Abstract
Background
Dose-sparing strategies are being explored for vaccines against pandemic influenza. We evaluated the dose-sparing potential of aluminum hydroxide (AlOH) adjuvant.
Methods
A total of 600 healthy subjects (age, 18–49 years) were randomized to receive 2 vaccinations 1 month apart with subvirion inactivated influenza A/H5N1 vaccine containing 7.5, 15, or 45 µg of hemagglutinin (HA), with or without 600 µg of aluminum hydroxide (AlOH), or 3.75 µg of HA, with or without 300 µg of AlOH. Serum specimens were obtained for antibody assays before and 1 month after each vaccination.
Results
All formulations were safe. Injection site discomfort was more frequent in groups given vaccines with AlOH. Dose-related increases in antibody responses were noted after both vaccinations (P < .001): geometric mean titers of hemagglutination inhibition antibody in vaccines with and without AlOH, respectively, were 5.4 and 5.4 for subjects who received 3.75 µg of HA, 7.7 and 5.3 for those who received 7.5 µg of HA, 8.1 and 8.5 for those who received 15 µg of HA, and 14.8 and 12 for those who received 45 µg of HA. A ≥4-fold increase in titer was observed in 2% and 2% of subjects who received 3.75 µg of HA with or without AlOH, respectively; in 14% and 0% who received 7 µg of HA; in 14% and 13% who received 15 µg of HA; and in 33% and 25% who received 45 µg of HA. Addition of AlOH enhanced responses only for subjects who received 7.5 µg of HA, but responses in subjects who received 7.5 µg of HA without AlOH were unexpectedly low.
Conclusion
Overall, a meaningful beneficial effect of AlOH adjuvant was not observed.
The emergence of novel influenza A virus strains (including subtype A/H5N1, H7N7, and H9N2 viruses) in human populations in recent years has resulted in a global effort to develop candidate vaccines, particularly those active against highly pathogenic influenza A/H5N1 viruses. Recent data suggest that vaccines containing greater-than-expected doses of inactivated influenza A/H5N1 are required to elicit detectable immune responses in a majority of subjects: 2 vaccinations with an inactivated subvirion vaccine containing 90 µg of hemagglutinin (HA) each were necessary to elicit antibody responses in less than half of the subjects [1]. Although vaccination with vaccine containing large doses of inactivated influenza virus is safe and enhances immunogenicity among healthy young adults and older persons [2], there is concern is that use of such large doses for mass vaccination will not be practical during a pandemic, especially if multiple inoculations are required, given the available worldwide capacity for influenza vaccine production. Therefore, dose-sparing approaches are being pursued [3].
Previous studies have shown that inclusion of an adjuvant can enhance immune responses to inactivated influenza A/H5N1 vaccines. IVVs formulated with MF59, a squalene-containing oil-in-water emulsion, are licensed for use in Europe [4]. However, the only type of adjuvant licensed for use in the United States is mineral-containing adjuvant, such as aluminum hydroxide (AlOH). AlOH is a common adjuvant used in many vaccines around the world. In a recent clinical trial, a candidate whole-virus influenza A/H2N2 vaccine containing as little as 2 µg of HA adjuvanted with AlOH was as immunogenic as a subvirion vaccine containing 15 µg of HA without AlOH [5]. Other investigators have recently explored the potential for aluminum-containing compounds to confer adjuvant effects for subvirion and whole-virus inactivated influenza A/H5N1 vaccines [6–11]. Results of these trials have been variable, and nonadjuvanted formulations were not always compared with adjuvanted formulations. In view of the dose-sparing potential of aluminum-containing vaccine formulations, we compared the reactogenicity and immunogenicity of a monovalent subvirion influenza A/H5N1 vaccine, formulated with or without AlOH, that included different doses of HA in healthy adults. In one previous study evaluating AlOH adjuvant with a very similar inactivated split-product H5 vaccine, there was no significant enhancement of the immune response with the addition of 600 µg of AlOH [6]. To confirm and extend these results, we performed an additional study in a larger number of subjects that involved a broader range of vaccine doses.
SUBJECTS, MATERIALS, AND METHODS
Vaccine doses
Inactivated subvirion influenza A/H5N1 vaccine was prepared using the A/Vietnam/1203/04 × A/PR/8/34 reassortant virus, derived by means of reverse-genetics techniques [1]. Three doses of vaccine containing 7.5, 15, or 45 µg of HA per 0.5 mL and one 0.25-mL dose of vaccine containing 3.75 µg of HA were formulated with or without AlOH adjuvant. The AlOH content was 600 µg for each 0.5-mL dose and 300 µg for the 0.25-mL dose (sanofi Pasteur).
Study design and subjects
We conducted a multicenter, randomized, double-blind, placebo-controlled clinical trial. Written informed consent was obtained from potential subjects before screening. Healthy nonpregnant adults between the ages of 18 and 49 years who had no known allergy to vaccine components (including eggs) and who had not previously received an influenza A/H5 vaccine were considered eligible. The study was conducted in accordance with protocols approved by institutional review boards at the participating study sites.
