascular AMD are being assessed in a phase II clinical trial.88 Daclizumab is reported to be relatively well tolerated with virtually no serious adverse effects following intravenous and subcutaneous injection.99, 100 Daclizumab may become a promising adjunctive therapy in neovascular AMD, especially for patients who do not respond to the conventional anti-VEGF agents.
Complement component inhibitors
The complement system is a key component of innate immunity. It is composed of at least 30 proteins and can be activated by the classical, alternative, and lectin pathways. The classical pathway is triggered by the interaction of antigen with specific antibody and other proteins, such as C-reactive protein, immunoglobulins, and complement components.101, 102 The alternative pathway is an antibody-independent route of complement activation and amplification. It is initiated by the hydrolysis of C3 in circulation and involves FB, factor D, and properdin. The alternative pathway is regulated by a number of complement proteins, such as CFH, decay accelerating factor, C4-binding protein, and complement receptor 1. The lectin pathway is triggered by mannose-binding lectin or a related series of termedficolin proteins. All three pathways converge on the activation of C3, and the deposition of C3b and C5b triggers the formation of the MAC, which promotes cell lysis. Many regulatory proteins involved with complement pathway activation are related to AMD progression and could be targeted during therapy.
Several preclinical and clinical trials have been designed to determine the safety and efficacy of complement regulators in AMD therapy. ARC1905 (a C5 inhibitor) and TNX-234 (a humanized antibody against factor D) have been developed in preclinical tests to treat neovascular AMD.103 An ongoing phase II trial is evaluating the effects of intravenous eculizumab (a humanized long-acting monoclonal antibody against C5) for the treatment of non-neovascular AMD.104 Other phase I clinical trials aim to provide initial safety and tolerability information on intravitreal POT-4 (a C3 inhibitor) for treatment of patients with neovascular AMD; however, the results are still inconclusive.105
Given the importance of the complement system in AMD development, complement components and regulators may be targeted in novel adjunctive therapies for non-neovascular and neovascular AMD. Additional clinical studies are needed to identify the long-term effects and potential ocular toxicity of the treatments.
Conclusion
The initial pathological changes in AMD appear in macular photoreceptors, RPE, Bruch's membrane, and choriocapillaris. Although their aetiology is not completely understood, it is indisputable that inflammation, both the innate immunity and autoimmune components, has a critical role in AMD pathogenesis and progression.3, 12, 106 Macrophages, microglia, complement components, and inflammatory cell-derived cytokines and chemokines are involved in both non-neovascular and neovascular AMD.8
Conventional therapy that focuses solely on inhibiting angiogenesis may not be optimal because of the inflammatory involvement in AMD. Inflammation that exacerbates AMD is likely not the initial aetiology. The fact that inflammation appears early in AMD pathology may explain why anti-inflammatory agents are beneficial as preventive or adjunctive therapies in combination with anti-VEGF therapy and/or PDT. Anti-inflammatory therapy also benefits AMD patients who do not respond to conventional anti-VEGF therapy. PDT treatment is reported to cause apoptosis of CNV endothelial cells as well as photoreceptors and RPE.107 The combined use of anti-inflammatory and antiangiogenic agents can also suppress apoptosis of photoreceptors and RPE, resulting in better visual outcomes than PDT alone.
Safety and toxicity are concerns in the topical and intraocular use of anti-inflammatory agents. Because most AMD patients are elderly and more prone to adverse effects of anti-inflammatory agents when compared with younger patients, large-scale and long-term follow-up studies must be considered for the safety and efficacy of novel anti-inflammatory agents.
In summary, the inflammatory process exacerbates AMD pathogenesis. Anti-inflammatory agents, which target specific inflammatory pathways and molecules, could be used as promising adjunct agents combined with anti-VEGF and/or PDT therapy for exudative neovascular AMD and potential therapies for GA AMD (Table 1). More studies are required to test the efficacy and safety of systemic, periocular, and intravitreal applications of each anti-inflammatory agent.
Table 1. Anti-inflammatory agents used in age-related macular degeneration (AMD).
