Abstract
In 1993, DeValois and DeValois proposed a “multi-stage color model” to explain how the cortex is ultimately able to deconfound the responses of neurons receiving input from three cone types in order to produce separate red-green and blue-yellow systems, as well as segregate luminance percepts (black-white) from color. This model extended the biological implementation of Hurvich and Jameson’s Opponent-Process Theory of color vision, a two-stage model encompassing the three cone types combined in a later opponent organization, which has been the accepted dogma in color vision. DeValois’ model attempts to satisfy the long-remaining question of how the visual system separates luminance information from color, but what are the cellular mechanisms that establish the complicated neural wiring and higher-order operations required by the Multi-stage Model? During the last decade and a half, results from molecular biology have shed new light on the evolution of primate color vision, thus constraining the possibilities for the visual circuits. The evolutionary constraints allow for an extension of DeValois' model that is more explicit about the biology of color vision circuitry, and it predicts that human red-green colorblindness can be cured using a retinal gene therapy approach to add the missing photopigment, without any additional changes to the post-synaptic circuitry.
1. INTRODUCTION
In 1993, DeValois and DeValois proposed a “multi-stage color model” to explain how the cortex is ultimately able to deconfound the responses of neurons receiving input from three cone types--short- (S-), middle- (M-), and long- (L-) wavelength sensitive--to produce separate red-green and blue-yellow systems, as well as segregate luminance percepts (black-white) from color (DeValois & DeValois, 1993). This model extended the biological implementation of Hurvich and Jameson’s Opponent-Process Theory of color vision (Hurvich & Jameson, 1957), a twostage model encompassing the three cone types combined in a later opponent organization, which has been the accepted dogma in color vision. The DeValois’ model attempts to satisfy the long-remaining question of how the visual system separates luminance information from color, but what are the cellular mechanisms that establish the complicated neural wiring and higherorder operations required by the Multi-Stage Model? Throughout the last decade and a half, results from molecular biology have shed new light on the evolution of primate color vision, thus constraining the possibilities for the circuitry underlying each of the six main hue percepts – red, green, blue, yellow, black, and white. The evolutionary constraints allow for an extension of DeValois' model that is more explicit about the biology of the circuitry, and it predicts that human red-green colorblindness can be cured using a retinal gene therapy approach to add the missing cone photopigment (M or L), without further modifications that would be required to transform neural circuits for luminance into ones for color.
2. A BRIEF HISTORY OF COLOR VISION THEORY
Prior to the emergence of modern biological techniques, breakthroughs in color vision research stemmed from careful consideration of perceptual experiences. The three-component theories of Young and Helmholtz, as well as Hering’s conflicting hypothesis of three paired, opponent color processes were developed in the 1800’s, long before the three types of cone photopigment were isolated and characterized within the retina. In the 1950’s Hurvich and Jameson proposed a resolution to the apparent conflict between earlier models by combining them in a two-stage theory of color vision (Hurvich & Jameson, 1957). The first stage was comprised of three cone types, the outputs of which were combined in an opponent organization at the second stage. Their model accounted for observations that there are four main hue percepts arranged in opponent pairs, red-green and blue-yellow, in addition to achromatic black-white opponency. Shortly thereafter, L/M “ON-” and “OFF-type” opponent cells were, indeed, discovered in the lateral geniculate nucleus (LGN) (DeValois, Abramov, & Jacobs, 1966; Wiesel & Hubel, 1966), providing a physiological substrate for the red-green circuitry proposed by Hurvich and Jameson. However, in later experiments, identifying a corresponding number of opponent cells with appropriate response characteristics for blue-yellow color vision, as would be expected based on the similar acuities of red-green and blue-yellow vision, has proved troublesome. To date, only a small percentage of cells responding with S-ON characteristics have been described (Dacey & Lee, 1994), and a corresponding number of S-OFF-type cells has remained elusive. An additional problem with the two-stage model is that most cells in the LGN respond to both color and luminance variations, and confound them, because of the spatial arrangement of their inputs from different cone types. That is, L/M ON- and OFF-opponent cells respond similarly to black-white luminance signals and to red-green chromatic signals, but logic tells us that the visual system is able to separate these confounded responses; otherwise, any time we are presented with a black-white pattern we would have spurious color percepts and vice versa. Furthermore, the idea that red-green color vision is based on comparisons between only L and M cones does not account for data from human psychophysics, which indicates that there is an Scone contribution to red-green, as well as blue-yellow color perception. These considerations lead DeValois & DeValois to use a bottom-up approach, based on anatomically-suggested connections known at the time, in proposing a third stage of cortical processing in which signals from S-opponent cells were added to, or subtracted from, the L- and M-opponent units to split and rotate the geniculate L/M response axis into separate red-green and yellow-blue color channels, and separate luminance from color (DeValois & DeValois, 1993). While an additional stage of higher-level cortical processing is one possibility, understanding how such complicated neural circuitry could have arisen during evolution has been difficult.
