Abstract
Currently, pharmacogenetic studies are at an impasse as the low prevalence (<2%) of most variants hinder their pharmacogenetic analysis with population sizes often inadequate for sufficiently powered studies. Grouping rare mutations by functional phenotype rather than mutation site can potentially increase sample size. Using human population-based studies (n = 1,761) to search for dysfunctional human prostacyclin receptor (hIP) variants, we recently discovered 18 non-synonymous mutations, all with frequencies less than 2% in our study cohort. Eight of the 18 had defects in binding, activation, and/or protein stability/folding. Mutations (M113T, L104R, and R279C) in three highly conserved positions demonstrated severe misfolding manifested by impaired binding and activation of cell surface receptors. To assess for association with coronary artery disease, we performed a case-control study comparing coronary angiographic results from patients with reduced cAMP production arising from the non-synonymous mutations (n = 23) with patients with non-synonymous mutations that had no reduction in cAMP (n = 17). Major coronary artery obstruction was significantly increased in the dysfunctional mutation group in comparison with the silent mutations. We then compared the 23 dysfunctional receptor patients with 69 age- and risk factor-matched controls (1:3). This verified the significantly increased coronary disease in the non-synonymous dysfunctional variant cohort. This study demonstrates the potential utility of in vitro functional characterization in predicting clinical phenotypes and represents the most comprehensive characterization of human prostacyclin receptor genetic variants to date.
Keywords: Atherosclerosis, G-protein-coupled Receptors (GPCR), Genetic Polymorphism, Heart, 7-Helix Receptor, Prostaglandins, Pharmacogenetics
Introduction
Single nucleotide polymorphisms (SNPs)3 occurring in DNA coding regions are sequence changes that result in either synonymous (i.e. no change in amino acid sequence) or non-synonymous (change in amino acid sequence) mutations. They are implicated in the pathogenesis of diseases (1), in the efficacy of drugs, and in drug-to-drug interactions (2–4). It is now generally believed that greater than 80% of the observed variability between individuals is the result of genetic variants (5). Numerous databases, including the National Center for Biotechnology Information SNP database, have accumulated millions of SNPs, and a growing list of clinical trials seeks to address the role of SNPs in pathophysiology and treatment response (6–8). Despite the rapid accumulation of data, surprisingly little has entered clinical practice. This may be in part due to the requirement for very large cohorts to perform an adequately powered study for the rarer mutations. Detailed genetic variant functional analysis in biochemical systems may provide a possible solution.
The human prostacyclin receptor gene (PTGIR) spans ∼7,000 bases along chromosome 19 (locus 19q13.3) and comprises three exons separated by two introns: one intron lies upstream from the ATG start codon, and the other lies at the end of the sixth transmembrane helix (9). The human prostacyclin receptor protein (hIP), composed of 386 amino acids, has a molecular mass of 37–41 kDa depending upon its glycosylation state (10). Expression of hIP is distributed throughout the body with predominate cardiovascular expression on platelets and vascular smooth muscle cells and is commonly associated with coupling to the Gαs subunit of the heterotrimeric G-protein. Upon receptor activation, Gαs stimulates membrane-bound adenylyl cyclase to catalyze formation of the second messenger, cAMP (11, 12). In the context of platelet function, this second messenger cascade leads to platelet inactivation and is a critical component of thrombogenic homeostasis. Based upon sequence homology, ligand structure, and overall receptor functionality, hIP is classified as a Class A rhodopsin-like G-protein-coupled receptor. We have previously identified the putative agonist binding pocket of the hIP receptor, which comprises four amino acid residues critical for receptor-ligand recognition. Furthermore, defined residues within the TM domain demarcate the potential molecular path by which the ligand binding-induced signal is transduced through the receptor protein to cytoplasmic effector molecules (13, 14).
Several lines of investigation have suggested that dysfunctional hIP mutations may have critical physiological consequences. Studies of prostacyclin receptor (IP) knock-out (IP−/−) mice have shown increased propensities toward thrombosis (15), intimal hyperplasia and restenosis (16), and reperfusion injury (17). More recently, prostacyclin receptor activity has been shown to have an atheroprotective effect in premenopausal knock-out (IP−/−) female mice (18). Such in vivo findings implicate dysfunctional IP activity in cardiovascular disorders, including stroke, myocardial infarction, and hypertension. Of note has been the worldwide withdrawal of the selective COX-2 inhibitors rofecoxib (VioxxTM) and valdecoxib (BextraTM) whose discriminating suppression of COX-2-derived prostacyclin (PGI2) and its hIP-mediated cardioprotective effects led to increased risk of cardiovascular events (e.g. myocardial infarction and thrombotic stroke), particularly in predisposed patients (19).