| Category | Examples | Recommendation |
|---|---|---|
| Corticosteroids | Dexamethasone | Used in conjunction with anti-VEGF agents and PDT to increase efficiency of treatment in neovascular AMD, when patients poorly respond or are resistant to anti-VEGF alone. |
| Triamcinolone acetonide (TA) | Not recommended for monotherapy of neovascular AMD, can be combined, when patients poorly respond or are resistant to anti-VEGF alone. | |
| NSAIDs | Bromfenac Nepafenac Diclofenac | Can be combined with anti-VEGF agents in neovascular AMD, more clinical studies are warranted. |
| Aspirin (low dose) | Can be used as long-term anti-inflammatory treatment, potential preventative power against AMD development. | |
| Immunosuppressant | Methotrexate | Alternative treatment for neovascular AMD patients who are resistant to anti-VEGF therapy, more clinical studies are warranted. |
| Rapamycin | Used in neovascular AMD patients and evaluated in clinical trials for non-neovascular and vascular AMD, more clinical studies are warranted. | |
| Anti-TNF-α agents | Infliximab Adalimumab | May be used in anti-VEGF resistant patients; more clinical studies are warranted. |
| IL-2 receptor antagonist | Daclizumab | Used in conjunction with anti-VEGF agents to decrease anti-VEGF treatment in neovascular AMD, more clinical studies are warranted. |
| Complement regulators | ARC1905 TNX-234 | Preclinical drugs developed for neovascular AMD, additional studies need to be conducted to determine the safety and efficacy of these regulators. |
| Eculizumab | Evaluated in clinical trials for non-neovascular AMD. | |
| POT-4 | Evaluated in clinical trials for neovascular AMD. |
Acknowledgments
The NEI Intramural Research Program provided the funding support. Mrs Pamela Sieving provided helpful editing assistance.
The authors declare no conflict of interest.
Footnotes
Part of the work was presented at the 50th Asia Pacific Academy of Ophthalmology Congress in Beijing, September 2010.
References
- World Health Organization Visual impairment and blindness 2009. Available at http://www.who.int/mediacentre/factsheets/fs282/en/index.html . Accessed 9 October 2010.
- Friedman DS, O'Colmain BJ, Munoz B, Tomany SC, McCarty C, de Jong PT, et al. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004;122:564–572. doi: 10.1001/archopht.122.4.564. [DOI] [PubMed] [Google Scholar]
- Coleman HR, Chan CC, Ferris FL, III, Chew EY. Age-related macular degeneration. Lancet. 2008;372:1835–1845. doi: 10.1016/S0140-6736(08)61759-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris FL, III, Fine SL, Hyman L. Age-related macular degeneration and blindness due to neovascular maculopathy. Arch Ophthalmol. 1984;102:1640–1642. doi: 10.1001/archopht.1984.01040031330019. [DOI] [PubMed] [Google Scholar]
- Klein R, Klein BE, Knudtson MD, Meuer SM, Swift M, Gangnon RE. Fifteen-year cumulative incidence of age-related macular degeneration: the Beaver Dam Eye Study. Ophthalmology. 2007;114:253–262. doi: 10.1016/j.ophtha.2006.10.040. [DOI] [PubMed] [Google Scholar]
- Klein R, Cruickshanks KJ, Nash SD, Krantz EM, Javier Nieto F, Huang GH, et al. The prevalence of age-related macular degeneration and associated risk factors. Arch Ophthalmol. 2010;128:750–758. doi: 10.1001/archophthalmol.2010.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding X, Patel M, Chan CC. Molecular pathology of age-related macular degeneration. Prog Retin Eye Res. 2009;28:1–18. doi: 10.1016/j.preteyeres.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel M, Chan CC. Immunopathological aspects of age-related macular degeneration. Semin Immunopathol. 2008;30:97–110. doi: 10.1007/s00281-008-0112-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein RJ, Zeiss C, Chew EY, Tsai JY, Sackler RS, Haynes C, et al. Complement factor H polymorphism in age-related macular degeneration. Science. 2005;308:385–389. doi: 10.1126/science.1109557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivaprasad S, Adewoyin T, Bailey TA, Dandekar SS, Jenkins S, Webster AR, et al. Estimation of systemic complement C3 activity in age-related macular degeneration. Arch Ophthalmol. 2007;125:515–519. doi: 10.1001/archopht.125.4.515. [DOI] [PubMed] [Google Scholar]