Study procedures
Eligible subjects were randomly assigned to receive 2 vaccinations with vaccine containing identical levels of HA in the deltoid muscle ~28 days apart. Vaccinations were administered by personnel who were not involved in the assessment of responses after vaccination. Subjects were observed for 30 min after each vaccination. For 7 days after each vaccination, subjects recorded their oral temperature and the presence and severity of injection site symptoms (pain, tenderness, redness, and swelling) and systemic symptoms (feverishness, malaise, myalgia, headache, and nausea) on a memory aid. Subjects were seen in the clinic on days 2 and 8 after each vaccination, at which time their memory aids were reviewed by study staff. Twenty-eight days after each vaccination and 6 months after the second vaccination, the interim medical history of each subject was reviewed. Blood samples for antibody assays were collected before and 1 month after each vaccination and 6 months after the second vaccination.
The severity of solicited adverse events (AEs) was scored on a scale from 0 to 3, where 0 was defined as no symptom, 1 as a mild symptom that did not interfere with activity, 2 as a moderate symptom that interfered with activity, and 3 as a severe, incapacitating symptom. Injection site redness and swelling were graded according to their diameters, as follows: 0, no redness or swelling (diameter, <0.5 cm); 1, small diameter (0.5–4.9 cm); 2, medium diameter (5–10 cm); and 3, large diameter (> 10 cm). Serious AEs (SAEs) were defined as life-threatening AEs, or AEs that resulted in significant or persistent disability, hospitalization, or death. All reported AEs that occurred during the first 2 months were recorded, as were all reported SAEs that occurred during the entire study period.
Laboratory assays
Hemagglutination inhibition (HAI) and neutralizing (Neut) antibody assays were performed at Southern Research Institute as described previously [1], with the exceptions that the same starting dilution was defined as 1:10 rather than 1:20 and that samples with negative test results were assigned a titer of 5. Therefore, an HAI antibody titer of 40 in the current study would correspond to an HAI titer of 80 in the study by Treanor et al. [1], Seroresponse was defined as an increase of ≥4-fold in antibody titer after vaccination (if antibody was detectable in the prevaccination sample) or an increase in antibody titer from <10 before vaccination to ≥40 after vaccination [12].
Statistical considerations
The primary objectives of the study were to determine the dose-related safety of subvirion-inactivated H5N1 vaccine adjuvanted with AlOH in healthy adults and to assess the potential for AlOH to enhance immune responses to an inactivated H5N1 vaccine in healthy adults. The prespecified primary reactogenicity end points were the frequencies and severities of AEs or SAEs solicited in the clinic and via memory aids and periodic targeted physical assessment. The primary immunogenicity end points included the proportion of subjects in each group who achieved a serum HAI or Neut antibody titer of ≥40 against influenza A/H5N1 virus 28 days after receipt of the second vaccination and the geometric mean titer (GMT) and frequency of significant HAI and Neut antibody responses in each group 28 days after receipt of the second vaccination. In multivariate analyses, the analytic model used to evaluate differences between groups for injection site/systemic reactogenicity and 4-fold increases in antibody titer (i.e., dichotomous outcomes, such as with pain or without pain) was a logistic regression model. The analytic model used to assess for differences in GMTs (continuous outcomes) was a generalized linear model, P values were not corrected for multiple comparisons.
Based on the assumption that participants who received non-adjuvanted vaccine with 45 µg of HA would have a response rate of ~40%, the study had a power of >80% to detect an increase of ≥50% in the response rate among those who received adjuvanted vaccine with 45 µg of HA.
RESULTS
A total of 600 subjects were enrolled between March and May 2006; 574 received 2 inoculations with vaccine, and 570 had serum specimens available for antibody assays after receipt of both vaccinations. Five subjects withdrew after enrollment (3 after receipt of the first vaccination, and 2 after receipt of the second vaccination). Four of these subjects were lost to follow-up, and 1 was unable to attend study visits. Three subjects received the incorrect dose of vaccine for the first or second vaccination and were excluded from safety and immunogenicity analyses. Baseline demographic characteristics of enrolled subjects are shown in table 1. No significant differences in baseline age, sex, or race were noted between the vaccine groups.
Table 1.
Demographic characteristics of a population vaccinated with inactivated influenza A/H5N1 vaccine, by hemagglutinin (HA) and aluminum hydroxide (AlOH) adjuvant levels.
| Characteristic | 3.75 µg HAa | 7.5 µg HA | 15 µg HA | 45 µg HA | P | ||||
|---|---|---|---|---|---|---|---|---|---|
| 300 µg AlOHa (n = 61) |
No AlOH (n = 59) |
600 µg AlOH (n = 60) |
No AlOH (n = 59) |
600 µg AlOH (n = 61) |
No AlOH (n = 58) |
600 µg AlOH (n = 120) |
No AlOH (n = 119) |
||
| Sex no of subjects | 76 | ||||||||
| Male | 23 | 29 | 21 | 27 | 24 | 24 | 55 | 49 | |
| Female | 38 | 30 | 39 | 32 | 37 | 34 | 65 | 70 | |
| Age, mean ± SD, years |
32.6 ± 9.0 | 31.6 ± 9.2 | 30.8 ± 8.2 | 33.7 ± 9.1 | 33.5 ± 8.2 | 32.8 ± 9.4 | 33.4 ± 9.1 | 32.9 ± 9.1 | .61 |
| Race no of subjects | 68 | ||||||||
| White | 46 | 43 | 48 | 47 | 51 | 50 | 100 | 92 | |
| Black | 8 | 7 | 8 | 6 | 7 | 5 | 9 | 15 | |
| Asian | 4 | 5 | 4 | 5 | 2 | 3 | 7 | 4 | |
| Other | 3 | 4 | 0 | 1 | 1 | 0 | 4 | 8 | |
NOTE. HA and AlOH levels are per 0.5-mL dose of vaccine, unless otherwise indicated.