Our recent discovery and characterization of the first known naturally occurring hIP variants (20) provide further insight into the molecular mechanism and cardioprotective function of this receptor. Since these initial observations, there has only been one similar publication describing two additional genetic variants in the coding region of PTGIR within a Japanese cohort (21). We recently initiated a large scale sequencing study to discover novel prostacyclin receptor genetic variants (22, 23). Here we report complete characterization of 18 rare non-synonymous mutations in a COS-1 overexpression system. Eight of these mutants exhibit deficiencies, providing insights into critical regions of receptor structure/function. Additionally, to demonstrate the utility of such characterization, we performed a case-controlled study focusing on disease severity in those patients for which we had coronary angiography results. Such insights may prove critical in the development and design of prostacyclin-based therapies targeted against cardiovascular disease.
EXPERIMENTAL PROCEDURES
Materials
Radiolabeled [3H]iloprost (17.0 Ci/mmol) and non-radiolabeled iloprost were purchased from Amersham Biosciences.
Site-directed PCR Mutagenesis
Variant hIP receptors were constructed using site-directed mutagenesis using human IP receptor cDNA cloned into the PMT4 vector (hIPPMT4) as described previously (24). In brief, oligonucleotide primers (Sigma-Genosys, The Woodlands, TX) in both sense and antisense directions extending 10–12 nucleotides 3′ and 5′ from the targeted mutation site were used along with Pfu buffer, dNTPs, and Pfu DNA polymerase (Stratagene, Austin TX). PCR mutagenesis was performed, initially denaturing at 96 °C for 5 min followed by 32 cycles of 96 °C for 1 min, annealing at 55 °C for 1 min, and polymerization at 68 °C for 12 min. PCR products were treated with DpnI restriction enzyme (Promega, Madison WI) to digest wild-type parent DNA. Competent DH5α Escherichia coli cells (∼2 × 109 cells) (Invitrogen) were transformed with the PCR mutagenesis product, and plasmid DNA was extracted from ampicillin-resistant colonies (Qiagen miniprep DNA isolation kit) and sequenced (Molecular Core Facility, Dartmouth Medical School, Hanover, NH).
Transformation of COS-1 Cells and Membrane Preparation
Transfection of COS-1 cells using diethylaminoethyl dextran (Sigma) has been described previously (25). Cells were plated on 15-cm plates and incubated at 37 °C in a 5% CO2 atmosphere to ∼60% confluence. Cells were transfected with mutant or wild-type cDNA (20 μg/plate). Following incubation for 48 h, membrane preparations were made from the transfected COS-1 cells using differential centrifugation (14).
Competition and Saturation Binding Studies
Ligand binding characteristics for the expressed receptors were initially determined through a series of competition binding assays using the radiolabeled ligand [3H]iloprost. Fifty micrograms of membrane and 15 nm [3H]iloprost along with one of 11 different concentrations of competing cold (non-radiolabeled) iloprost ranging from 10 μm to 0.1 nm were used. The reaction was stopped after 1.5 h by the addition of ice-cold 10 mm Tris/HCl buffer (pH 7.4) and filtered onto Whatman® GF/C glass fiber filters using a Brandel® cell harvester. The filters were washed five times with ice-cold Tris/HCl buffer, and radioactivity was measured in the presence of 5 ml of EcoscintTM H scintillation fluid (National Diagnostics, Atlanta, GA). A 500-fold excess of non-radiolabeled iloprost was used to determine nonspecific binding. GraphPad Prism® software (GraphPad Software, Inc., San Diego, CA) was used to analyze data. The Cheng-Prusoff equation was used to convert IC50 values to Ki values (mean ± S.E.). For saturation binding experiments, six different concentrations of [3H]iloprost were varied from 1 to 100 nm. Nonspecific binding was determined also by the addition of a 500-fold excess of non-radiolabeled iloprost. Data were analyzed using non-linear regression (GraphPad Prism software).