- Dastgheib K, Green WR. Granulomatous reaction to Bruch's membrane in age-related macular degeneration. Arch Ophthalmol. 1994;112:813–818. doi: 10.1001/archopht.1994.01090180111045. [DOI] [PubMed] [Google Scholar]
- Penfold PL, Wong JG, Gyory J, Billson FA. Effects of triamcinolone acetonide on microglial morphology and quantitative expression of MHC-II in exudative age-related macular degeneration. Clin Experiment Ophthalmol. 2001;29:188–192. doi: 10.1046/j.1442-9071.2001.00407.x. [DOI] [PubMed] [Google Scholar]
- Apte RS. Regulation of angiogenesis by macrophages. Adv Exp Med Biol. 2010;664:15–19. doi: 10.1007/978-1-4419-1399-9_2. [DOI] [PubMed] [Google Scholar]
- Langmann T. Microglia activation in retinal degeneration. J Leukoc Biol. 2007;81:1345–1351. doi: 10.1189/jlb.0207114. [DOI] [PubMed] [Google Scholar]
- Gupta N, Brown KE, Milam AH. Activated microglia in human retinitis pigmentosa, late-onset retinal degeneration, and age-related macular degeneration. Exp Eye Res. 2003;76:463–471. doi: 10.1016/s0014-4835(02)00332-9. [DOI] [PubMed] [Google Scholar]
- Penfold PL, Provis JM, Furby JH, Gatenby PA, Billson FA. Autoantibodies to retinal astrocytes associated with age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol. 1990;228:270–274. doi: 10.1007/BF00920033. [DOI] [PubMed] [Google Scholar]
- Gu X, Meer SG, Miyagi M, Rayborn ME, Hollyfield JG, Crabb JW, et al. Carboxyethylpyrrole protein adducts and autoantibodies, biomarkers for age-related macular degeneration. J Biol Chem. 2003;278:42027–42035. doi: 10.1074/jbc.M305460200. [DOI] [PubMed] [Google Scholar]
- Morohoshi K, Goodwin AM, Ohbayashi M, Ono SJ. Autoimmunity in retinal degeneration: autoimmune retinopathy and age-related macular degeneration. J Autoimmun. 2009;33:247–254. doi: 10.1016/j.jaut.2009.09.003. [DOI] [PubMed] [Google Scholar]
- Haas P, Steindl K, Schmid-Kubista KE, Aggermann T, Krugluger W, Hageman GS, et al. Complement factor H gene polymorphisms and Chlamydia pneumoniae infection in age-related macular degeneration. Eye (Lond) 2009;23:2228–2232. doi: 10.1038/eye.2008.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen D, Tuo J, Patel M, Herzlich AA, Ding X, Chew EY, et al. Chlamydia pneumoniae infection, complement factor H variants and age-related macular degeneration. Br J Ophthalmol. 2009;93:405–408. doi: 10.1136/bjo.2008.145383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalayoglu MV, Miller JW. Infection, inflammation and age-related macular degeneration. Clin Experiment Ophthalmol. 2007;35:3–4. doi: 10.1111/j.1442-9071.2007.01432.x. [DOI] [PubMed] [Google Scholar]
- Guymer R, Robman L. Chlamydia pneumoniae and age-related macular degeneration: a role in pathogenesis or merely a chance association. Clin Experiment Ophthalmol. 2007;35:89–93. doi: 10.1111/j.1442-9071.2006.01392.x. [DOI] [PubMed] [Google Scholar]
- Fujimoto T, Sonoda KH, Hijioka K, Sato K, Takeda A, Hasegawa E, et al. Choroidal neovascularization enhanced by Chlamydia pneumoniae via Toll-like receptor 2 in the retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2010;51:4694–4702. doi: 10.1167/iovs.09-4464. [DOI] [PubMed] [Google Scholar]
- Baird PN, Robman LD, Richardson AJ, Dimitrov PN, Tikellis G, McCarty CA, et al. Gene-environment interaction in progression of AMD: the CFH gene, smoking and exposure to chronic infection. Hum Mol Genet. 2008;17:1299–1305. doi: 10.1093/hmg/ddn018. [DOI] [PubMed] [Google Scholar]
- Miller DM, Espinosa-Heidmann DG, Legra J, Dubovy SR, Suner IJ, Sedmak DD, et al. The association of prior cytomegalovirus infection with neovascular age-related macular degeneration. Am J Ophthalmol. 2004;138:323–328. doi: 10.1016/j.ajo.2004.03.018. [DOI] [PubMed] [Google Scholar]
