Abstract
Gene therapy has emerged as a promising strategy for treatment of various diseases. However, widespread implementation is hampered by difficulties in assessing the success of transfection in the target tissue and the longevity of gene expression. Thus, there is increasing interest in the development of non-invasive in vivo reporter techniques to assay gene expression. We recently demonstrated the ability to detect β-galactosidase activity in stably transfected human prostate tumor xenografts in mice in vivo using 19F NMR. We now extend the studies to human MCF7 breast cancer cells growing as xenografts in nude mice. Moreover, by using two spectrally resolved reporters (o-fluoro-p-nitrophenyl-β-D-galactopyranoside and an isomer) two tumors could be interrogated simultaneously revealing lacZ transgene activity in a stably transfected tumor versus no activity in a wild type tumor. Most significantly hydrolytic activity observed by 19F NMR corresponded with differential activity in lacZ expressing tumors.
Keywords: β-galactosidase, 19F NMR, lacZ, reporter molecules, breast cancer, pH
Introduction
Reporter genes are widely used to study gene transfection, regulation, and tumor development in biological systems. A requirement is that the gene not be normally expressed in the tissue of interest. Popular reporter genes generate β-galactosidase (β-gal), β-glucuronidase (GUS), the sodium iodine symporter (hNIS), firefly luciferase, or fluorescent proteins (e.g., GFP) (1-3). Several of these reporter genes are used routinely in vivo, in particular for studies of mice where optical approaches are effective. In large animals and for deep structures radiological approaches using PET, SPECT or MRI may be required. NMR approaches avoid issues of radioactive substrates with inherent complications of limited shelf life and waste disposal, although NMR reporters must generally be used at higher concentrations. In early work it was recognized that liver lacks creatine kinase (CK), and thus, transfection with CK could be evaluated based on 31P NMR of phosphocreatine, though application was limited to liver. More recently, 1H contrast methods have been developed based on transferrin and ferritin accumulation in transfected tumors. Promises and pitfalls of various approaches were recently reviewed (4).
The lacZ gene encoding β-gal was the first reporter gene to be widely used and remains exceedingly popular (5-7). LacZ has not only been used in cell culture, but applications have extended to clinical trials (8,9). There are many commercial colorimetric indicators available for detecting β-gal activity with diverse properties concerning color, thermal stability and enzyme sensitivity (10-13). However, these indicators are not suitable for applications in vivo. Therefore, development of reporter molecules for non-invasive in vivo detection of lacZ transgene expression would be of considerable value for research and potentially for future clinical gene therapy trials as well. A characteristic of β-gal is its extreme promiscuity (lack of substrate specificity), which can be exploited with a variety of substrate structures. Recently, Tung et al. (14) presented a near infrared fluorescent optical approach based on 9H-(1,3-dichloro-9,9-dimethylacridin-2-one-7-yl) β-D-galactopyranoside, Lee et al. (15) described a radionuclide substrate 2-(4-[125I/123I]iodophenyl)ethyl-1-thio-β-D-galactopyranoside, and Louie et al. (16) reported a Gd(III)-based 1H MRI approach using 1-[2-(β-D-galactopyranosyloxy)propyl]-4,7,10-tris(carboxymethyl)-1,4,7,10-tetraazacyclododecane) gadolinium (III), to assess β-gal activity in vivo.
Seeking the benefits of an NMR approach (deep tissue detectability of stable isotopes), a series of reporter molecules suitable for 19F NMR detection of β-gal activity based on the 19F chemical shift change accompanying enzyme activated cleavage was designed and synthesized (17-22). As an in vivo probe 19F NMR has several virtues: a high magnetogyric ratio, 100% natural abundance, a large chemical shift dispersion and essentially no background signal (23). Recognizing that addition of a fluorine atom to the traditional colorimetric reporter nitrophenyl-β-D-galactopyranosides (ONPG) could generate a sensitive NMR reporter p-fluoro-o-nitrophenyl-β-D-galactopyranoside (PFONPG) was tested as a first example in vitro with β-gal enzyme, and with β-gal expressing prostate cancer cells in culture (17). Subsequently, other fluorinated substrates and isomers of PFONPG, such as o-fluoro-p-nitrophenyl-β-D-galactopyranoside (OFPNPG) were shown to be excellent enzyme substrates exhibiting a single 19F NMR signal with a Δδ of 6 to 10 ppm upon β-gal catalyzed cleavage (19). Recently, OFPNPG was used to interrogate human prostate tumors growing in mice (24). We now demonstrate for the first time, the ability to simultaneously examine and differentiate MCF7-lacZ and wild type (WT) cells growing as separate tumors in mice using this 19F NMR approach.
Materials and Methods
19F NMR substrates for β-gal
The reporter molecules p-fluoro-o-nitrophenyl-β-D-galactopyranoside (PFONPG), o-fluoro-p-nitrophenyl-β-D-galactopyranoside (OFPNPG), p-trifluoromethyl-o-nitrophenyl-β-D-galactopyranoside (PCF3ONPG) and p-fluoro-o-nitrophenyl-β-D-glucopyranoside (PFONPGu, incorporating glucose in place of galactose) were synthesized by us (18-21)1.
Cell preparation
E. coli lacZ gene (from pSV-β-gal vector, Promega, Madison, WI) was inserted into high expression human cytomegalovirus (CMV) immediate-early enhancer/promoter vector phCMV (Gene Therapy Systems, San Diego, CA) giving a recombinant vector phCMV/lacZ, which was used to transfect human MCF7 breast cancer cells using GenePORTER2 (Gene Therapy Systems). The highest β-gal expressing colony was selected using the antibiotic G418 disulfate (800 μg/ml; aminoglycoside antibiotic, Research Products International Corp, Mt. Prospect, IL) and G418 (200 μg/ml) was also included for routine culture.
B-gal activity
The β-gal activity of tumor cells and tissues in mice was measured using a β-gal assay kit (Promega, Madison, WI) with yellow o-nitrophenyl-β-D-galactopyranoside (ONPG).
Western blot
Protein was extracted from tumors and other normal organs and was quantified by a protein assay (Bio-Rad, Hercules, CA) based on the Bradford method (25). Each well was loaded with 30 μg protein and separated by 10% SDS-PAGE (Nu-PAGE) and transferred to a polyvinylidene fluoride (PVDF) membrane. Primary monoclonal anti-β-gal antibody (Promega) and anti-actin antibody (Sigma) were used as probes at a dilution of 1:5000, and reacting protein was detected using a horseradish peroxidase-conjugated secondary antibody and ECL (Enhanced chemiluminescent) detection (Amersham, Piscataway, NJ).