Receptor Activation cAMP Determination
The wild-type and genetic variant constructs were analyzed for activation using COS-1 cells transfected with 2 μg of receptor DNA (25-mm plates) (22) and iloprost as the agonist. [3H]cAMP was used in competition for a cAMP-binding protein against known concentrations of non-radiolabeled cAMP followed by determination of the unknowns. Results were analyzed with GraphPad Prism software. For the dose response, a non-linear, curve-fitting program (GraphPad Prism) was used, and the EC50 (mean ± S.E.) was determined for wild-type hIP1D4 and mutant constructs.
Confocal Immunofluorescence Microscopy
COS-1 cells were seeded into 6-well tissue culture plates containing sterilized poly-l-lysine (Sigma)-treated glass coverslips and transfected with 1.0 μg/ml wild-type or mutant hIP DNA. Cells were fixed and permeabilized 48 h post-transfection in ice-cold methanol. Prostacyclin receptors were labeled using anti-1D4 monoclonal antibody (C-terminal tagged hIP) followed by goat anti-mouse IgG F(ab′)2 Alexa Fluor® 568 fluorescent antibody (Molecular Probes, Inc.). The endoplasmic reticulum was labeled using anti-calnexin polyclonal antibody (Stressgen Bioreagents) followed by goat anti-rabbit IgG Alexa Fluor 488 fluorescent antibody (Molecular Probes, Inc.). Cells were postfixed with 4% paraformaldehyde (Fluka) for 5 min, mounted with ProLong Gold with DAPI, and examined via confocal microscopy using a Zeiss LSM 510 Meta laser scanning confocal microscope system (i.e. inverted Zeiss Axiovert 200 microscope with two conventional photomultipliers and one meta polychromatic multichannel detector) (Carl Zeiss Microimaging, Inc., Thornwood, NY). Representative cells were selected, and images were captured at high resolution (63×) for cytoplasmic or plasma membrane localization.
Molecular Modeling of hIP Receptor
Binding, activation, and expression analysis are interpreted in the context of computer modeling (based upon our current model of hIP) to rationalize structural reasons for observed defects. To assist with interpretation of structural interactions altered by non-synonymous variants, we developed a theoretical three-dimensional homology model of the seven transmembrane α-helices of the hIP receptor using the internet-based protein-modeling server SWISS-MODEL (GlaxoSmithKline, Geneva, Switzerland) (26). The homology model was generated using the 2.8-Å-resolution x-ray crystallographic structure of the bovine rhodopsin receptor as the template (Protein Data Bank code 1HZX). The transmembrane domains were energy-minimized utilizing the Gromos96 force field to improve the stereochemistry of the model and remove unfavorable clashes (SWISS-MODEL). Extracellular loops were constructed based on Jpred consensus predictions. The constructed loops were minimized and attached to the modeled transmembrane domains. Although our model is a useful first pass tool in providing preliminary insights and predictions of hIP structure/function, biochemical and molecular pharmacological techniques are necessary to confirm our hypotheses. Structural predictions are thus based upon analysis of the naturally occurring mutations with further assessments based upon integrated predictions between mutations, ligand binding, receptor activation, and molecular modeling studies.
Nested Case-Control Study
For most of the patients, coronary angiography results were available to assess severity of any coronary artery blockages. There are three main coronary arteries that supply the heart, and thus significant blockages in each (greater than or equal to 50% diameter narrowing) are deemed clinically significant. Thus, they are classified from 0 (normal clean vessels) to 3 (significant blockage of all three vessels) with 3 being the most severe form of coronary artery disease. We had coronary angiographic results for 40 patients with non-synonymous hIP variants (6 × V15A, 1 × V25M, 1 × L104R, 1 × G181A, 20 × R212C, 2 × R215C, 7 × P226T, and 2 × S319W). All patients were heterozygotes except one R212C patient who was homozygous for the mutation. The remaining characterized mutants were identified in “healthy volunteers” below the normal age of onset of cardiovascular disease symptoms (e.g. M113T was found in a 27-year-old Mexican male, R279C was found in a 47-year-old Mexican male, R77C was found in an 18-year-old Chinese female, R212H was found in a 22-year-old Chinese male, and I293N was found in a 24-year-old Chinese male). As such, there were no angiographic data for these individuals. Whether these individuals might go on to develop cardiovascular disease with increasing age remains to be determined. For those whom we had complete angiographic results, as the numbers were small we divided them into those with functionally defective mutations (23 patients with reduction in cAMP production; 20 × R212C, 2 × R215C, and 1 × L104R) and variants with no significant reduction in cAMP (17 patients; 6 × V15A, 1 × V25M, 7 × P226T, 2 × S319W, and 1 × G181A). Results in each group were pooled and initially analyzed using Student's t tests. There was no significant difference in age between the two groups. We then assessed separately for association with disease followed by association with disease severity.