Per 0.25-mL dose of vaccine.
Safety and Reactogenicity
Safety
Five SAEs were reported during the study period, none of which was considered to be associated with vaccination: breast cancer; gallstone pancreatitis; acute appendicitis; teno-synovitis; and gastroenteritis requiring hospitalization. All events were reported ≥70 days after receipt of the second vaccination. No deaths were reported.
Injection site reactogenicity
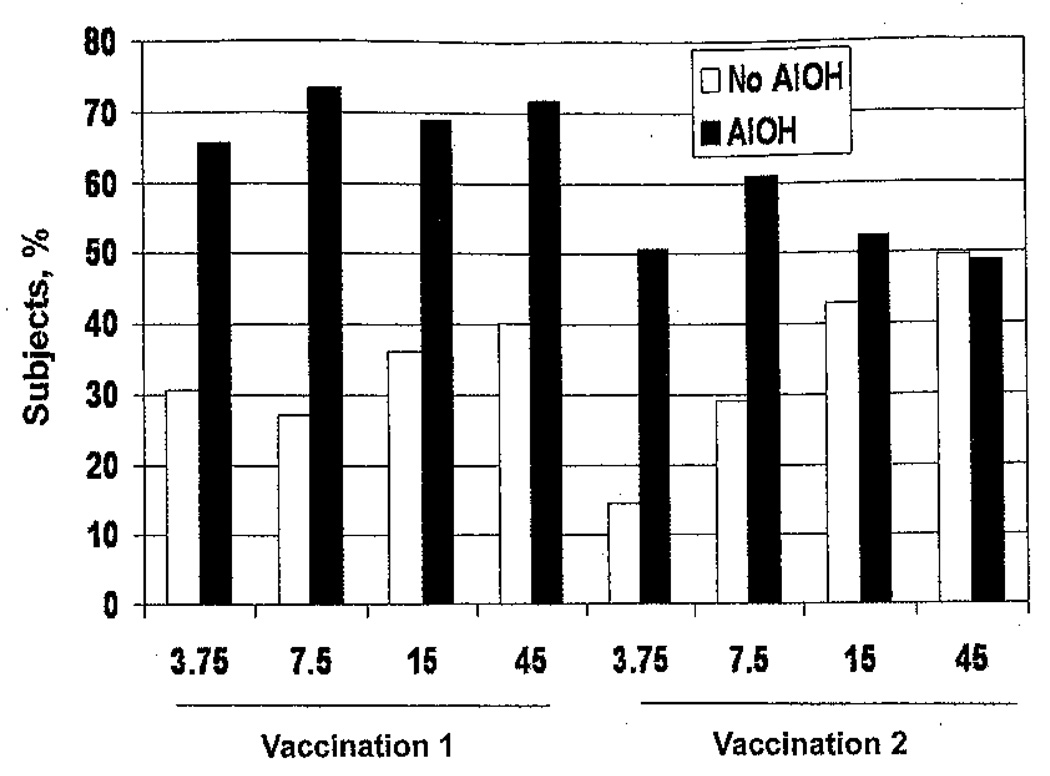
Pain and tenderness at the injection site were the most common solicited AEs. The frequencies of injection site tenderness during the week after receipt of each vaccination are shown in figure 1. Most injection site symptoms were mild and peaked in frequency on day 0 or 1. Dose-related increases in the frequencies of injection site pain after the first vaccination were observed regardless of nonadjuvanted (P < .0001) or adjuvanted (P < .07) vaccine status (data not shown). For each dose of HA, the frequencies of injection site pain and tenderness in groups that received adjuvanted vaccine were significantly higher than those in groups that received non-adjuvanted vaccine (P < .005 for all comparisons). After the second vaccination, increases in the frequencies of pain or tenderness were observed only in the groups that received nonadjuvanted vaccine (P ≤ 001 for both comparisons). The frequencies of pain and tenderness in the groups that received 3.75 µg of HA plus adjuvant and 7.5 µg of HA plus adjuvant were greater than in the groups that received nonadjuvanted vaccine with corresponding HA doses (P < .01 for both comparisons). The frequency of injection site pain after the second vaccination in the group that received 15 µg of HA plus adjuvant was less than that after the first vaccination (P = .04), and the frequencies of pain and tenderness after the second vaccination in the group that received 45 µg of HA plus adjuvant were less than those after the first vaccination (P = .0015 and P = .0005, respectively) (data not shown). In logistic regression analyses, increased HA dose, inclusion of adjuvant, and younger age were independently associated with a higher frequency of injection site pain or tenderness after the first and second vaccinations; female sex was associated with a higher frequency of injection site discomfort after the second vaccination only (data not shown).
Figure 1.