In vitro NMR
NMR spectra were acquired using a vertical bore Varian Unity INOVA 400 spectrometer (376 MHz for 19F) with a dilute solution of sodium trifluoroacetate (NaTFA) in a capillary as external standard (δ = 0 ppm). Mixtures of PFONPGu (2.5 mg, 7.8 μmol) and PFONPG or OFPNPG (2.2 mg, 6.9 μmol) were prepared in PBS (0.1 M, pH 7.4 or 4.5, 500 μl) in 5 mm NMR tubes to assess substrate selectivity. Enzyme was added (typically, 10 μl, 1 unit/ μl of β-galactosidase E801A (Promega, Madison, WI, USA, pH 7.4) or G5160 (Sigma, pH 4.5) or β-glucosidase (Sigma, G0395, pH 5.0) in PBS buffer (0.1 M, pH 7.4 or 4.5) at 22 °C or 37 °C and 19F NMR spectra were acquired immediately and for a period between 20 mins and 20 h.
Control wild type and lacZ-transfected tumor cells were grown in culture dishes under standard conditions in DMEM (Dulbecco's Modification of Eagle's Medium, Mediatech, Inc, Herndon, VA), 10% FBS (Fetal bovine serum, Hyclone, Logan, UT) with 1% penicillin-streptomycin solution (Mediatech) at 37 °C and 5%CO2 in an incubator. Tumor cells were harvested with trypsin/EDTA and suspended in PBS.
Reporter molecule (5 μmol) in PBS (100 μL) was added to suspensions of 6×106 cells in PBS (500 μL) in a 5 mm NMR tube and 19F NMR data were acquired immediately and over a period of 40 mins at 37 °C.
In vivo NMR
Based on the observations in cells, OFPNPG was chosen for initial in vivo investigations and PFONPG selected as a paired agent. For in vivo studies MCF7 cells (2×106 wild type or transfected to stably express lacZ) were implanted subcutaneously in thighs of nude mice. NMR studies were performed when the tumors reached ∼0.8 cm in diameter. Mice were anesthetized (1.5% isoflurane /air @ 1 dm/m3) and placed in the horizontal bore of a 4.7 T Varian Unity INOVA spectrometer (188.2 MHz for 19F) with a 2 cm diameter home built volume coil placed around the tumor. Shimming was performed on the tissue water proton signal. The mouse and surrounding coil were removed from the magnet and an OFPNPG solution (8.2 mg in 100 μL DMSO/PBS 1:1 V/V′ with 1 mg NaTFA) was injected directly into the tumor in a “fan” pattern. Addition of DMSO substantially reduced the total volume required for solvation and injection. The mouse was replaced in the magnet and time course 19F NMR spectra were obtained immediately. Each spectrum was acquired in 2.5 min (pulse width 40 μs, angle 45°) with 128 acquisitions across a spectral width=100 ppm, to cover NaTFA and the reporter molecule. Before Fourier transformation a 40 Hz exponential line broadening was applied.
For in vivo studies with paired reporter molecules, 106 wild type and 106 lacZ expressing cells were implanted subcutaneously in right and left thighs, respectively of 4 nude mice. When tumors reached a diameter of about 0.8 cm, the mice were anesthetized with a ketamine/xylazine cocktail and OFPNPG or PFONPG (50 μl, 0.24 M in DMSO/PBS 1:1 v/v) was injected intratumorally. In these experiments neither NaTFA nor fluorinated anesthetic was used to avoid additional fluorine signals allowing the spectral width to be reduced. The substrate signals were used as internal chemical shift standards. The animal torso was then inserted into a 3.5 cm diameter home-built single turn solenoid volume coil such that both tumors were inside the coil. Time-course 19F NMR data were obtained immediately, as described above.
Histology
For post mortem verification of β-gal, the tumors were excised after the final NMR study an, and cut into 8 μm sections. The sections were fixed with 4% formaldehyde + 0.2% glutaraldehyde in PBS for 10 min, and washed three times in PBS (pH 7.4), then transferred to β–gal staining solution (1 mg/ml of 5-bromo-4-chloro-3-indolyl-β–D-galactopyranoside (X-gal), 2 mM MgCl2, and 5 mM K4Fe(CN)6, and 5 mM K3Fe(CN)6) for various times (20 min to 1 hr) at room temperature, and then stained with Nuclear fast (POLY Scientific, Bay Shore, NY).
Results
Evaluation of reporters in vitro
As reported previously (19,20) each of the agents exhibited a single line in solution with chemical shift invariant with pH. PFONPG, OFPNPG, and PCF3ONPG give a single 19F NMR signal at -42.9, -54.9, and 13.4 ppm, respectively. No line splitting due to proton coupling was observed. Upon addition of β-gal enzyme hydrolysis occurred with a range of rates generating new resolved resonances with a pH dependant chemical shift at -52.7 ppm (PFONP), -61.0 ppm (OFPNP) and 14.5 ppm (PCF3ONP).
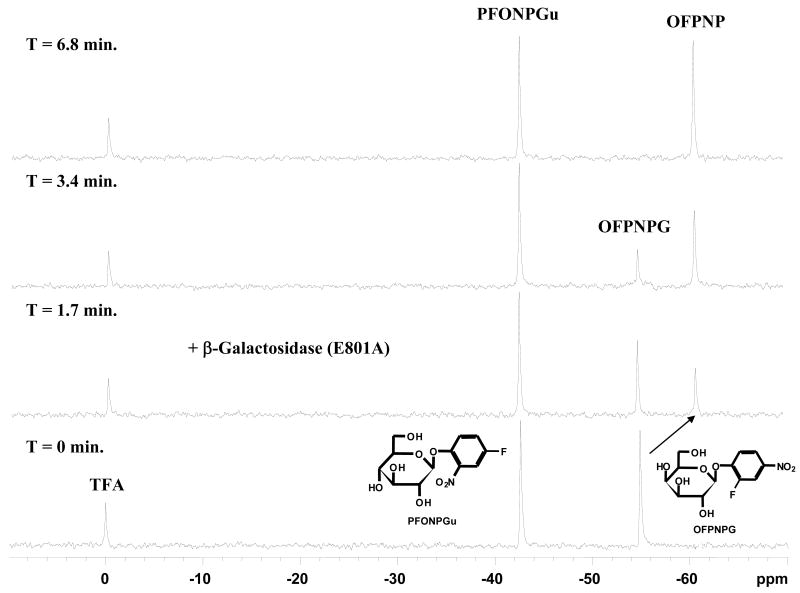
Selectivity for the sugar isomers galactose and glucose was compared with respect to β-galactosidases and β-glucosidase. At pH 7.4 the optimum value for activity of β-gal E801A both β-D-galactosides (OFPNPG and PFONPG) were cleaved, but no hydrolysis was seen for the β-D-glucoside PFONPGu (shown for OFPNPG and PFONPGu in Figure 1). At pH=4.5 the optimal value for activity of β-gal G5160 6.8 μmol of OFPNPG were completely hydrolyzed by 5.7 units in less than 7 mins at 37 °C, whereas PFONPGu showed no hydrolysis at this time and less than 50% after 20 h. At pH 4.5 the glucosidase did cause rapid hydrolysis of both the β-glucoside and β-galactoside.