A second case-control study was performed using a newly established data base of 5,560 coronary angiograms. Controls were selected to match the 23 cases (3:1 ratio; 69:23) for sex, age, body mass index, and risk factors, including hypertension, diabetes, hyperlipidemia, and smoking. Controls were the best matches that had the minimum risk factors to that of each case, and thus overall, the controls had more risk factors than the cases. The numbers of significant occlusions (>50%) of the three major arteries (left anterior descending artery, left circumflex artery, and right coronary artery) were compared for both groups.
Statistical Analysis
For the in vitro testing and the nested case-control study, analysis of variance (Newman-Keuls multiple comparison post hoc test) delineated statistically significant differences (p < 0.05) between multiple groups, or an unpaired Student's t test was used for direct comparison between two groups. All experiments were repeated at least three times (in duplicate) with an internal wild-type control. For the analysis of disease association, a χ2 test was initially used to determine an odd ratio and 95% confidence limits. Vessel involvement was compared between cases and matched controls and reported here using the likelihood ratio statistic from conditional logistic regression. In addition, we compared the data using generalized estimating equations specifying cases and their matched controls as clusters as well as paired t tests of the difference between cases and the average of their matched controls. All of these approaches yielded very similar results. Statistical significance was determined to be a p value <0.05.
RESULTS
We have thus far detected 18 rare (<2% prevalence) non-synonymous mutations in the coding region of the human prostacyclin receptor gene upon sequencing 1,761 human subjects (Fig. 1). Our initial goal for the current study was to analyze receptor variants for ligand binding (KD) and activation (cAMP EC50) in parallel using an in vitro COS-1 overexpression system previously used to successfully characterize five mutations (V25M, R77C, R212C, R212H, and R279C) (20, 23, 27, 28). We now report the characterization of the 13 newly identified non-synonymous mutations, including two variants (L104R and M113T) at highly conserved positions (100%) across all prostanoid receptors. Such conserved residues, in addition to residues unique to the hIP, provide insights into critical structural requirements for hIP and prostanoid receptor function. This is particularly important clinically as prostacyclin dysfunction (reduced prostacyclin production or a defective receptor) has recently been shown to lead to acceleration of cardiovascular disease.
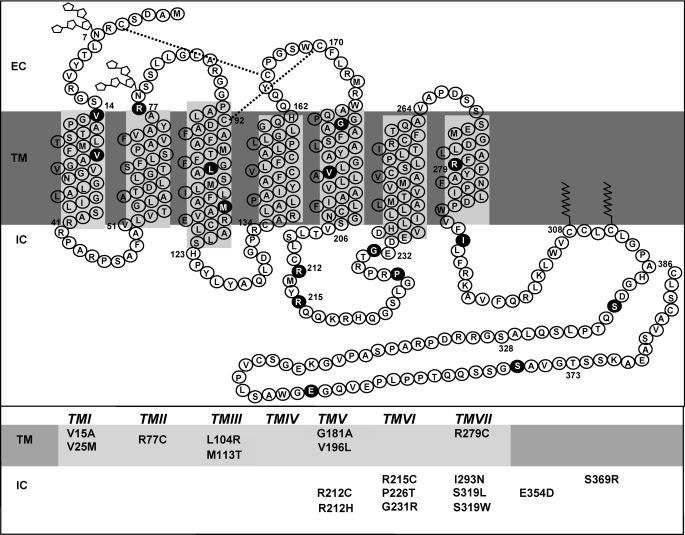
FIGURE 1.