Subjects with injection site tenderness during the week after receipt of inactivated influenza A/H5N1 vaccine with or without aluminum hydroxide (AlOH) adjuvant.
Systemic reactogenicity
No dose-related increases in systemic symptoms were observed in the nonadjuvanted or adjuvanted groups after the first or second vaccination. No significant differences in frequencies of systemic reactions were seen when comparing adjuvanted and nonadjuvanted groups at any dose after the first or second vaccination, with the exception of the groups that received 15 µg of HA, for which the frequencies of nausea after the first vaccination and headache after the second vaccination were higher among those who received adjuvanted vaccine (P = .03 and P = .04, respectively) (data not shown). In logistic regression analyses, female sex was associated with a higher frequency of malaise, myalgia, and headache after both vaccinations; younger age was associated with a higher frequency of malaise after the first vaccination; and inclusion of adjuvant was associated with a higher frequency of headache after the second vaccination (data not shown).
Immunogenicity
Dose-response relationships after vaccination
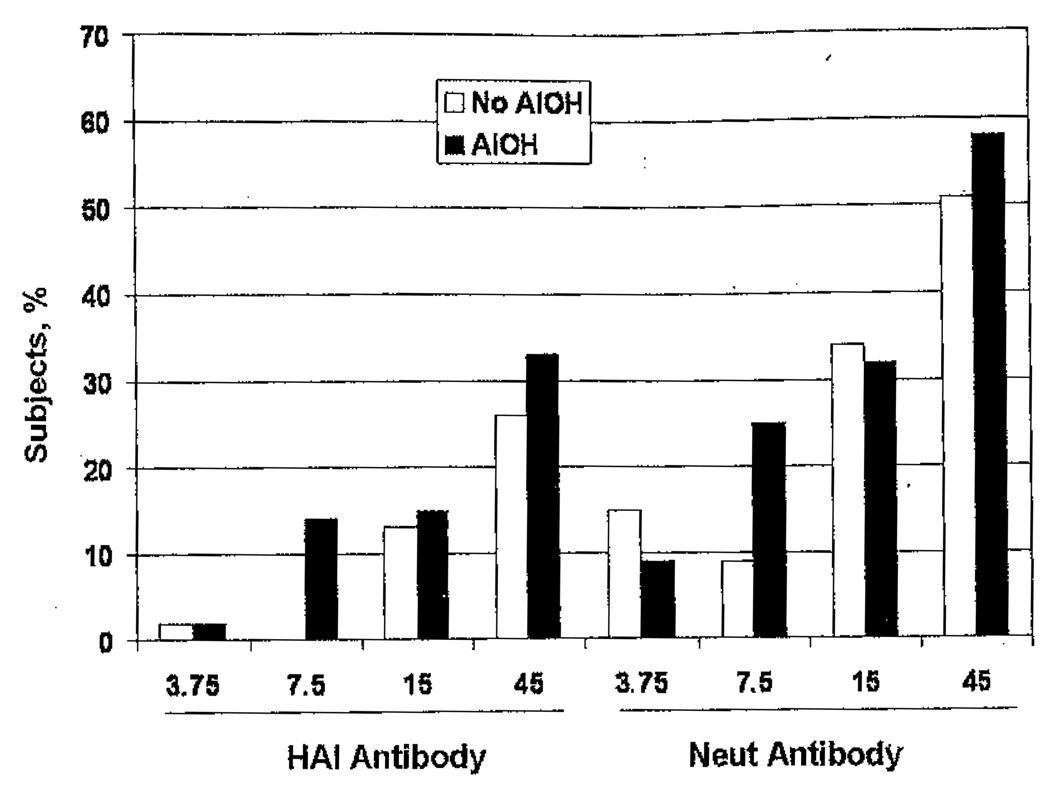
GMTs of serum HAI and Neut antibody before vaccination were similar among all groups (range, 5.0–5.3 for HAI antibody and 5.4–5.8 for Neut antibody; P = not significant [data not shown]). Serum antibody responses 1 month after the first and second vaccinations are shown in table 2 and figure 2. After the first vaccination, dose-related increases in the GMTs of serum HAI and Neut antibody were observed for the groups given adjuvanted vaccine (P < .01 and P < .001, respectively, by analysis of variance); similar dose-related increases in GMTs of serum Neut antibody also were observed for groups given nonadjuvanted vaccines (P < .001, by analysis of variance). Dose-related increases in the proportions of subjects with a ≥4-fold increase in HAI antibody titer and the proportions of subjects with an HAI antibody titer of ≥40 after receipt of the first vaccination were observed for the groups given adjuvanted vaccines (P = .018 for both comparisons, by the Fisher exact test). Dose-related increases in the proportions of subjects with a ≥4-fold increase in Neut antibody titer and the proportions of subjects with a titer of ≥40 after receipt of the first vaccination were observed for the groups given nonadjuvanted vaccines (P < .001 for both comparisons, by the Fisher exact test). Significant dose-response relationships for serum HAI and Neut antibody responses (GMTs, the proportions of subjects with a ≥4-fold increase in titer, and the proportions of subjects with a titer of ≥40) were observed after receipt of the second vaccination, regardless of the inclusion of adjuvant (P < .001 for all comparisons).
Table 2.