3. COLOR VISION FROM AN EVOLUTIONARY PERSPECTIVE
Our dichromatic, or red-green colorblind, primate ancestors had only two types of cone, S and L cones and, presumably, they also had similar circuits underlying their vision as modern-day dichromats. Results from molecular genetics indicate that trichromatic color vision arose relatively recently from a gene duplication event that added M cones. In order for this low probability genetic event to get passed-on and eventually confer routine trichromacy to Old World primates, including humans, it must have produced an immediate advantage for the primate ancestor by adapting some pre-existing visual circuit for a new purpose. One candidate circuit would have been the high-acuity spatial vision circuit; the primate midget system or its precursor, which compared L vs. L cones to provide achromatic luminance signals. A second candidate would have been the pre-existing color vision circuit which compared S vs. L cones to provide blue-yellow color vision. If the first possibility is correct, then additional changes in the post-synaptic circuitry would have been required over time to separate red-green color signals from achromatic luminance signals. However, the ancient, pre-existing blue-yellow circuitry had already evolved mechanisms for filtering-out luminance signals and only responding to color. Thus, a more plausible explanation is that rather than “hijacking” pre-existing luminance circuitry, the new class of M cone changed the input to the pre-existing blue-yellow circuit such that it automatically gave rise to a new dimension of red-green color vision. That is, in a dichromat with only S and L cones, any circuits across the retina that compared spectrally different cones would have the same S vs. L opponency, but the addition of M cones would introduce a variety of possible comparisons, providing two different L/M receptive field organizations. When combined with S cones in the existing chromatic pathway, they produced two different chromatic signatures, one corresponding to red-green and another to blue-yellow color vision. This idea implies that luminance and color information become segregated as early as the initial inputs into primary visual cortex, and that the specialized functions of the higherorder cortical circuits are imposed by the character of the peripheral receptor mosaic.
4. EVOLUTIONARY CONSTRAINTS LEAD TO AN EXTENSION OF DEVALOIS’ MODEL
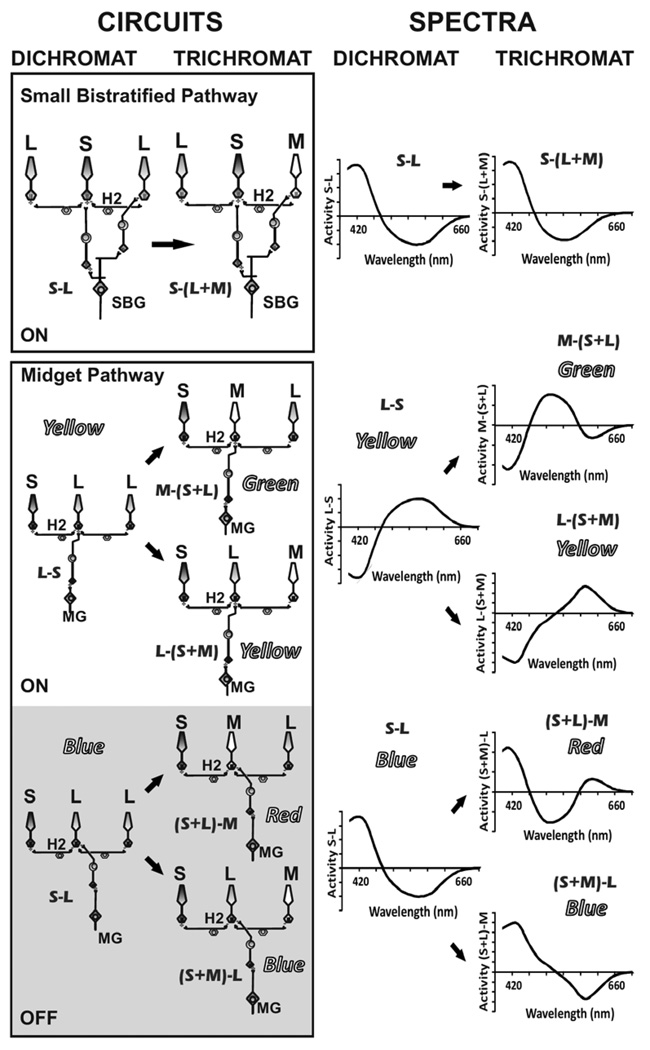
Short wavelength cones are an evolutionarily ancient photoreceptor type and blue-yellow color vision, based on comparisons between S cones and longer-wavelength cones, appears to be the ancestor from which all other color vision evolved. DeValois and DeValois explained their multi-stage model in terms of S-cone input being either added to or subtracted from the redgreen system. However, from an evolutionary perspective, it was actually a new M cone input that was either added to the “center” or the “surround” of pre-existing blue-yellow opponent receptive fields, in order to give rise to separate red-green and blue-yellow systems. In primates, the S cones have only two significant outputs: 1) a straight-through output to the receptive field center of S-cone-specific bipolars which, in turn, output to the small bistratified ganglion cells (Figure 1, upper panels) providing a “blue-on” signal (Dacey & Lee, 1994), and 2) input via H2 horizontal cells to adjacent cones (Dacey, Lee, Stafford, Pokorny, & Smith, 1996), which then output to ganglion cells via L/M cone specific bipolar cells (Figure 1, lower panels). The blue- ON bistratified ganglion cell is considered to be a candidate for providing the basis for blueyellow color vision. However, this cell type would have been little affected in its spectral response characteristics by the addition of M cones to the retina; the center would remain unaltered due to the S-cone specific connections made by small bistratified cells, and the surround would be transformed from “L” to “M+L.” Thus, the simple addition of a third cone type would not “split” this ganglion cell class into two spectral types, as required by the evolutionary constraint that the addition of a third cone type produced an immediate advantage for primate ancestors by adding a new dimension of color vision.