Detected non-synonymous mutations of hIP. Shown is the secondary structure of the human prostacyclin receptor divided into the three major domains: intracellular or cytoplasmic (IC), TM, and extracellular (EC). Glycosylation sites are depicted as ring structures, the dual disulfide bonds are indicated with dashed lines, and the palmitoylation sites in the C-terminal tail are shown as jagged lines. Shown in circles and in the table are the relative positions of the 18 detected non-synonymous mutations analyzed in this study.
Mutations at Highly Conserved Sites (L104R, M113T, and R279C)
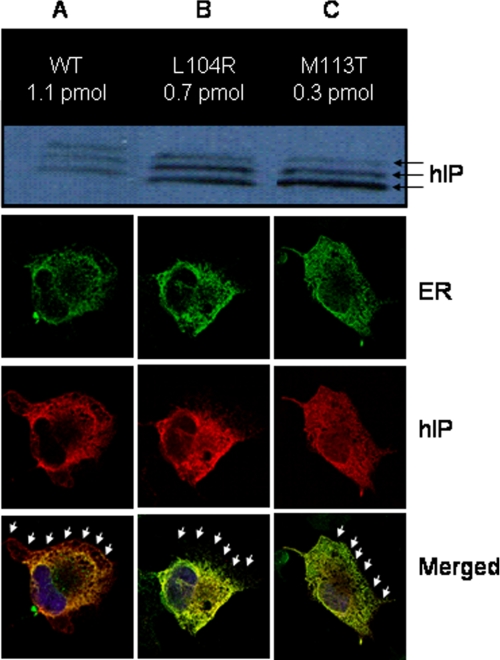
Positions Leu-104 (TMIII), Met-113 (TMIII), and Arg-279 (TMVII) are 100% conserved across all prostanoid receptors, suggesting that these positions are critical for both hIP and prostanoid receptor structure and function. It was therefore not surprising that alterations at each of these positions produced severe defects, particularly in ligand binding as well as in activation and protein expression (Table 1). We have demonstrated previously that Arg-279 is an important counter-ion for the C1 carboxylate residue of prostacyclin (29) and that an SNP with a cysteine in this position has deleterious effects on binding and activation (22). The novel L104R and M113T mutations, located in transmembrane helix 3, each exhibited a lack of specific counts upon competition binding (Table 1), indicating severely compromised ligand binding. Western analysis (Fig. 2) on membrane preparations showed abundant protein relative to wild-type hIP expressed in COS-1 cells; however, saturation binding on the same preparations showed reduced ligand recognition suggestive of protein misfolding. Confocal microscopy analysis confirmed significant endoplasmic reticulum retention and decreased cell surface expression (Fig. 2), particularly for M113T. Activation analysis, using cAMP detection, was consistent with the reduced binding and misfolding. This was particularly notable in M113T where increasing concentrations of iloprost failed to increase cAMP signaling above background (Fig. 3 and Table 1).
TABLE 1.
Characterization of detected non-synonymous hIP genetic variants
Agonist binding and cAMP generation were performed with iloprost as described under “Experimental Procedures.” The variants are abbreviated as follows. The first letter represents the amino acid code for the wild-type amino acid, numbers represent amino acid position, and the last letter identifies the amino acid polymorphism. The symbol % represents conservation at the equivalent position across all prostanoid receptors (high conservation, >50%). Binding constants are given as Ki in nm, and cAMP activity is given as EC50. Bold results represent mutations that have defective signaling. Their full dose response is provided in Fig 3. ND, not detectible; >50, no significant counts on competition binding despite the presence of significant protein on Western analysis.