Serum hemagglutination inhibition (HAI) and neutralizing antibody responses 1 month after the first and second vaccinations with inactivated influenza A/H5N1 vaccine, by hemagglutinin (HA) and aluminum hydroxide (AlOH) adjuvant levels.
| Variable | 3.75 µg HAa | 7.5 µg HA | 15 µg HA | 45 µg HA | ||||
|---|---|---|---|---|---|---|---|---|
| 300 µg AlOHa | No AlOH | 600 µg AlOH | No AlOH | 600 µg AlOH | No AlOH | 600 µg AlOH | No AlOH | |
| HAI antibody | ||||||||
| Geometric mean titer | ||||||||
| After vaccination 1 | 5.5 (4.8–6.2) | 5.4 (4.7–6.2) | 5.5 (5.0–6.0) | 5.5 (4.9–6.1) | 5.3 (4.9–5.9) | 6.5 (5.1–8.3) | 7.6 (6.2–9.3) | 7.3 (5.9–9.1) |
| After vaccination 2 | 5.4 (4.8–6.0) | 5.4 (4.9–6.0) | 7.7 (6.0–9.9) | 5.3 (4.9–5.8) | 8.1 (6.3–10.6) | 8.5 (6.3–11.4) | 14.8 (11.2–19.6) | 12.0 (9.3–15.4) |
| Increase in titer,b % of subjects | ||||||||
| After vaccination 1 | 3 | 2 | 0 | 2 | 2 | 7 | 10 | 11 |
| After vaccination 2 | 2 | 2 | 14 | 0 | 14 | 13 | 33 | 15 |
| Neutralizing antibody | ||||||||
| Geometric mean titer | ||||||||
| After vaccination 1 | 6.2 (5.3–7.2) | 7.1 (5.8–8.7) | 7.2 (6.0–8.7) | 6.1 (5.4–6.9) | 6.3 (5.4–7.3) | 8.3 (6.6–10.5) | 9.8 (8.0–12.0) | 11.4 (9.1–14.2) |
| After vaccination 2 | 11.2 (9.1–13.8) | 10.1 (7.8–13.2) | 18.5 (14.2–24.2) | 8.8 (7.2–10.8) | 22.3 (17.3–28.7) | 18.3 (13.7–24.4) | 41.5 (34.4–50.0) | 31.0 (25.6–39.2) |
| Increase in titer,a % of subjects | ||||||||
| After vaccination 1 | 3 | 4 | 3 | 0 | 2 | 5 | 11 | 20 |
| After vaccination 2 | 7 | 13 | 24 | 9 | 31 | 34 | 58 | 51 |
Note. HA and AlOH levels are per 0.5-mL does of vaccine, unless otherwise indicated.
Per 0.25-mL does of vaccine.
Defined as achievement of a ≥4 fold increase or an increase from <10 to ≥40.
Figure 2.
Subjects achieving a serum hemagglutination inhibition (HAI) or neutralizing (Neut) antibody titer of ≥40 after receipt of 2 vaccinations with inactivated influenza A/H5N1 vaccine with or without aluminum hydroxide (AlOH) adjuvant.
Effect of AlOH on immune responses
No significant differences in GMTs of serum HAI or Neut antibody were observed after the first vaccination between adjuvanted and nonadjuvanted groups given similar doses of HA, Only the 7.5-µg HA dose was associated with a significant difference in serum antibody response after the second vaccination, with the response among those who received nonadjuvanted vaccine significantly lower than the response among those who received adjuvanted vaccine. No subject given the 7.5-µg dose of HA without adjuvant developed a ≥4-fold increase in HAI titer after the second vaccination, compared with 8 (14%) of 59 subjects given the same HA dose with adjuvant (P < .01, by the Fisher exact test). The Neut antibody titer among subjects who received 7.5 µg of HA increased by ≥4-fold in 5 (8%) of 55 subjects who received nonadjuvanted vaccine, compared with 14 (24%) of 59 who received adjuvanted vaccine (P = .045, by the Fisher exact test). In regression analyses, increased HA dose, female sex, and younger age were associated with higher serum HAI and Neut antibody response frequencies and/or GMTs after the second vaccination; a significant effect of adjuvant was noted for GMTs of serum Neut antibody (data not shown).
DISCUSSION
Aluminum-containing adjuvants have been shown to enhance antibody responses to a number of protein antigens. Several groups have previously investigated the effect of adsorbing influenza vaccines other than those targeting influenza A/H5N1 virus on aluminum-containing adjuvants. Hennessy and Davenport [13] demonstrated that an aluminum phosphate-adsorbed vaccine elicited higher mean antibody levels following booster vaccination of infants, compared with infants given aqueous vaccines; however, overall response frequencies were similar. In contrast, Davenport et al. [14] observed no difference in previously primed adults. Gerth and Mok-Hsu [15] reported that an AlOH-adsorbed subvirion vaccine elicited a higher frequency of injection site reactions than did aqueous vaccine, but they were unable to demonstrate significant enhancement of antibody responses. Conversely, Pressler et al. [16] compared an aluminum oxide-adjuvanted influenza vaccine and its aqueous counterpart Modest enhancement in antibody responses was reported among subjects given adsorbed vaccine. However, the vaccine was incompletely described, and no statistical comparisons were provided. Potter et al. [17] were unable to demonstrate an adjuvant-associated effect. Nicholson et al. [18] reported clinical trials of aqueous and AlOH-adsorbed monovalent A/USSR/77 (H1N1) vaccine. Responses were somewhat greater among unprimed subjects (age, 12–25 years) who were given adsorbed vaccine containing 9 µg of influenza A/H1N1 HA: GMTs of HAI antibody and percentages of subjects with a titer of ≥40 following the second vaccination were 124 and 85%, respectively, for the adjuvanted group (51 subjects) and 75 and 70%, respectively, for the group given aqueous vaccine (29 subjects). No statistical comparisons were provided [18].