FIGURE 1.
The addition of M cones to a dichromat only changes the “surrounds” of small bistratified ganglion (SBG) cell receptive fields, and all SBGs continue to have similar spectral response properties, making them an unlikely substrate for hue sensations of red, green, blue, and yellow. In contrast, both the “center” and “surround” of midget ganglion (MG) cell receptive fields become altered by the addition of M cones, splitting the pre-existing blue and yellow circuits each into two organizations with distinct spectral response properties and providing a biological basis for separate blue-yellow and red-green systems, with only a single change in the cone mosaic. H2 horizontal cells are labeled.
From an anatomical perspective, there is only one other plausible circuit that could be the basis for blue-yellow perception: L/M midget ON and OFF ganglion cells that receive S-cone input in their receptive field surrounds via H2 horizontal cells. Most retinal electrophysiologists would argue from recordings in both the retina and LGN that this type of S-cone input is not observed. Given the paucity of S cones in the retina, it is conceivable that the S-inputs that are transmitted by a correspondingly small percentage of midget cells may have been overlooked in earlier electrophysiological recordings; however, midget cells with these response characteristics have recently been reported by Tailby and colleagues (Tailby, Solomon, & Lennie, 2008). If midget ganglion cells with S-cone-surround input are the basis for the primordial blue-yellow system, the evolutionary steps leading to a new dimension of red-green color vision were as follows. In the dichromatic primate fovea, each L cone has a private connection with two different bipolar cells and two midget ganglion cells, providing an ON- and OFF-pathway for each L cone. The cones are also interconnected by an inhibitory network in which lateral connections are provided by horizontal cells. This is the functional unit of the retina; it acts to compare the light absorption of a single cone to the average light absorption of its neighbors. It is set in such a way that if the activity of a cone is equal to its neighbors, then the antagonistic interaction between center and surround results in no signal. If the center cone is more active than the surrounding cells, then a signal is sent through the ON pathway, and if the center is less active than the surround, the OFF pathway becomes activated. This initial round of lateral inhibition between adjacent cones gives rise to two classes of midget ganglion cells. One class receiving input from an L-center, L-surround receptive field would be spatially-opponent and responsible for signaling high resolution spatial luminance contrast; presumably, “whiteness” through the ON-pathway and “blackness” through the OFF-pathway. These signals are then propagated to the lateral geniculate nucleus and then on to primary visual cortex. In order for this luminance system to function properly, there must also be a second round of lateral antagonism at the level of the visual cortex. The reason for this is that intrinsic noise is very high in cone photoreceptors and ganglion cells produce random action potentials at a high rate in the dark. If cortical-level antagonism did not filter-out these random signals, they would produce a constant pattern of white noise that would be interpreted as a constantly changing blotchy luminance pattern.