Figure 1. 19F NMR evaluation of enzyme selectivity towards substrates.
A series of 19F NMR spectra were acquired at 376 MHz of a mixture of PFONPGu (2.5 mg, 7.8 μmol), OFPNPG (2.2 mg, 6.9 μmol) and NaTFA (0.11 mg) in PBS (0.1 M, pH 7.4, 600 μl) with respect to addition of β-gal (E801A, 10 units) at 22 °C. Rapid hydrolysis of OFPNPG was seen generating a new signal for the aglycone, whereas PFONPGu remained unchanged.
Evaluation of reporters in cells
Incubation of PFONPG or OFPNPG with wild-type human MCF7 breast tumor cells for 5 h in PBS at 37 °C under 5% CO2 in air with 95% humidity showed no changes in the 19F NMR spectra. Incubation with lacZ-expressing cells led to release of aglycone products revealed by rapid spectral changes (e.g., OFPNPG in Figure 2). Since each reporter has a unique chemical shift, several reporters can be observed simultaneously, as shown in Figure 3, although the high cumulative concentration caused much slower reactivity. When used individually p-trifluoromethyl-o-nitrophenyl-β-D-galactopyranoside (PCF3ONPG) was most reactive with β-gal expressing cells, but provided the smallest chemical shift response (Δδ=1.14 ppm). Western blot and traditional biochemical assay showed at least 10 fold higher β-gal activity in lacZ-than the WT-cells.
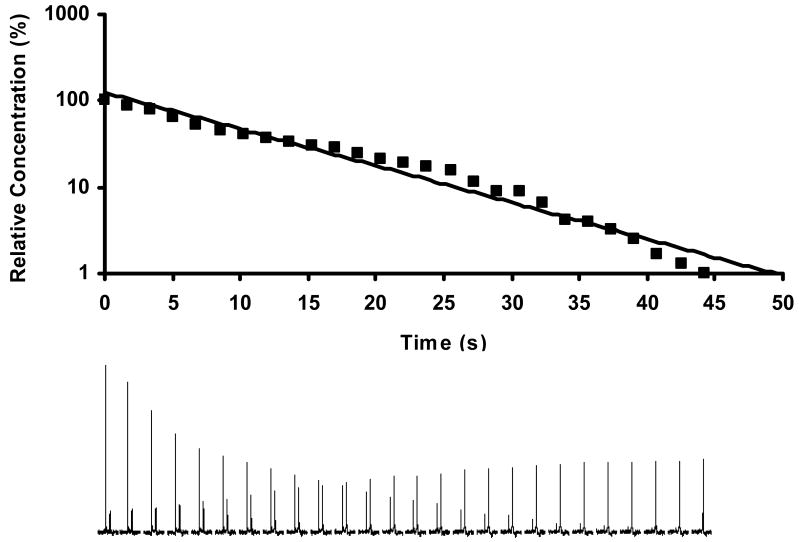
Figure 2. 19F NMR time-course of OFPNPG hydrolysis by MCF7-lacZ cells.
OFPNPG (5.4 mg, 17.0 μmol) was added to stably transfected MCF7-lacZ cells (2×107) in PBS (0.1 M, pH=7.4, 600 μL) at 37 °C. a) 19F NMR spectra were acquired consecutively in 102 s each, and enhanced with an exponential line broadening (40 Hz). Decline of the OFPNPG is apparent accompanied by appearance of the new upfield signal for the aglycone OFPNP. b) Logarithmic fit to signal intensity of OFPNPG indicating first order kinetics with a rate of 17 nmol/106 cells/min.
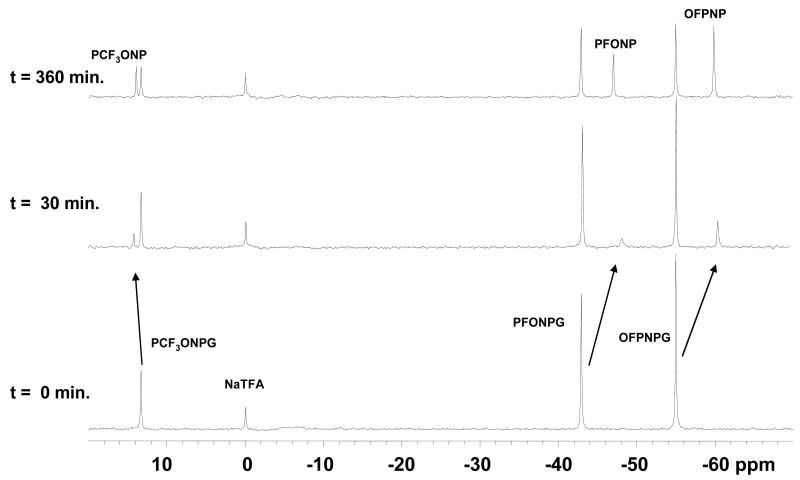
Figure 3. Detection of β-gal using multiple reporter molecules simultaneously in breast tumor cells.