AlOH adjuvant has been shown to confer significant antigen-sparing effects in a mouse model of H5N1 vaccination [19]. Dose-sparing approaches are now being evaluated in clinical trials of influenza A/H5N1 vaccines in view of the high levels of antigen required to elicit detectable immune responses. Bresson et al. [6] reported clinical and serologic responses among 300 healthy younger subjects given a subvirion influenza A/H5N1 vaccine containing 7.5, 15, or 30 µg of HA with or without AlOH adjuvant. Immune responses were not significantly improved with the addition of adjuvant, although point estimates suggested that response rates were modestly higher for the adjuvanted formulation containing 30 µg of HA and somewhat lower for the adjuvanted formulation containing 7.5 µg of HA, compared with nonadjuvanted formulations at the corresponding HA doses. Bernstein et al. [7] noted that inclusion of AlOH resulted in reduction of immune responses among healthy adults given inactivated vaccine containing 15 or 30 µg of influenza A/H5N1. Nolan et al. [8] noted a modest enhancement in the proportion of subjects who achieved a Neut antibody titer ≥20 after vaccination with inactivated subvirion H5N1 vaccine containing aluminum phosphate adjuvant (AlPO4): 51% and 37% of subjects given 7.5 µg with or without AlPO4, respectively, and 54% and 51% given 15 µg with or without AlPO4, respectively, achieved this titer after 2 vaccinations. Several groups have reported the immunogenicity of whole-virus influenza A/H5N1 vaccines containing AlOH; however, no comparison with nonadjuvanted vaccine was provided [9, 10]. Finally, inclusion of AlOH adjuvant in a Vero cell culture–grown whole-virus H5N1 vaccine recently was shown to reduce the immunogenicity of the vaccine [11].
Our results confirm and extend previous observations related to the use of aluminum-containing adjuvants to improve the immunogenicity of IVVs. The frequencies of injection site reactogenicity were increased in groups given AlOH-adjuvanted vaccine, and dose-related increases in the frequencies of injection site discomfort were noted, as observed previously [1, 2, 6]. Clinically meaningful increases in immunogenicity were not observed when responses among subjects given adjuvanted preparations were compared with responses among subjects given similar doses containing no AlOH. The low response rates among subjects given the nonadjuvanted vaccine containing 7.5 µg of HA were unexpected. Of note, a similar study that used the same vaccine formulations was conducted among persons who were ≥65 years old and found that immune responses to this dose were similar among groups given vaccine with or without adjuvant [20]. By comparison, response rates among healthy younger adults given 2 doses of a similar nonadjuvanted vaccine containing 90 µg of HA each were recently reported to be 44%, using the new definition of the starting dilution we used for our analyses [21]
In contrast to aluminum-containing adjuvants, oil-in-water emulsions have conferred significant adjuvant effects on candidate pandemic IVVs. Nicholson et al. [22] described the safety and immunogenicity of a purified surface antigen (PSA) vaccine derived from nonpathogenic influenza A/Duck/Singapore (H5N3). Serum HAI and Neut antibody responses were observed in 6 and 8 of 10 healthy adult subjects, respectively, who were given 2 vaccinations with MF 59–adjuvanted vaccine containing 7.5 µg of HA. Nonadjuvanted vaccine was poorly immunogenic. Similar results were reported by Atmar et al. [23], using a vaccine prepared from another potential pandemic virus, A/Hong Kong/97 (H9N2): 2 MF 59-adjuvanted doses containing as little as 3.75 µg of HA stimulated responses in >75% of subjects. The significant adjuvant effect of an oil-in-water adjuvant system on immune responses following vaccination with an inactivated A/H5N1 vaccine resulted in a European Union license application for a prepandemic vaccine earlier this year [24]. More than 80% of healthy adult subjects given two 3.8-µg doses of adjuvanted vaccine responded. As observed in the study by Atmar et al. [23], no dose-response relationships for adjuvanted vaccine were apparent.
The mechanisms by which aluminum-containing adjuvants enhance immune responses are related to the structure of the specific mineral salt, the properties of the adjuvant, and the adsorption mechanism [25]. The reasons for the general failure of AlOH adjuvants to enhance immune responses to influenza virus HA in humans are unknown but may be related to a number of factors, including the strength of adsorption of the HA to the adjuvant, the ratio of antigen to adjuvant, and the interactions of the adjuvanted preparation with interstitial fluid. Combination of an aluminum-containing adjuvant with another type of adjuvant may enhance immune responses; these approaches deserve additional study. On the basis of the variable effects on immunogenicity, dose dependence, and at-best modest effects of AlOH on enhancing the immunogenicity of influenza vaccines, we conclude that alternative dose-sparing approaches must be pursued in the development of vaccines for influenza A/H5N1.