- Xu H, Chen M, Forrester JV. Para-inflammation in the aging retina. Prog Retin Eye Res. 2009;28:348–368. doi: 10.1016/j.preteyeres.2009.06.001. [DOI] [PubMed] [Google Scholar]
- Jermak CM, Dellacroce JT, Heffez J, Peyman GA. Triamcinolone acetonide in ocular therapeutics. Surv Ophthalmol. 2007;52:503–522. doi: 10.1016/j.survophthal.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Cronstein BN, Kimmel SC, Levin RI, Martiniuk F, Weissmann G. A mechanism for the antiinflammatory effects of corticosteroids: the glucocorticoid receptor regulates leukocyte adhesion to endothelial cells and expression of endothelial-leukocyte adhesion molecule 1 and intercellular adhesion molecule 1. Proc Natl Acad Sci USA. 1992;89:9991–9995. doi: 10.1073/pnas.89.21.9991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YS, Friedrichs U, Eichler W, Hoffmann S, Wiedemann P. Inhibitory effects of triamcinolone acetonide on bFGF-induced migration and tube formation in choroidal microvascular endothelial cells. Graefes Arch Clin Exp Ophthalmol. 2002;240:42–48. doi: 10.1007/s00417-001-0398-y. [DOI] [PubMed] [Google Scholar]
- Bakri SJ, Couch SM, McCannel CA, Edwards AO. Same-day triple therapy with photodynamic therapy, intravitreal dexamethasone, and bevacizumab in wet age-related macular degeneration. Retina. 2009;29:573–578. doi: 10.1097/IAE.0b013e3181a46a8a. [DOI] [PubMed] [Google Scholar]
- Augustin AJ, Puls S, Offermann I. Triple therapy for choroidal neovascularization due to age-related macular degeneration: verteporfin PDT, bevacizumab, and dexamethasone. Retina. 2007;27:133–140. doi: 10.1097/IAE.0b013e3180323de7. [DOI] [PubMed] [Google Scholar]
- Ehmann D, Garcia R. Triple therapy for neovascular age-related macular degeneration (verteporfin photodynamic therapy, intravitreal dexamethasone, and intravitreal bevacizumab) Can J Ophthalmol. 2010;45:36–40. doi: 10.3129/i09-243. [DOI] [PubMed] [Google Scholar]
- Graham RO, Peyman GA. Intravitreal injection of dexamethasone. Treatment of experimentally induced endophthalmitis. Arch Ophthalmol. 1974;92:149–154. doi: 10.1001/archopht.1974.01010010155016. [DOI] [PubMed] [Google Scholar]
- Francis BA, Chang EL, Haik BG. Particle size and drug interactions of injectable corticosteroids used in ophthalmic practice. Ophthalmology. 1996;103:1884–1888. doi: 10.1016/s0161-6420(96)30411-9. [DOI] [PubMed] [Google Scholar]
- Gillies MC, Simpson JM, Luo W, Penfold P, Hunyor AB, Chua W, et al. A randomized clinical trial of a single dose of intravitreal triamcinolone acetonide for neovascular age-related macular degeneration: one-year results. Arch Ophthalmol. 2003;121:667–673. doi: 10.1001/archopht.121.5.667. [DOI] [PubMed] [Google Scholar]
- Jonas JB, Degenring RF, Kreissig I, Friedemann T, Akkoyun I. Exudative age-related macular degeneration treated by intravitreal triamcinolone acetonide. A prospective comparative nonrandomized study. Eye (Lond) 2005;19:163–170. doi: 10.1038/sj.eye.6701438. [DOI] [PubMed] [Google Scholar]
- Jonas JB, Kreissig I, Degenring RF. Factors influencing visual acuity after intravitreal triamcinolone acetonide as treatment of exudative age related macular degeneration. Br J Ophthalmol. 2004;88:1557–1562. doi: 10.1136/bjo.2003.039552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danis RP, Ciulla TA, Pratt LM, Anliker W. Intravitreal triamcinolone acetonide in exudative age-related macular degeneration. Retina. 2000;20:244–250. [PubMed] [Google Scholar]
- Jonas JB, Spandau UH, Kamppeter BA, Harder B. Follow-up after intravitreal triamcinolone acetonide for exudative age-related macular degeneration. Eye (Lond) 2007;21:387–394. doi: 10.1038/sj.eye.6702222. [DOI] [PubMed] [Google Scholar]
- Gilson MM, Bressler NM, Jabs DA, Solomon SD, Thorne JE, Wilson DJ. Periocular triamcinolone and photodynamic therapy for subfoveal choroidal neovascularization in age-related macular degeneration. Ophthalmology. 2007;114:1713–1721. doi: 10.1016/j.ophtha.2007.03.071. [DOI] [PubMed] [Google Scholar]