A mixture of PCF3ONPG (1.7 mg, 4.6 μmol), PFONPG (4.6 mg, 14.5 μmol) and OFPNPG (4.8 mg, 15.1 μmol) in 100 μl PBS was added to stably transfected MCF7-lacZ cells (3.0×106) in PBS (0.1 M, pH=7.4, 500 μL) and incubated at 37 °C. 19F NMR spectra (376 MHz) were acquired in 16 min each starting at the times shown, and enhanced with an exponential line broadening (40 Hz). In this case the presence of high concentrations of the three reporter molecules seemed to inhibit activity, probably due to acidification. Within 30 minutes the pH sensitive aglycone product chemical shifts were δF(PCF3ONP) = 14.26 ppm, δF(PFONP) = -47.82 ppm, and δF(OFPNP) = -59.92 ppm each corresponding to pH = 6.10 ± 0.05.
Evaluation of reporters in vivo
Following direct intratumoral injection of OFPNPG (0.24 M, 100 μl) in a (1:1) DMSO/PBS solution into tumors, the hydrolysis of OFPNPG to OFPNP was clearly observed within 10 mins with Δδ -6 ppm in β-gal-expressing tumors (Figure 4). No conversion was seen over the same time period in MCF7 wild-type tumors. Following intratumoral injection of PFONPG into a WT-tumor and OFPNPG into the contralateral lacZ-transfected tumor, extensive conversion of OFPNPG was seen in 30 min, while no conversion of PFONPG was observed (Figure 5). When agents were switched between WT- and lacZ-transfected tumors, both showed rapid hydrolysis in the lacZ-tumor, but neither showed conversion in the wild-type tumor (Figure 6a and b). X-gal and Nuclear fast staining of tumor sections showed intense blue stain for the MCF7-lacZ tumor section only confirming β-gal activity (Figure 6c and d).
Figure 4. In vivo detection of β-gal in breast tumors.
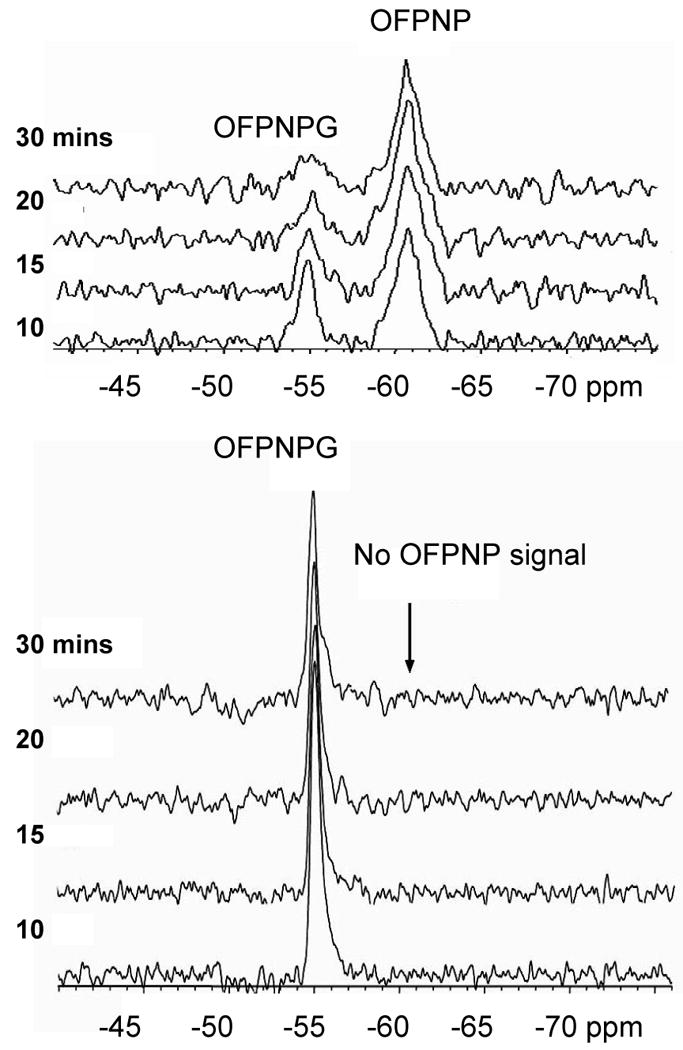
Expansions of 19F NMR (188 MHz) spectra acquired following introduction of OFPNPG into tumors (signals for TFA and isoflurane occurred downfield and are not shown).
a) A solution (8.2 mg in 100 μL 1:1 DMSO/PBS with 10 mg/ml NaTFA) was injected intratumorally in a “fan” pattern into an MCF7-lacZ tumor (0.8×1.1×1.2 cm) in a mouse. Serial spectra were acquired at 4.7 T (188 MHz) with TR=1.0 s and 256 acquisitions (5 min. each) showing liberation of aglycone.
b) As for (a), but wild-type tumor (0.4×0.6×0.9 cm).
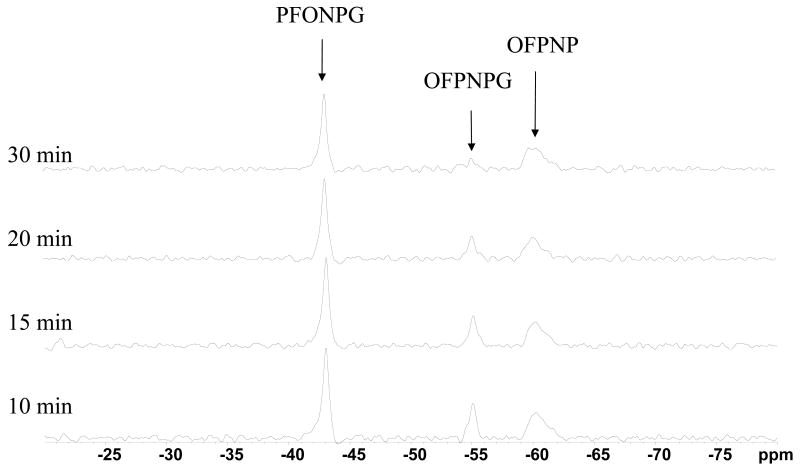
Figure 5. Differentiation of lacZ- and WT-breast tumors in vivo.
19F NMR spectra (188 MHz) of a mouse bearing two thigh tumors. Following intratumoral injection of an aqueous DMSO solution of PFONPG (50 μl) in wild type (WT) tumor and OFPNPG (50 μl) in lacZ transfected tumor, complete conversion of OFPNPG was seen in 30 min, while no conversion of PFONPG was observed. Each spectrum was acquired in 5 mins and 60 Hz exponential line broadening was applied.