Acknowledgments
We are grateful to the following persons for their contributions to the study: Annette Nagel and Kirtida Patel (Baylor College of Medicine); Mary Lou Mullen and Lisa Chrisley (University of Maryland); Carrie Nolan and D’arcy Gaisser (University of Rochester); Lynn Harrington, Rowena Dolor, and Beth Patterson (Duke University); Robin Cessna (EMMES); Cathy Hanley, Teresa Taffer, and Christy Gillette (PPD); and Tracy Williams and E. Lucile White (Southern Research Institute). We also thank the members of the Safety Monitoring Committee (Karen Kotloff, Chair; Mark Udden, Richard Frothingham, and Michael Keefer), for their valuable input, and their colleagues at the Division of Microbiology and Infectious Diseases, National Institute of Allergy and Infectious Diseases, National Institutes of Health (Linda Lambert, Roland Levandowski, Rosemary McCown, Katherine Muth, and Shy Shorer).
Financial support: Division of Microbiology and Infectious Diseases, National Institute of Allergy and Infectious Diseases, National Institutes of Health (contracts AI-25465, AI-25461, and AI-25460); University of Maryland General Clinical Research Center (grant M01 RR165001); University of Rochester Clinical and Translational Science Institute (grant UL1 RR024160 from the National Center for Research Resources, National Institutes of Health).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Trial registration. ClinicaITrials.gov identifier: NCT00296634.
Potential conflicts of interest W.A.K. and S.M.P. have received research support from Protein Sciences, and research support from Novartis and GlaxoSmithKline is pending. J.J.T. has received research support from Protein Sciences, Merck, Wyeth, GlaxoSmithKline, Sanofi Pasteur, Antigen Express, Mercia Pharma, and Vaxlnnate and has served as a consultant for AlphaVax, Epimmune, and Dynavax. E.B.W, is a speaker for Sanofi Pasteur and Merck and has served as a consultant for Merck and GlaxoSmithKline. All other authors: none reported. Presented in part; Options for the Control of Influenza VI Conference, Toronto, Canada, 17–23 June 2007 (poster 722).
References
- 1.Treanor JJ, Campbell JD, Zangwill KM, Rowe T, Wolff M. Safety and immunogenicity of an inactivated subvirion influenza A (H5N1) vaccine. New Engl J Med. 2006;354:1343–1351. doi: 10.1056/NEJMoa055778. [DOI] [PubMed] [Google Scholar]
- 2.Keitel WA, Couch RB, Cate TR, et al. High doses of purified influenza A virus hemagglutinin significantly augment serum and nasal secretion antibody responses in healthy young adults. J Clin Microbiol. 1994;32:2468–2473. doi: 10.1128/jcm.32.10.2468-2473.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Keitel W, Atmar R. Preparing for a possible pandemic; influenza A/H5N1 vaccine development. Curr Opin Pharmacol. 2007;7:484–490. doi: 10.1016/j.coph.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 4.Minutello M, Senatore F, Cecchinelli G, Bianchi M, Andreani T, Podda A, Crovari P. Safety and immunogenicity of an inactivated subunit influenza virus vaccine combined with MF59 adjuvant emulsion in elderly subjects, immunized for three consecutive influenza seasons. Vaccine. 1999;17:99–104. doi: 10.1016/s0264-410x(98)00185-6. [DOI] [PubMed] [Google Scholar]
- 5.Hehme N, Engelmann H, Kuenzel W, Neumeier E, Saenger R. Immunogenicity of a monovalent, aluminum-adjuvanted influenza whole virus vaccine for pandemic use. Virus Res. 2004;103:163–171. doi: 10.1016/j.virusres.2004.02.029. [DOI] [PubMed] [Google Scholar]
- 6.Bresson JL, Perronne C, Launay O, et al. Safety and immunogenicity of an inactivated split-virion influenza A/Vietnam/1194/2004 (H5N1) vaccine: phase I randomised trial. Lancet. 2006;367:1657–1664. doi: 10.1016/S0140-6736(06)68656-X. [DOI] [PubMed] [Google Scholar]
- 7.Bernstein DI, Edwards KM, Dekker CL, et al. Effects of adjuvants on the safety and immunogenicity of an avian influenza (H5N1) vaccine in adults. J Infect Dis. 2008;197:667–675. doi: 10.1086/527489. [DOI] [PubMed] [Google Scholar]
- 8.Nolan T, Richmond P, Papanoum K, et al. Phase I and II trials of a prototype adjuvanted inactivated split-virion influenza A/Vietnam/1194/2004NIBRG (H5N1) vaccine (abstract P726). Program and abstracts of the Options for the Control of Influenza VI Conference; 17–23 June 2007; Toronto, Canada. [Google Scholar]
- 9.Lin J, Zhang J, Dong X, et al. Safety and immunogenicity of an inactivated adjuvanted whole-virion influenza A (H5N1) vaccine: a phase I randomised controlled trial. Lancet. 2006;368:991–997. doi: 10.1016/S0140-6736(06)69294-5. [DOI] [PubMed] [Google Scholar]
- 10.Vajo Z, Kosa L, Visontay I, et al. Inactivated whole virus influenza A (H5N1) vaccine. Emerg Infect Dis. 2007;13:807–808. doi: 10.3201/eid1305.061248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Keitel W, Dekker C, Mink C, Campbell J, Edwards K, Patel S. A phase I, randomized, double-blind, placebo-controlled dose ranging clinical trial of the safety, reactogenicity and immunogenicity of immunization with inactivated Vero cell culture-derived influenza A/H5N1 vaccine given with or without aluminum hydroxide to healthy young adults (abstract S2). Program and abstracts of the 11th Annual Conference on Vaccine Research; 5–7 May 2008; Baltimore, MD. [Google Scholar]
- 12.Center for Biologics Evaluation and Research, Food and Drug Administration, US Department of Health and Human Services. [Accessed 2 September 2008];Clinical data needed to support the licensure of pandemic influenza vaccines: guidance for industry. 2007 :1–18. Available at http://www.fda.gov/cber/gdlns/panfluvac.pdf.