In the same dichromatic primate, the second, smaller population of midget ganglion cells that receives input from an L-center, S + L surround receptive field would be spectrally-opponent. Diffuse blue light would cause the L-OFF midget ganglion cell to become activated via S-cone input from the surround, which is inverted at the H2 horizontal cell synapse; thus paradoxically, this OFF-cell would also be carrying an S-ON signal. Likewise, diffuse yellow light would cause the L-ON midget ganglion cell to fire, and it would have an S-OFF response characteristic due to the sign-reversal via H2 horizontal cells. In the circuitry proposed here, there is a total of four midget cell types--luminance “ON” and “OFF,” and S “ON” and “OFF” cells--exactly as is required as the substrate for the sensations of white, black, blue, and yellow, respectively. In contrast to the “blue-ON” small bistratified ganglion cells discussed above, the opponent midget ganglion cells satisfy the evolutionary constraint that the addition of a third class of cone produced the immediate advantage of a new dimension of red-green color vision. That is, when M cones were randomly added to the pre-existing midget system, the spectrally-opponent ganglion cells would have automatically become segregated into two different classes. The center of the receptive field would become either “L” or “M;” accordingly, the receptive field surrounds were transformed to either “M + S” or “L + S,” automatically producing midget cells with two different spectral response characteristics--one corresponding to red-green and the other to blue-yellow color vision (Figure 1, lower panels).
Following the DeValois and DeValois multi-stage color model, all four hue percepts are the result of circuits with input from S cones, and the relationship between cone inputs and hue is as follows: the perception of red comes from a neural comparison between (S+L)-M; green from M-(S+L); blue from (S+M)-L; and yellow from L-(S+M). As explained above, it is possible to extend DeValois’ model in proposing a straightforward mechanism to form these circuits. Simply, midget ganglion cells that have inhibitory S-cone input in their surround become the basis for hue circuits in the cortex. The cone forming the receptive field center can be either L or M, and the ganglion cells can be either ON or OFF center. The resulting four possible combinations correspond to the four main hue perceptions. An ON-center ganglion cell receiving input from an M cone center with S and L cones in the surround makes M-(S+L)-- green; the same receptive field through an OFF-center ganglion cell makes (S+L)-M--red. An L cone center with an M and S surround makes L-(S+M)--yellow; that same receptive field through an OFF-center ganglion cell produces (S+M)-L--blue. Accordingly, cortical neurons receiving input from receptive fields containing only M and L cones are the basis for luminance circuitry and give rise to achromatic white percepts through the ON pathway and black percepts via the OFF pathway. Thus, even though L/M opponent receptive fields result in midget ganglion cells and LGN cells that respond both to color and luminance, the second round of lateral antagonism at the level of visual cortex nulls the spurious color signals, leaving this circuit dedicated to the original purpose for which it evolved--responding to luminance signals only.
5. THE POSSIBILITY OF GENE THERAPY TO CURE RED-GREEN COLORBLINDNESS
An important implication of the color vision model described here is that it should be possible to cure human red-green colorblindness, even in adults. A rapidly progressing field in molecular biology has been viral vector-mediated gene therapy, with great strides being made in the area of vision disorders, in particular. We have recently demonstrated that it is possible to target therapeutic transgenes specifically to cone photoreceptors in primates (Mancuso et al., 2007). Because all of the circuitry required for taking advantage of a third cone type is already present in dichromatic individuals, it should be possible to transform an adult dichromat to a trichromat with full red-green color vision through the simple addition of the missing photopigment to the retina. The model described here implies that gene therapy would recapitulate what occurred during the evolution of trichromatic color vision in our primate ancestors, and the addition of a third cone type would split the dichromat's existing blue-yellow circuits into two classes, one for red-green and the other for blue-yellow color vision.