- Lee J, Freeman WR, Azen SP, Chung EJ, Koh HJ. Prospective, randomized clinical trial of intravitreal triamcinolone treatment of neovascular age-related macular degeneration: one-year results. Retina. 2007;27:1205–1213. doi: 10.1097/IAE.0b013e31815ec367. [DOI] [PubMed] [Google Scholar]
- Nicolo M, Ghiglione D, Lai S, Calabria G. Intravitreal triamcinolone in the treatment of serous pigment epithelial detachment and occult choroidal neovascularization secondary to age-related macular degeneration. Eur J Ophthalmol. 2005;15:415–419. [PubMed] [Google Scholar]
- Jonas JB, Ihloff AK, Harder B, Kreissig I, Schlichtenbrede F, Libondi T, et al. Intravitreal bevacizumab versus triamcinolone acetonide for exudative age-related macular degeneration. Ophthalmic Res. 2009;41:21–27. doi: 10.1159/000162113. [DOI] [PubMed] [Google Scholar]
- Agurto-Rivera R, Diaz-Rubio J, Torres-Bernal L, Macky TA, Colina-Luquez J, Papa-Oliva G, et al. Intravitreal triamcinolone with transpupillary therapy for subfoveal choroidal neovascularization in age related macular degeneration. A randomized controlled pilot study (ISRCTN74123635) BMC Ophthalmol. 2005;5:27. doi: 10.1186/1471-2415-5-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber U. Exsudative AMD: regression analysis of transpupillary thermotherapy with and without intravitreal injection of triamcinolone, bevacizumab and ranibizumab. Klin Monbl Augenheilkd. 2010;227 (11:897–904. doi: 10.1055/s-0029-1245370. [DOI] [PubMed] [Google Scholar]
- Chaudhary V, Mao A, Hooper PL, Sheidow TG. Triamcinolone acetonide as adjunctive treatment to verteporfin in neovascular age-related macular degeneration: a prospective randomized trial. Ophthalmology. 2007;114:2183–2189. doi: 10.1016/j.ophtha.2007.02.013. [DOI] [PubMed] [Google Scholar]
- Chan A, Blumenkranz MS, Wu KH, Wang G, Berker N, Parast LM, et al. Photodynamic therapy with and without adjunctive intravitreal triamcinolone acetonide: a retrospective comparative study. Ophthalmic Surg Lasers Imaging. 2009;40:561–569. doi: 10.3928/15428877-20091030-05. [DOI] [PubMed] [Google Scholar]
- Hatta Y, Ishikawa K, Nishihara H, Ozawa S, Ito Y, Terasaki H. Effect of photodynamic therapy alone or combined with posterior subtenon triamcinolone acetonide or intravitreal bevacizumab on choroidal hypofluorescence by indocyanine green angiography. Retina. 2010;30:495–502. doi: 10.1097/IAE.0b013e3181bcedbe. [DOI] [PubMed] [Google Scholar]
- Lie S, Aue A, Sacu S, Simader C, Polak K, Schmidt-Erfurth U. Time-course and characteristic morphology of retinal changes following combination of verteporfin therapy and intravitreal triamcinolone in neovascular age-related macular degeneration. Acta Ophthalmol. 2010;88:212–217. doi: 10.1111/j.1755-3768.2008.01405.x. [DOI] [PubMed] [Google Scholar]
- Ruiz-Moreno JM, Montero JA, Zarbin MA. Photodynamic therapy and high-dose intravitreal triamcinolone to treat exudative age-related macular degeneration: 2-year outcome. Retina. 2007;27:458–461. doi: 10.1097/IAE.0b013e318030c77c. [DOI] [PubMed] [Google Scholar]
- el Matri L, Chebil A, Kort F, Bouraoui R, Baklouti K, Mghaieth F. Intravitreal injection of triamcinolone combined with bevacizumab for choroidal neovascularization associated with large retinal pigment epithelial detachment in age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol. 2010;248:779–784. doi: 10.1007/s00417-010-1302-4. [DOI] [PubMed] [Google Scholar]
- Schaal S, Kaplan HJ, Tezel TH. Is there tachyphylaxis to intravitreal anti-vascular endothelial growth factor pharmacotherapy in age-related macular degeneration. Ophthalmology. 2008;115:2199–2205. doi: 10.1016/j.ophtha.2008.07.007. [DOI] [PubMed] [Google Scholar]
- Yip PP, Woo CF, Tang HH, Ho CK. Triple therapy for neovascular age-related macular degeneration using single-session photodynamic therapy combined with intravitreal bevacizumab and triamcinolone. Br J Ophthalmol. 2009;93:754–758. doi: 10.1136/bjo.2008.150987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaide RF, Sorenson J, Maranan L. Photodynamic therapy with verteporfin combined with intravitreal injection of triamcinolone acetonide for choroidal neovascularization. Ophthalmology. 2005;112:301–304. doi: 10.1016/j.ophtha.2004.08.012. [DOI] [PubMed] [Google Scholar]