- 13.Hennessy AV, Davenport FM. Studies on vaccination of infants against influenza with influenza hemagglutinin. Proc Soc Exp Biol Med. 1974;146:200–204. doi: 10.3181/00379727-146-38069. [DOI] [PubMed] [Google Scholar]
- 14.Davenport FM, Hennessy AV, Askin FB. Lack of adjuvant effect of AIPO4 on purified influenza virus hemagglutinins in man. J Immunol. 1968;100:1139–1140. [PubMed] [Google Scholar]
- 15.Gerth H-J, Mok-Hsu YCh. Reactogenicity and serological response to polyvalent aqueous and Al (OH)3 adsorbed Tween-ether split product influenza vaccine in young adults 1979. Infection. 1981;9:85. [Google Scholar]
- 16.Pressler K, Peukert M, Schenk D, Borgono M. Comparison of the anti-genicity and tolerance of an influenza aluminium oxide adsorbate vaccine with an aqueous vaccine. Pharmatherapeutica. 1982;3:195–200. [PubMed] [Google Scholar]
- 17.Potter CW. Inactivated influenza virus vaccine. In: Beare AS, editor. Basic and applied influenza research. Boca Raton: CRC Press; 1982. pp. 119–158. [Google Scholar]
- 18.Nicholson KG, Tyrrell DA, Harrison P, et al. Clinical studies of monovalent inactivated whole virus and subunit A/USSR/77 (H1N1) vaccine: serological responses and clinical reactions. J Biol Stand. 1979;7:123–136. doi: 10.1016/s0092-1157(79)80044-x. [DOI] [PubMed] [Google Scholar]
- 19.Ninomiya A, Imai M, Tashiro M, Odagiri T. Inactivated influenza H5N1 whole-virus vaccine with aluminum adjuvant induces homologous and heterologous protective in a mouse model. Vaccine. 2007;25:3554–3560. doi: 10.1016/j.vaccine.2007.01.083. [DOI] [PubMed] [Google Scholar]
- 20.Brady RC, Treanor JJ, Atmar RL, Chen WH, Winokur P, Belshe R. A phase I-II, randomized, controlled, dose-ranging study of the safety, reactogenicity, and immunogenicity of intramuscular inactivated influenza A/H5N1 vaccine given alone or with aluminum hydroxide to healthy elderly adults (abstract P739). Program and abstracts of the Options for the Control of Influenza VI Conference; 17–23 June 2007; Toronto, Canada. [Google Scholar]
- 21.Treanor J, Bernstein D, Edwards K, Zangwill K, Noah D. Evaluation of inactivated monovalent rga/Vietnam/1203/04 X PR8 subvirion vaccine in healthy elderly adults (abstract P731). Program and abstracts of the Options for the Control of Influenza VI Conference; 17–23 June 2007; Toronto, Canada. [Google Scholar]
- Nicholson KG, Colegate AE, Podda A, et al. Safety and antigenicity of non-adjuvanted and MF59-adjuvanted influenza A/Duck/Singapore/97 (H5N3) vaccine: a randomised trial of two potential vaccines against H5N1 influenza. Lancet. 2001;357:1937–1943. doi: 10.1016/S0140-6736(00)05066-2. [DOI] [PubMed] [Google Scholar]
- 23.Atmar RL, Keitel WA, Patel SM, et al. Safety and immunogenicity of nonadjuvanted and MF59-adjuvanted influenza A/H9N2 vaccine preparations. Clin Infect Dis. 2006;43:1135–1142. doi: 10.1086/508174. [DOI] [PubMed] [Google Scholar]
- 24.Leroux-Roels I, Borkowski A, Vanwolleghem T, et al. Antigen sparing and cross-reactive immunity with an adjuvanted rH5N1 prototype pandemic influenza vaccine: a randomized controlled trial. Lancet. 2007;370:580–589. doi: 10.1016/S0140-6736(07)61297-5. [DOI] [PubMed] [Google Scholar]
- 25.Hem SL, HogenEsch H. Relationship between physical and chemical properties of aluminum-containing adjuvants and immunopotentiation. Expert Rev Vaccines. 2007;6:685–698. doi: 10.1586/14760584.6.5.685. [DOI] [PubMed] [Google Scholar]