Figure 6. 19F NMR Assessment of β-gal expression in tumors in vivo and histological confirmation.
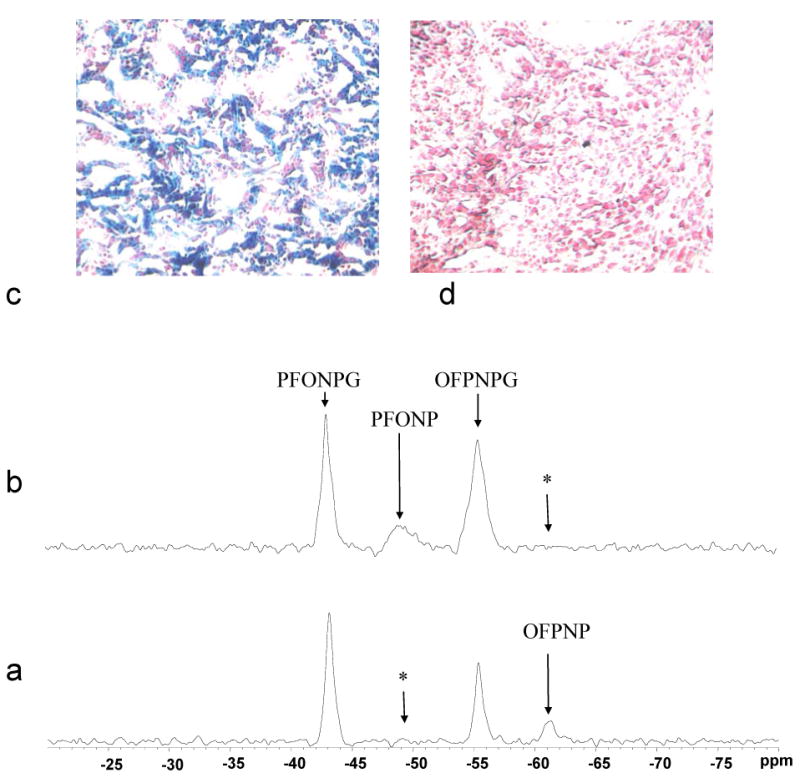
a) Solutions of PFONPG and OFPNPG were injected into tumors, as for Figure 5. Release of OFPNP was observed confirming β-gal expression. * shows location of other aglycone, which was not observed.
b) Five hours later, following clearance of substrates and product, agents were again injected, but into the opposite tumors. Now PFONP was observed, but no OFPNP.
X-gal and Nuclear fast staining of slices from the tumors obtained post mortem (c) MCF7-lacZ (100×) and (d) MCF7-WT(100×). Intense blue stain showed β-gal activity for the MCF7-lacZ tumor section only.
In a series of 8 investigations of paired MCF7-lacZ and –WT tumors in four mice (including repeat measurements as tumors grew) WT tumors never showed any conversion of 19F substrates (OFPNPG or PFONPG), i.e., 100% negative specificity. For lacZ-tumors 5 of 8 measurements showed hydrolysis with appearance of product aglycone, but intriguingly three did not. Where possible, histology was performed soon after the NMR with X-gal staining to evaluate β-gal activity. Lack of β-gal activity was clear in WT tumors, e.g., Figure 6d. In tumors which showed 19F NMR activity strong blue staining was observed within 20 mins exposure time, though color intensity developed further at later times (Figure 7). In the lacZ-tumors, which unexpectedly showed no 19F NMR activity, histology showed little or no blue stain at 20 mins, though there was some development at later times (Figure 7).
Figure 7. Observing differential β-gal activity in MCF7-lacZ tumors.
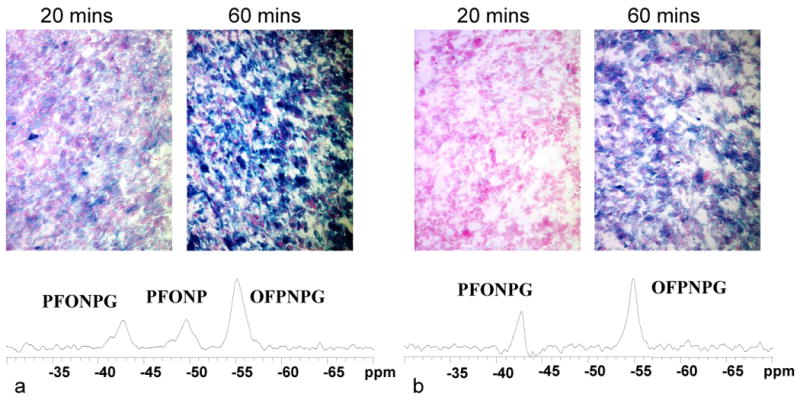
19F NMR spectra are shown for two mice each with a WT- and a lacZ-tumor in contralateral thighs. In each case PFONPG was injected into the lacZ-tumor and OFPNPG into the WT. a) In the first mouse rapid hydrolysis was observed in the lacZ-tumor and β-gal activity was confirmed by intense blue staining with X-gal post mortem (20 and 60 minute development times). b) In the second mouse neither reporter was hydrolyzed indicating lack of β-gal activity in lacZ- or WT-tumor. Histological staining post mortem with X-gal confirmed lower activity, as revealed by the rate and intensity of blue staining.
Discussion
The broad specificity of β-gal allows diverse molecular substrates to be developed as potential reporter molecules. Here, agents incorporating a 19F reporter moiety reveal β-gal activity in cell suspensions and breast tumor xenografts in mice. Each substrate and product is characterized by a unique chemical shift, so that multiple agents can be detected simultaneously.
OFPNPG is stable in buffer or with wild type cells. When injected directly into a wild type MCF7 tumor no conversion was detected over 30 mins, although the substrate was found to decline slightly, likely due to wash out (Figure 4). By comparison, the MCF7-lacZ tumor immediately showed a large product peak. Over the next 30 mins the substrate declined, presumably due to further conversion and some washout, while the product increased slightly. Our first test in vivo used OFPNPG since it is more reactive than PFONPG and the pKa of the aglycone (6.03) is outside the normal physiological range minimizing potential line broadening in tumors due to heterogeneous pH. The aglycone of PFONPG has pKa= 6.87 and in vivo spectra showed a broader signal for the aglycone PFONP compared to OFPNP, but not unduly so (Figure 6), compared with the substrate peaks with linewidths ca. about 250 Hz. Our initial studies in vivo used NaTFA as an internal chemical shift reference and isoflurane as the anesthetic. It rapidly became apparent that the reporter molecules themselves could serve as references for identification of cleavage product aglycones, and thus NaTFA could be omitted. Isoflurane was well resolved from substrates and products of these β-gal reporters, but was often visible with two resonances at -5 and -11 ppm, relative to NaTFA = 0 ppm, as described previously (23). In later experiments we used a non-fluorinated injectable anesthetic to avoid potential signal aliasing when using small spectral acquisition windows (e.g., Fig. 6).