| hIP | % | Ki (nm) (n) | EC50 (nm) (n) | Bmax (pmol/mg protein) (n) |
|---|---|---|---|---|
| Newly characterized hIP variants | ||||
| WT | 7.9 ± 1.7 (9) | 1.2 ± 0.1 (10) | 1.1 ± 0.1 (3) | |
| V15A | 36 | 5.5 ± 4.1 (3) | 1.7 ± 1.0 (3) | 1.5 ± 0.1 (3) |
| L104R | 100 | >50 (6)a | 3.5 ± 0.2 (4)b | 0.7 ± 0.2 (3) |
| M113T | 100 | >50 (4)a | ND (3)a | 0.3 ± 0.06 (3)c |
| G181A | 10 | 11.8 ± 4.8 (3) | 2.0 ± 0.9 (3) | 1.1 ± 0.1 (3) |
| V196L | 33 | 4.5 ± 0.6 (3) | 0.6 ± 0.3 (3) | 1.6 ± 0.2 (3) |
| R215C | 64 | 9.4 ± 1.1 (3) | 1.1 ± 0.1 (6) | 0.7 ± 0.05 (3)b |
| P226T | 10 | 8.0 ± 0.4 (4) | 0.7 ± 0.2 (3) | 1.0 ± 0.2 (4) |
| G231R | 29 | 4.4 ± 1.6 (3) | 0.9 ± 0.4 (3) | 0.7 ± 0.2 (3) |
| I293N | 69 | >50 (3)a | 1.9 ± 0.6 (3) | 0.3 ± 0.05 (6)c |
| S319W | 17 | 9.0 ± 1.7 (3) | 0.7 ± 0.2 (4) | 1.4 ± 0.2 (3) |
| S319L | 17 | 8.0 ± 1.3 (3) | 1.0 ± 0.2 (3) | 1.4 ± 0.1 (3) |
| E354D | 10 | 8.3 ± 0.7 (3) | 1.1 ± 0.5 (3) | 1.1 ± 0.1 (3) |
| S369R | 10 | 15.7 ± 5.9 (4) | 0.7 ± 0.3 (3) | 0.8 ± 0.1 (4) |
| Previously characterized hIP variants | ||||
| WT | 7.9 ± 1.7 (9) | 1.2 ± 0.1 (10) | 1.1 ± 0.1 (3) | |
| V25M | 21 | 6.1 ± 2.3 (4) | 0.9 ± 0.4 (3) | 1.5 ± 0.4 (3) |
| R77C | 19 | 6.4 ± 1.1 (3) | 0.9 ± 0.3 (3) | 0.3 ± 0.04 (3)c |
| R212C | 50 | 4.3 ± 1.1 (7) | 2.6 ± 0.7 (9)b | 0.5 ± 0.03 (3)c |
| R212H | 50 | 2.2 ± 1.2 (4) | 2.8 ± 0.5 (5)b | 0.8 ± 0.2 (3) |
| R279C | 100 | >50 (4)a | 34.0 ± 3.0 (5)a | 0.3 ± 0.02 (3)c |
a p < 0.001.
b p < 0.05.
c p < 0.01.
FIGURE 2.
Assessment of expression for L104R and M113T and wild type (WT). A, results from saturation binding (mean of at least three separate experiments; pmol/mg of membrane protein) and Western analysis (representative of at least three separate experiments) from the same membrane preparations. The lower band of the western (37 kDa) is the protein backbone, and the upper two bands (39 and 41 kDa) are the glycosylated forms. The green confocal microscopic picture represents anti-calnexin polyclonal antibody (endoplasmic reticulum marker), the red is the hIP receptor (1D4 monoclonal antibody), and the blue represents nuclear DAPI staining. The superimpositions of the micrographs contain arrows that show the outer limit of the cell. B, using the same techniques described in A for L104R. C, using the same techniques described in A for M113T.
FIGURE 3.
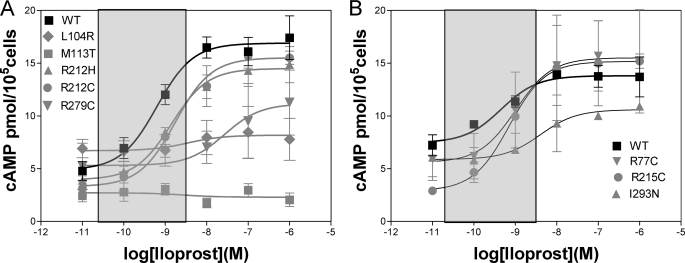
Iloprost dose response for selected mutants and WT. Mutant and wild-type hIP were expressed in COS-1 cells, and dose response was analyzed using six different concentrations of iloprost (1 μm to 0.01 nm), measuring cAMP response (mean ± SE). Shown are the pooled results from four separate experiments with each performed in duplicate. Shown in the shaded boxes are the concentration ranges likely to be physiologically relevant (around EC50). All mutations shown have reduced cAMP at that concentration. A, WT, L104R, M113T, R212H, R212C, and R279C are a series of mutants that demonstrated abnormal EC50 (Table 1) and reduced cAMP production at the putative physiological concentrations of agonist (shaded box). B, WT, R77C, R215C, and I293N are mutants that demonstrated a normal EC50 but had clear defects in cAMP production at the putative physiological agonist levels (shaded box).