- Gillies MC, Simpson JM, Billson FA, Luo W, Penfold P, Chua W, et al. Safety of an intravitreal injection of triamcinolone: results from a randomized clinical trial. Arch Ophthalmol. 2004;122:336–340. doi: 10.1001/archopht.122.3.336. [DOI] [PubMed] [Google Scholar]
- Jonas JB, Degenring RF, Kreissig I, Akkoyun I, Kamppeter BA. Intraocular pressure elevation after intravitreal triamcinolone acetonide injection. Ophthalmology. 2005;112:593–598. doi: 10.1016/j.ophtha.2004.10.042. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Flach AJ, Jampol LM. Nonsteroidal anti-inflammatory drugs in ophthalmology. Surv Ophthalmol. 2010;55:108–133. doi: 10.1016/j.survophthal.2009.07.005. [DOI] [PubMed] [Google Scholar]
- Vane JR, Botting RM.Mechanism of action of nonsteroidal anti-inflammatory drugs Am J Med 19981042S–8S.discussion 21S–22S. [DOI] [PubMed] [Google Scholar]
- Vane JR, Bakhle YS, Botting RM. Cyclooxygenases 1 and 2. Annu Rev Pharmacol Toxicol. 1998;38:97–120. doi: 10.1146/annurev.pharmtox.38.1.97. [DOI] [PubMed] [Google Scholar]
- Botting RM. Vane's discovery of the mechanism of action of aspirin changed our understanding of its clinical pharmacology. Pharmacol Rep. 2010;62:518–525. doi: 10.1016/s1734-1140(10)70308-x. [DOI] [PubMed] [Google Scholar]
- Maloney SC, Fernandes BF, Castiglione E, Antecka E, Martins C, Marshall JC, et al. Expression of cyclooxygenase-2 in choroidal neovascular membranes from age-related macular degeneration patients. Retina. 2009;29:176–180. doi: 10.1097/IAE.0b013e3181884fa6. [DOI] [PubMed] [Google Scholar]
- Gamache DA, Graff G, Brady MT, Spellman JM, Yanni JM. Nepafenac, a unique nonsteroidal prodrug with potential utility in the treatment of trauma-induced ocular inflammation: I. Assessment of anti-inflammatory efficacy. Inflammation. 2000;24:357–370. doi: 10.1023/a:1007049015148. [DOI] [PubMed] [Google Scholar]
- Chakraborti AK, Garg SK, Kumar R, Motiwala HF, Jadhavar PS. Progress in COX-2 inhibitors: a journey so far. Curr Med Chem. 2010;17:1563–1593. doi: 10.2174/092986710790979980. [DOI] [PubMed] [Google Scholar]
- Ku EC, Lee W, Kothari HV, Scholer DW. Effect of diclofenac sodium on the arachidonic acid cascade. Am J Med. 1986;80:18–23. doi: 10.1016/0002-9343(86)90074-4. [DOI] [PubMed] [Google Scholar]
- Christen WG, Glynn RJ, Chew EY, Buring JE. Low-dose aspirin and medical record-confirmed age-related macular degeneration in a randomized trial of women. Ophthalmology. 2009;116:2386–2392. doi: 10.1016/j.ophtha.2009.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeer PL, Sibley J. Sparing of age-related macular degeneration in rheumatoid arthritis. Neurobiol Aging. 2005;26:1199–1203. doi: 10.1016/j.neurobiolaging.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Libondi T, Jonas JB. Topical nepafenac for treatment of exudative age-related macular degeneration. Acta Ophthalmol. 2010;88:e32–e33. doi: 10.1111/j.1755-3768.2008.01491.x. [DOI] [PubMed] [Google Scholar]
- Zweifel SA, Engelbert M, Khan S, Freund KB. Retrospective review of the efficacy of topical bromfenac (0.09%) as an adjunctive therapy for patients with neovascular age-related macular degeneration. Retina. 2009;29:1527–1531. doi: 10.1097/IAE.0b013e3181b32f4c. [DOI] [PubMed] [Google Scholar]
- Boyer DS, Beer PM, Joffe L, Koester JM, Marx JL, Weisberger A, et al. Effect of adjunctive diclofenac with verteporfin therapy to treat choroidal neovascularization due to age-related macular degeneration: phase II study. Retina. 2007;27:693–700. doi: 10.1097/IAE.0b013e318030e519. [DOI] [PubMed] [Google Scholar]
- ClinicalTrials.gov identifier: NCT00805233. Combination ranibizumab and bromfenac for neovascular age-related macular degeneration 2009. Available at http://www.clinicaltrials.gov/ct2/show/NCT00805233 . Accessed 9 October 2010.