An ultimate goal is to image β-gal activity and this was previously demonstrated in cell culture (20,22), but currently the signal to noise ratio precludes effective MRI in vivo. In the current study spatial discrimination was based on injection of two spectrally distinct substrates into separate contralateral tumors. Thus, both tumors could be interrogated simultaneously and independently. Alternatively, localized spectroscopy could be applied using a single substrate, but this is technically more challenging and requires gradients. For this study OFPNPG and PFONPG were selected since they have similar 19F NMR signals and kinetic characteristics. Discrimination of the contralateral lacZ- and WT-tumors was immediately obvious upon injection (Figures 5 and 6). However, since the hydrolysis rate for OFPNPG was found to be about twice that of PFONPG in MCF7-lacZ cell cultures, there was concern that a differential response in vivo might reflect only the difference in uptake/hydrolysis rates for the two substrates. Thus, investigations were also performed in two phases with substrate switching (Figure 6). In the first phase, OFPNPG showed activity in the lacZ-tumor whereas PFONPG showed none in the WT-tumor. Upon alternating the substrates for the second phase five hours later, consistent results were observed with conversion of the PFONPG in the lacZ-tumor. For the group of four mice with wild type-tumors we achieved 100% negative specificity, i.e., none showed any substrate hydrolysis. Unexpectedly, some MCF7-lacZ tumors showed no apparent β-gal activity by 19F NMR. However, in tumors where histological comparison was available it was reassuring that these tumors also exhibited much less β-gal activity assessed by X-gal staining.
The current approach reveals relative expression in stably transfected tumor xenografts (Figure 7), but in vivo application of reporter genes would likely be related to in situ transfection, where extensive heterogeneity would be expected in terms of cellular expression. The current approach would likely not differentiate widespread low levels of expression versus highly localized intense expression of transgenes. Indeed, differential bystander effects present a critical issue in enzyme activated pro-drug therapy as encountered previously by Corban-Wilhelm et al. (26) with respect to cytosine deaminase activation of 5FC.
The current approach was prompted by the report of Louie et al. (16), who presented a 1H MRI approach to detecting β-gal activity, but their substrate required direct intracellular injection. The 19F NMR agents reveal β-gal activity based on intratumoral injection, which is feasible for in vivo preclinical investigations. Ultimately, substrates should be developed, which can be delivered systemically (IP, SC or IV). A key requirement is accumulation or trapping of the enzyme activated reporters at the site of activity. For comparison nuclear imaging has been successfully applied to evaluate thymidine kinase based on accumulation of phosphorylated purine nucleosides (3). To date, we have observed weak signals of the fluorinated agents presented here in tumors following IP administration, but no conversion, which we attribute to rapid product washout. 19F MRI of xenobiotic metabolism has been reported, albeit usually at low resolution, as for example with 5FU (27). These studies required 32 mins per image following 200 mg/kg IP in tumor bearing rats. Stegman et al. (28) observed conversion of 5FC to 5FU by cytosine deaminase in tumors following IP administration, but required 20 mins to achieve spectra at 7 T following administration of 1 g/kg. Others (29) resorted to intra tumoral injection of substrate, as we have used here for detection of β-gal activity. Imaging should be more feasible with higher concentration of reporter molecule. In early studies using β-gal enzyme in solution we showed straightforward Michaelis Menten kinetics for these substrates (17). However, the product aglycones are toxic (19) and excessive concentrations seem to inhibit enzyme activity (Figure 3). Enhanced signal to noise can be achieved by using a CF3 moiety in place of a single fluorine atom, but we have shown that the chemical shift difference is much smaller, and although we have imaged conversion in vitro, it is probably not feasible in vivo (20). Higher magnetic fields should also be advantageous.
Proton MRI approaches are likely to be more suitable for MRI, as reported by others using cells transfected to express transferrin or ferritin (30-32). In some cases cells were labeled with ferric irons prior to injection into animals (30), while in other cases contrast developed in tumors during growth of xenografts (31). We have achieved preliminary results detecting β-gal activity in tumors by 1H MRI based on intra tumoral injection of the histology stain S-gal together with ferric ammonium citrate (33). 1H MRI offers prodigious signal, but detection of transgene activity depends on the contrast-to-noise ratio, which may be difficult to interpret in highly heterogeneous tissues. 19F NMR has a much lower effective SNR, but the lack of background signal aids interpretation; thus, both approaches have merit.
While β-gal exhibits broad specificity it is reassuring to find that there was no activity by either β-galactosidases (G5160 or E801) at the respective optimal pHs towards the glucoside (PFONPGu, Figure 1). Previously, we had shown lack of activity towards α-galactosides (19). We had hoped to verify that the β-D-galactosides would resist activity of other enzymes. Unfortunately, we have been unable to obtain β-glucosidases, which are active at physiological pH. When G0395 was tested at its optimal pH=4.5 both β-D-glucosides and β-D-galactosides were hydrolyzed. Reassuringly no hydrolysis of the β-D-galactosides has been seen in WT cells or tumors even after many hours.
In summary, these results further demonstrate and validate 19F NMR gene reporter molecules for detection of β-gal activity. We have used lacZ, but other genes (viz. enzymes) such as glucosidases and glucuronidases would be expected to behave similarly. Initial observations with enzymes in solution have been translated to cells in culture and ultimately tumors growing in vivo. The most important result may be not just identification of WT versus stably transfected tumors, but rather the ability to visualize differential expression.
Acknowledgments
Supported in part by grants from the DOD Breast Cancer Initiative IDEA award DAMD17-03-1-0343 and the Cancer Imaging Program, NIH P20 CA 86354 and U24 CA126608. NMR experiments were conducted at the Advanced Imaging Research Center, NIH BTRP facility P41 RR02584. Jennifer McAnally and Xianghui Wang provided outstanding technical assistance.