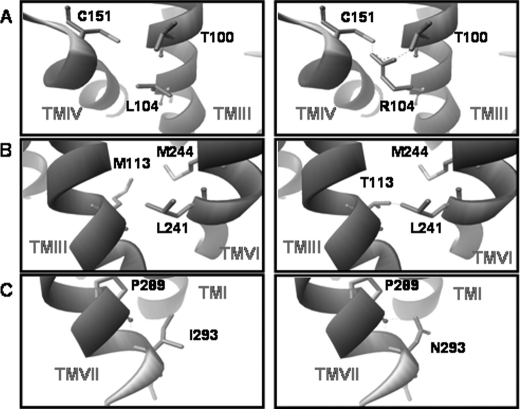
Molecular modeling revealed probable defects in receptor structure. The completely conserved leucine at position 104 in TMIII may play a critical role in interhelical interactions between TMIII and TMIV (Fig. 4A). The completely conserved methionine at position 113 in TMIII when changed to Thr appears to generate a possible steric hindrance with TMVI (Fig. 4B). Thus, two completely conserved residues, leucine 104 and methionine 113, likely serve critical roles mediating interhelical interactions between transmembrane domains 3 and 6, the disruption of which leads to severe protein misfolding. Of note, movement of TMIII and TMVI are critical in the activation of rhodopsin and probably G-protein-coupled receptors in general (30). It should be emphasized that our observations are based upon the best homology model we have available to date and not a structure, and as such, the accuracy remains questionable until more definitive structural data become available (e.g. through crystallography or NMR).
FIGURE 4.
Molecular modeling of mutations. The molecular model of the hIP based upon the crystal structure of rhodopsin and previous extensive mutagenesis studies (as described under “Experimental Procedures”) is shown. The left panels highlight the positions of the native amino acid, and the right panels highlight the mutation with potential steric hindrance with neighboring residues. A, L104R; B, M113T; C, I293N.
Lessons from Other Functionally Defective Polymorphisms (R77C, R212C, R212H, R215C, and I293N)
Of the remaining functionally defective mutations, the 293 position (cytoplasmic end of TMVII) is conserved among 69% of all prostanoid receptors. There were clear defects in binding and expression with additional increases in EC50, although they were not statistically significant (Table 1). cAMP production was clearly reduced in comparison with wild-type levels (Fig. 3B). The isoleucine at position 293 at the interface of TMVII and the C-terminal tail may interact with Pro-289 in TMVII (Fig. 4). Pro-289 is a highly conserved residue (98% conservation) found within TMVII and part of the important highly conserved NPXXY motif (in the case of the hIP, DPXXF motif) (14).
Both R77C and R215C exhibited reduced cell surface expression upon saturation binding (0.3 ± 0.04 pmol/mg of membrane protein, n = 3, p < 0.01 and 0.3 ± 0.05 pmol/mg of membrane protein, n = 6, p < 0.01, respectively). Both demonstrated reduced cAMP production at physiologically relevant concentrations of agonist (Fig. 3B). The remaining two mutations (R212C and R212H), which have been included to complete the set of 18, have previously been characterized (20, 27, 31) and exhibit clear activation defects (Table 1 and Fig. 3).
Functionally Silent Polymorphisms
Of the 18 non-synonymous mutations, 10 of them exhibit no significant activation or expression problems (Table 1). We were somewhat surprised at this number despite some dramatic physicochemical amino acid changes. The G231R and P226T variants are both located in the critical third intracellular loop (known interaction site with heterotrimeric G-protein), whereas S319W, E354D, and S369R are located in the C-terminal tail. It is possible that these silent amino acid changes are accommodated in the hIP structure. However, because we analyzed the mutations in a non-native overexpression system, we cannot rule out that these mutations may have defects in other pathways (particularly hIP transport) that may be of clinical importance to other disease processes (e.g. asthma or bleeding).