As discussed above, an unresolved problem in color vision has stemmed from the fact that most cells in the lateral geniculate nucleus respond to both color and luminance variations and confound them. When considering this problem, DeValois and DeValois commented, “The Standard Model [i.e., the two-stage opponent-process theory] has one color system (the RG system) based on the outputs of the L and M cones, some 90–95% of the cone population, whereas the whole YB system is centered on just the remaining 5–10% of the cones, the S cones. Such an imbalance seems inherently implausible, and one of the considerations that led us to our current model was that of attempting to arrive at a more balanced arrangement between the inputs to the red-green and the yellow-blue color systems. One can reasonably argue that the preponderance of L and M cones reflects the fact that these cone types alone are used for luminance detection. However, with current color models, this still leaves one with either an imbalance between the two chromatic systems or the equally distasteful suggestion that only a fraction of the spectrally-opponent information from L and M cones contributes to color vision.” A key difference between our model and DeValois’ model is that evolutionary constraints force us to the notion (albeit "distasteful") that most of the spectrally-opponent information from L and M cones is disregarded by the visual system and that all LGN cells that receive input from L and M, but not S cones, ultimately give rise to achromatic luminance percepts. Consideration of known retinal anatomy and observations from human color perception has guided us to the conclusion that both the red-green and blue-yellow systems are based on the S cones; all four hue percepts require S-cone input early in the visual pathway, rectifying the apparent imbalance between each color system. In summary, trichromats have six different circuits that extract six distinct percepts from an original mosaic containing three cone types. All six circuits arise as the natural consequence of generic cortical circuitry that indiscriminately compares the activity of a cell in the center of its cortical receptive field with the receptive fields of its immediate neighbors. Therefore, the specialized functions of the cortical circuits are imposed by the character of the peripheral receptor mosaic and by post-receptoral elements early in the neural pathway.
ACKNOWLEDGMENTS
Supported by the National Institutes of Health grants R01EY016861, R01EY09303, and Research to Prevent Blindness.
REFERENCES
- Dacey DM, Lee BB. The blue-ON opponent pathway in primate retina originates from a distinct bistratified ganglion cell type. Nature. 1994;367:731–735. doi: 10.1038/367731a0. [DOI] [PubMed] [Google Scholar]
- Dacey DM, Lee BB, Stafford DK, Pokorny J, Smith VC. Horizontal cells of the primate retina: cone specificity without spectral opponency. Science. 1996;271:656–659. doi: 10.1126/science.271.5249.656. [DOI] [PubMed] [Google Scholar]
- DeValois RL, Abramov I, Jacobs GH. Analysis of response patterns of LGN cells. Journal of the Optical Society of America. 1966;56:966–977. doi: 10.1364/josa.56.000966. [DOI] [PubMed] [Google Scholar]
- DeValois RL, DeValois KK. A multi-stage color model. Vision Research. 1993;33(8):1053–1065. doi: 10.1016/0042-6989(93)90240-w. [DOI] [PubMed] [Google Scholar]
- Hurvich LM, Jameson D. An opponent process theory of color vision. Psychological Review. 1957;64:384–404. doi: 10.1037/h0041403. [DOI] [PubMed] [Google Scholar]
- Mancuso K, Hendrickson AE, Connor TB, Jr, Mauck MC, Kinsella JJ, Hauswirth WW, et al. Recombinant adeno-associated virus targets passenger gene expression to cones in primate retina. Journal of the Optical Society of America A Optics, Image Science, and Vision. 2007;24:1411–1416. doi: 10.1364/josaa.24.001411. [DOI] [PubMed] [Google Scholar]
- Tailby C, Solomon SG, Lennie P. Functional asymmetries in visual pathways carrying S-cone signals in macaque. J Neurosci. 2008;28(15):4078–4087. doi: 10.1523/JNEUROSCI.5338-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesel TN, Hubel DH. Spatial and chromatic interactions in the lateral geniculate body of the rhesus monkey. J Neurophysiol. 1966;29(6):1115–1156. doi: 10.1152/jn.1966.29.6.1115. [DOI] [PubMed] [Google Scholar]