Non-standard Abbreviations
- 5FC
5 fluorocytosine
- 5FU
5-fluorouracil
- β-gal
β-galactosidase
- CK
creatine kinase
- CMV
cytomegalovirus
- DMEM
Dulbecco's Modification of Eagle's Medium
- DMSO
dimethyl sulphoxide
- DOD
Department of Defense
- ECL
enhanced chemiluminescent
- FBS
fetal bovine serum
- GFP
green fluorescent protein
- GUS
β-glucuronidase
- hNIS
sodium iodine symporter
- IP
intra peritoneal
- IV
intra venous
- NIH
National Institutes of Health
- NaTFA
sodium trifluoroacetate
- OFPNP
o-fluoro-p-nitrophenol
- OFPNPG
o-fluoro-p-nitrophenyl-β-D-galactopyranoside
- ONPG
o-nitrophenyl-β-D-galactopyranoside
- PBS
phosphate buffered saline
- PCF3ONP
p-trifluoromethyl-o-nitrophenol
- PCF3ONPG
p-trifluoromethyl-o-nitrophenyl-β-D-galactopyranoside
- PET
positron emission tomography
- PFONP
p-fluoro-o-nitrophenol
- PFONPG
p-fluoro-o-nitrophenyl-β-D-galactopyranoside
- PFONPGu
p-fluoro-o-nitrophenyl-β-D-glucopyranoside
- PVDF
polyvinylidene fluoride
- SC
subcutaneous
- SDS-PAGE
sodium dodecyl sulfate polyacrylamide gel electrophoresis
- SPECT
single photon emission computed tomography
- WT
wild type
- X-gal
5-bromo-4-chloro-3-indolyl-β–D-galactopyranoside
Footnotes
Description of the synthesis of OFPNPGu is in preparation.
References
- 1.Hoffman R. Green fluorescent protein imaging of tumour growth, metastasis, and angiogenesis in mouse models. Lancet Oncol. 2002;3:546–556. doi: 10.1016/s1470-2045(02)00848-3. [DOI] [PubMed] [Google Scholar]
- 2.Contag CH, Ross BD. It's not just about anatomy: In vivo bioluminescence imaging as an eyepiece into biology. JMRI. 2002;16:378–387. doi: 10.1002/jmri.10178. [DOI] [PubMed] [Google Scholar]
- 3.Haberkorn U, Mier W, Eisenhut M. Scintigraphic imaging of gene expression and gene transfer. Curr Med Chem. 2005;12:779–794. doi: 10.2174/0929867053507351. [DOI] [PubMed] [Google Scholar]
- 4.Gilad AA, Winnard PT, van Zijl PCM, Bulte JWM. Developing MR reporter genes: promises and pitfalls. NMR Biomed. 2007;20(3):275–290. doi: 10.1002/nbm.1134. [DOI] [PubMed] [Google Scholar]
- 5.Jacob F, Monod J. Genetic regulatory mechanisms in the synthesis of proteins. J Mol Biol. 1961;3:318–356. doi: 10.1016/s0022-2836(61)80072-7. [DOI] [PubMed] [Google Scholar]
- 6.Murakami T, Kobayashi E. Color-engineered rats and luminescent LacZ imaging: a new platform to visualize biological processes. J Biomed Opt. 2005;10:041204. doi: 10.1117/1.2007947. [DOI] [PubMed] [Google Scholar]
- 7.Olesen CEM, Yan YX, Liu B, Martin D, D'Eon B, Judware R, Chris Martin C, Voyta JC, Bronstein I. Novel methods for chemiluminescent detection of reporter enzymes. Methods Enzymol. 2000;326:175–202. doi: 10.1016/s0076-6879(00)26055-2. [DOI] [PubMed] [Google Scholar]
- 8.Griscelli F, Opolon P, Saulnier P, Mami-Chouaib F, Gautier E, Echchakir H, Angevin E, Le Chevalier T, Bataille V, Squiban P, Tursz T, Escudier B. Recombinant adenovirus shedding after intratumoral gene transfer in lung cancer patients. Gene Ther. 2003;10:386–395. doi: 10.1038/sj.gt.3301928. [DOI] [PubMed] [Google Scholar]
- 9.Puumalainen AM, Vapalahti M, Agrawal RS, Kossila M, Laukkanen J, Lehtolainen P, Viita H, Paljarvi L, Vanninen R, Yla-Herttuala S. Beta-galactosidase gene transfer to human malignant glioma in vivo using replication-deficient retroviruses and adenoviruses. Human Gene Ther. 1998;9:1769–1774. doi: 10.1089/hum.1998.9.12-1769. [DOI] [PubMed] [Google Scholar]
- 10.Eustice DC, Feldman PA, Colberg-Poley AM, Buckery RM, Neubauer RH. A sensitive method for the detection of beta-galactosidase in transfected mammalian cells. Biotechniques. 1991;11:739–740. [PubMed] [Google Scholar]
- 11.James AL, Perry JD, Chilvers K, Robson IS, Armstrong L, Orr KE. Alizarin-beta-D-galactoside: a new substrate for the detection of bacterial beta-galactosidase. Letters Appl Microbiol. 2000;30:336–340. doi: 10.1046/j.1472-765x.2000.00669.x. [DOI] [PubMed] [Google Scholar]
- 12.Pocsi I, Taylor SA, Richardson AC, Smith BV, Price RG. Comparison of several new chromogenic galactosides as substrates for various beta-D-galactosidases. Biochim Biophys Acta. 1993;1163:54–60. doi: 10.1016/0167-4838(93)90278-y. [DOI] [PubMed] [Google Scholar]
- 13.Chilvers KF, Perry JD, James AL, Reed RH. Synthesis and evaluation of novel fluorogenic substrates for the detection of bacterial beta-galactosidase. J Appl Microbiol. 2001;91(6):1118–1130. doi: 10.1046/j.1365-2672.2001.01484.x. [DOI] [PubMed] [Google Scholar]
- 14.Tung CH, Zeng Q, Shah K, Kim DE, Schellingerhout D, Weissleder R. In vivo imaging of beta-galactosidase activity using far red fluorescent switch. Cancer Res. 2004;64:1579–1583. doi: 10.1158/0008-5472.can-03-3226. [DOI] [PubMed] [Google Scholar]
- 15.Lee KH, Byun SS, Choi JH, Paik JY, Choe YS, Kim BT. Targeting of lacZ reporter gene expression with radioiodine-labelled phenylethyl-beta-d-thiogalactopyranoside. Eur J Nucl Med Mol Imaging. 2004;31:433–438. doi: 10.1007/s00259-003-1395-7. [DOI] [PubMed] [Google Scholar]