- Baranano DE, Kim SJ, Edelhauser HF, Durairaj C, Kompella UB, Handa JT. Efficacy and pharmacokinetics of intravitreal non-steroidal anti-inflammatory drugs for intraocular inflammation. Br J Ophthalmol. 2009;93:1387–1390. doi: 10.1136/bjo.2009.157297. [DOI] [PubMed] [Google Scholar]
- Soheilian M, Karimi S, Ramezani A, Peyman GA. Pilot study of intravitreal injection of diclofenac for treatment of macular edema of various etiologies. Retina. 2010;30:509–515. doi: 10.1097/IAE.0b013e3181bdfa43. [DOI] [PubMed] [Google Scholar]
- Snir M, Axer-Siegel R, Friling R, Weinberger D. Efficacy of diclofenac versus dexamethasone for treatment after strabismus surgery. Ophthalmology. 2000;107:1884–1888. doi: 10.1016/s0161-6420(00)00350-x. [DOI] [PubMed] [Google Scholar]
- Komarowska I, Heilweil G, Rosenfeld PJ, Perlman I, Loewenstein A. Retinal toxicity of commercially available intravitreal ketorolac in albino rabbits. Retina. 2009;29:98–105. doi: 10.1097/IAE.0b013e31817d8c09. [DOI] [PubMed] [Google Scholar]
- Pe'er J, Hochberg FH, Foster CS. Clinical review: treatment of vitreoretinal lymphoma. Ocul Immunol Inflamm. 2009;17:299–306. doi: 10.3109/09273940903370755. [DOI] [PubMed] [Google Scholar]
- Weinblatt ME, Coblyn JS, Fox DA, Fraser PA, Holdsworth DE, Glass DN, et al. Efficacy of low-dose methotrexate in rheumatoid arthritis. N Engl J Med. 1985;312:818–822. doi: 10.1056/NEJM198503283121303. [DOI] [PubMed] [Google Scholar]
- Montesinos MC, Takedachi M, Thompson LF, Wilder TF, Fernandez P, Cronstein BN. The antiinflammatory mechanism of methotrexate depends on extracellular conversion of adenine nucleotides to adenosine by ecto-5′-nucleotidase: findings in a study of ecto-5′-nucleotidase gene-deficient mice. Arthritis Rheum. 2007;56:1440–1445. doi: 10.1002/art.22643. [DOI] [PubMed] [Google Scholar]
- Phillips DC, Woollard KJ, Griffiths HR. The anti-inflammatory actions of methotrexate are critically dependent upon the production of reactive oxygen species. Br J Pharmacol. 2003;138:501–511. doi: 10.1038/sj.bjp.0705054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronstein BN. Low-dose methotrexate: a mainstay in the treatment of rheumatoid arthritis. Pharmacol Rev. 2005;57:163–172. doi: 10.1124/pr.57.2.3. [DOI] [PubMed] [Google Scholar]
- Kurup SK, Gee C, Greven CM. Intravitreal methotrexate in therapeutically resistant exudative age-related macular degeneration. Acta Ophthalmol. 2010;88:e145–e146. doi: 10.1111/j.1755-3768.2009.01560.x. [DOI] [PubMed] [Google Scholar]
- Gingras AC, Raught B, Sonenberg N. Regulation of translation initiation by FRAP/mTOR. Genes Dev. 2001;15:807–826. doi: 10.1101/gad.887201. [DOI] [PubMed] [Google Scholar]
- Zohlnhofer D, Nuhrenberg TG, Neumann FJ, Richter T, May AE, Schmidt R, et al. Rapamycin effects transcriptional programs in smooth muscle cells controlling proliferative and inflammatory properties. Mol Pharmacol. 2004;65:880–889. doi: 10.1124/mol.65.4.880. [DOI] [PubMed] [Google Scholar]
- Schmelzle T, Hall MN. TOR, a central controller of cell growth. Cell. 2000;103:253–262. doi: 10.1016/s0092-8674(00)00117-3. [DOI] [PubMed] [Google Scholar]
- Attur MG, Patel R, Thakker G, Vyas P, Levartovsky D, Patel P, et al. Differential anti-inflammatory effects of immunosuppressive drugs: cyclosporin, rapamycin and FK-506 on inducible nitric oxide synthase, nitric oxide, cyclooxygenase-2 and PGE2 production. Inflamm Res. 2000;49:20–26. doi: 10.1007/PL00000199. [DOI] [PubMed] [Google Scholar]
- Shanmuganathan VA, Casely EM, Raj D, Powell RJ, Joseph A, Amoaku WM, et al. The efficacy of sirolimus in the treatment of patients with refractory uveitis. Br J Ophthalmol. 2005;89:666–669. doi: 10.1136/bjo.2004.048199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nussenblatt RB, Byrnes G, Nida H, Yeh S, Faia L, Meyerle C, et al. A randomized pilot study of systemic immunosuppression in the treatment of age-related macular degeneration with choroidal neovascularization. Retina. 2010;30 (10:1579–1587. doi: 10.1097/IAE.0b013e3181e7978e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ClinicalTrials.gov identifier: NCT00766649 Sirolimus to treat geographic atrophy associated with age-related macular degeneration 2010. Available at http://www.clinicaltrial.gov/ct2/show/NCT00766649 . Accessed 9 October 2010.
- ClinicalTrials.gov identifier: NCT00304954 Infliximab, Sirolimus and Daclizumab to Treat Age-Related Macular Degeneration 2010. Available at http://www.clinicaltrial.gov/ct2/show/NCT00304954 . Accessed 9 October 2010.