- 16.Louie AY, Huber MM, Ahrens ET, Rothbacher U, Moats R, Jacobs RE, Fraser SE, Meade TJ. In vivo visualization of gene expression using magnetic resonance imaging. Nature Biotechnol. 2000;18:321–325. doi: 10.1038/73780. [DOI] [PubMed] [Google Scholar]
- 17.Cui W, Otten P, Li Y, Koeneman K, Yu J, Mason RP. A novel NMR approach to assessing gene transfection: 4-fluoro-2-nitrophenyl-β-D-galactopyranoside as a prototype reporter molecule for β-galactosidase. Magn Reson Med. 2004;51:616–620. doi: 10.1002/mrm.10719. [DOI] [PubMed] [Google Scholar]
- 18.Yu JX, Mason RP. Synthesis and Characterization of Novel lacZ Gene Reporter Molecules: Detection of β-Galactosidase Activity Using 19F NMR of Polyglycosylated Fluorinated Vitamin B6. J Med Chem. 2006;49:1991–1999. doi: 10.1021/jm051049o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu JX, Otten P, Ma Z, Cui W, Liu L, Mason RP. A Novel NMR Platform for Detecting Gene Transfection: Synthesis and Evaluation of Fluorinated Phenyl β-D-Galactosides with Potential Application for Assessing LacZ Gene Expression. Bioconj Chem. 2004;15(6):1334–1341. doi: 10.1021/bc049936d. [DOI] [PubMed] [Google Scholar]
- 20.Yu JX, Liu L, Kodibagkar VD, Cui W, Mason RP. Synthesis and Evaluation of Novel Enhanced Gene Reporter Molecules: Detection of β-Galactosidase Activity Using 19F NMR of Trifluoromethylated Aryl β-D-Galactopyranosides. Bioorg Med Chem. 2006;14:326–333. doi: 10.1016/j.bmc.2005.08.021. [DOI] [PubMed] [Google Scholar]
- 21.Yu JX, Ma Z, Li Y, Koeneman KS, Liu L, Mason RP. Synthesis and Evaluation of a Novel Gene Reporter Molecule: Detection of β-galactosidase activity Using 19F NMR of a Fluorinated Vitamin B6 conjugate. Med Chem. 2005;1(3):255–262. doi: 10.2174/1573406053765495. [DOI] [PubMed] [Google Scholar]
- 22.Kodibagkar VD, Yu J, Liu L, Hetherington HP, Mason RP. Imaging β-galactosidase activity using 19F chemical shift imaging of LacZ gene-reporter molecule 2-fluoro-4-nitrophenol-β-D-galactopyranoside. Magn Reson Imaging. 2006;24(7):959–962. doi: 10.1016/j.mri.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 23.Yu JX, Kodibagkar V, Cui W, Mason RP. 19F: a versatile reporter for non-invasive physiology and pharmacology using magnetic resonance. Curr Med Chem. 2005;12:818–848. doi: 10.2174/0929867053507342. [DOI] [PubMed] [Google Scholar]
- 24.Liu L, Kodibagkar VD, Yu JX, Mason RP. 19F-NMR detection of lacZ gene expression via the enzymic hydrolysis of 2-fluoro-4-nitrophenyl β-D-galactopyranoside in vivo in PC3 prostate tumor xenografts in the mouse. FASEB J. 2007;21:2014–2019. doi: 10.1096/fj.06-7366lsf. [DOI] [PubMed] [Google Scholar]
- 25.Bradford MM. A rapid and sensitive for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analyt Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 26.Corban-Wilhelm H, Hull WE, Becker G, Bauder-Wust U, Greulich D, Debus J. Cytosine deaminase and thymidine kinase gene therapy in a Dunning rat prostate tumour model: absence of bystander effects and characterisation of 5-fluorocytosine metabolism with 19F-NMR spectroscopy. Gene Therapy. 2002;9:1564–1575. doi: 10.1038/sj.gt.3301834. [DOI] [PubMed] [Google Scholar]
- 27.Brix G, Bellemann ME, Gerlach L, Haberkorn U. Direct detection of intratumoral 5-fluorouracil trapping using metabolic F-19 MR imaging. Magn Reson Imaging. 1999;17(1):151–155. doi: 10.1016/s0730-725x(98)00115-5. [DOI] [PubMed] [Google Scholar]
- 28.Stegman LD, Rehemtulla A, Beattie B, Kievit E, Lawrence TS, Blasberg RG, Tjuvajev JG, Ross BD. Noninvasive quantitation of cytosine deaminase transgene expression in human tumor xenografts with in vivo magnetic resonance spectroscopy. PNAS (USA) 1999;96:9821–9826. doi: 10.1073/pnas.96.17.9821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dresselaers T, Theys J, Nuyts S, Wouters B, de Bruijn E, Anne J, Lambin P, Van Hecke P, Landuyt W. Non-invasive F-19 MR spectroscopy of 5-fluorocytosine to 5-fluorouracil conversion by recombinant Salmonella in tumours. Br J Cancer. 2003;89(9):1796–1801. doi: 10.1038/sj.bjc.6601345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deans AE, Wadghiri YZ, Bernas LM, Yu X, Rutt BK, Turnbull DH. Cellular MRI contrast via coexpression of transferrin receptor and ferritin. Magn Reson Med. 2006;56(1):51–59. doi: 10.1002/mrm.20914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cohen B, Dafni H, Meir G, Harmelin A, Neeman M. Ferritin as an endogenous MRI reporter for noninvasive imaging of gene expression in C6 glioma tumors. Neoplasia (New York) 2005;7:109–117. doi: 10.1593/neo.04436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Genove G, Demarco U, Xu H, Goins WF, Ahrens ET. A new transgene reporter for in vivo magnetic resonance imaging. Nature Med. 2005;11:450–454. doi: 10.1038/nm1208. [DOI] [PubMed] [Google Scholar]
- 33.Cui W, Ma Z, Mason RP. S-Gal™, a novel 1H MRI reporter for β-galactosidase. Kyoto, Japan: 2004. p. 1712. [Google Scholar]