- Sfikakis PP. The first decade of biologic TNF antagonists in clinical practice: lessons learned, unresolved issues and future directions. Curr Dir Autoimmun. 2010;11:180–210. doi: 10.1159/000289205. [DOI] [PubMed] [Google Scholar]
- Markomichelakis NN, Theodossiadis PG, Sfikakis PP. Regression of neovascular age-related macular degeneration following infliximab therapy. Am J Ophthalmol. 2005;139:537–540. doi: 10.1016/j.ajo.2004.09.058. [DOI] [PubMed] [Google Scholar]
- Theodossiadis PG, Liarakos VS, Sfikakis PP, Vergados IA, Theodossiadis GP.Intravitreal administration of the anti-tumor necrosis factor agent infliximab for neovascular age-related macular degeneration Am J Ophthalmol 2009147825–830.830 e821. [DOI] [PubMed] [Google Scholar]
- Giganti M, Beer PM, Lemanski N, Hartman C, Schartman J, Falk N. Adverse events after intravitreal infliximab (Remicade) Retina. 2010;30:71–80. doi: 10.1097/IAE.0b013e3181bcef3b. [DOI] [PubMed] [Google Scholar]
- ClinicalTrials.gov identifier: NCT00695682 Intravitreal infliximab for diabetic macular edema (DME) and choroidal neovascularization (CNV) (ITVR) 2008. Available at http://www.clinicaltrial.gov/ct2/show/NCT00695682 . Accessed 9 October 2010.
- ClinicalTrials.gov identifier: NCT01136252 Intravitreal adalimumab in patients with choroidal neovascularization secondary to age-related macular degeneration 2010. Available at http://www.clinicaltrial.gov/ct2/show/NCT01136252 . Accessed 9 October 2010.
- Mikuls TR, Weaver AL. Lessons learned in the use of tumor necrosis factor-alpha inhibitors in the treatment of rheumatoid arthritis. Curr Rheumatol Rep. 2003;5:270–277. doi: 10.1007/s11926-003-0005-9. [DOI] [PubMed] [Google Scholar]
- Rodrigues EB, Farah ME, Maia M, Penha FM, Regatieri C, Melo GB, et al. Therapeutic monoclonal antibodies in ophthalmology. Prog Retin Eye Res. 2009;28:117–144. doi: 10.1016/j.preteyeres.2008.11.005. [DOI] [PubMed] [Google Scholar]
- Goebel J, Stevens E, Forrest K, Roszman TL. Daclizumab (Zenapax) inhibits early interleukin-2 receptor signal transduction events. Transpl Immunol. 2000;8:153–159. doi: 10.1016/s0966-3274(00)00021-6. [DOI] [PubMed] [Google Scholar]
- McDyer JF, Li Z, John S, Yu X, Wu CY, Ragheb JA. IL-2 receptor blockade inhibits late, but not early, IFN-gamma and CD40 ligand expression in human T cells: disruption of both IL-12-dependent and -independent pathways of IFN-gamma production. J Immunol. 2002;169:2736–2746. doi: 10.4049/jimmunol.169.5.2736. [DOI] [PubMed] [Google Scholar]
- Nussenblatt RB, Peterson JS, Foster CS, Rao NA, See RF, Letko E, et al. Initial evaluation of subcutaneous daclizumab treatments for noninfectious uveitis: a multicenter noncomparative interventional case series. Ophthalmology. 2005;112:764–770. doi: 10.1016/j.ophtha.2004.12.034. [DOI] [PubMed] [Google Scholar]
- Mottershead M, Neuberger J. Daclizumab. Expert Opin Biol Ther. 2007;7:1583–1596. doi: 10.1517/14712598.7.10.1583. [DOI] [PubMed] [Google Scholar]
- Jha P, Bora PS, Bora NS. The role of complement system in ocular diseases including uveitis and macular degeneration. Mol Immunol. 2007;44:3901–3908. doi: 10.1016/j.molimm.2007.06.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehrs KM, Jackson JR, Brown EN, Allikmets R, Hageman GS. Complement, age-related macular degeneration and a vision of the future. Arch Ophthalmol. 2010;128:349–358. doi: 10.1001/archophthalmol.2010.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricklin D, Lambris JD. Complement-targeted therapeutics. Nat Biotechnol. 2007;25:1265–1275. doi: 10.1038/nbt1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ClinicalTrials.gov identifier: NCT00935883 Complement inhibition with eculizumab for the treatment of non-exudative macular degeneration (AMD) (COMPLETE) 2009. Available at http://www.clinicaltrial.gov/ct2/show/NCT00935883 . Accessed 9 October 2010.
- ClinicalTrials.gov identifier: NCT00473928 Safety of intravitreal POT-4 therapy for patients with neovascular age-related macular degeneration (AMD) (ASaP) 2010. Available at http://www.clinicaltrial.gov/ct2/show/NCT00473928 . Accessed 9 October 2010.
- Grossniklaus HE, Green WR. Choroidal neovascularization. Am J Ophthalmol. 2004;137:496–503. doi: 10.1016/j.ajo.2003.09.042. [DOI] [PubMed] [Google Scholar]
- Miller JW. Higher irradiance and photodynamic therapy for age-related macular degeneration (an AOS thesis) Trans Am Ophthalmol Soc. 2008;106:357–382. [PMC free article] [PubMed] [Google Scholar]