Production of cAMP at Physiological Agonist Concentration: the Importance of Full Dose-Response Analysis
From the modeling and secondary structure, individual mutations can lead to quite different structural perturbations; however, we believe that the resultant signaling defect is of greatest importance for association with clinical disease. It was therefore mandatory to initially estimate physiological concentrations of agonist to determine whether there is a reduction in hIP-stimulated cAMP production at those concentrations in human coronary vascular smooth muscle cells. As the half-life of prostacyclin is very short (seconds to minutes), we used an ELISA for the stable metabolic product of prostacyclin, 6-keto-PGF1α (Cayman Chemical, Ann Arbor, MI) (32). In human patient blood, we were able to detect basal levels of 0.018 ± 0.001 nm (n = 8 cardiology patients). Local concentrations in regions of stimulated prostacyclin release are likely to be considerably higher in the range of the observed EC50 (5 nm) determined using agonist on human coronary vascular smooth muscle cells (Fig. 5A). This result is consistent with the EC50 and binding affinities (nm range) for hIP expressed in COS-1 (Fig. 3 and Table 1). Detailed full dose-response curves for the eight dysfunctional mutations are therefore provided in Fig. 3, A and B. Reduced cAMP production was prominent at physiological agonist concentrations (shaded box) for L104R, M113T, R212H, R212C, and R279C (Fig. 3A), all of which had abnormal EC50. For the mutations R77C, R215C, and I293N, which had normal EC50 (Table 1), there were also clear defects in cAMP production at the physiological range (Fig. 3B).
FIGURE 5.
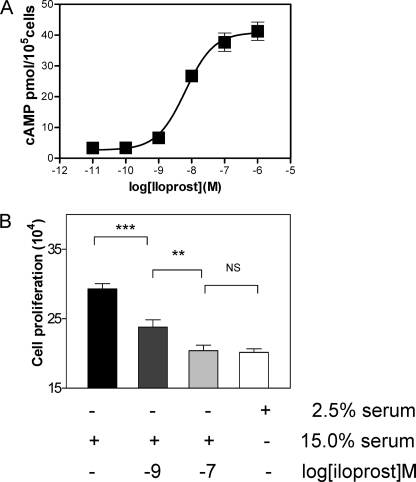
Dose response for inhibition of proliferation. Iloprost (1 and 100 nm) was used to assess its effect on human coronary smooth muscle cell proliferation. 15% serum was used to promote proliferation (mean ± SE), and 2.5% serum was used to assess for stasis. Analysis of variance was performed to assess for significant differences between the groups using post-test Newman-Keuls analysis (**, p < 0.01; ***, p < 0.001; NS, not significant).
Prostacyclin Receptor Function in Coronary Vascular Smooth Muscle Cells
We then proceeded to assess the functional importance of prostacyclin receptor activation by nm concentrations of agonist in both coronary vascular smooth muscle cell proliferation and differentiation as these functional characteristics are important in retarding the formation of an atherosclerotic plaque occlusion. The iloprost dose response for cAMP production in primary human coronary vascular smooth muscle cells showed an EC50 of 5.5 ± 2.5 nm (means ± S.E., n = 4) (Fig. 5A). Iloprost inhibited the serum-induced proliferation of human coronary vascular smooth muscle cells in a dose-dependent manner (1 and 100 nm, all p < 0.001 versus 15% FBS positive control, Student's t test) (Fig. 5B). Iloprost also induced a dose-dependent increase in the SM2 isoform of smooth muscle myosin heavy chain and calponin, recognized markers of smooth muscle cell differentiation (33) (Fig. 6). Reduced hIP signaling would therefore lead to hyperproliferation and dedifferentiation, hallmarks of atherosclerosis and coronary vessel occlusion. Thus, normal signaling (via cAMP) at nm concentrations of hIP agonist plays an important cardioprotective role.
FIGURE 6.
Analysis of coronary vascular smooth muscle cell to assess for markers of differentiation. A, Western analysis for dose-response (iloprost) activation of calponin, a marker for vascular smooth muscle cell differentiation. GAPDH was used as the loading control. B, quantification of calponin results from three separate experiments. C, Western analysis for dose-response (iloprost) activation of SM2 myosin heavy chain (MHC), another major marker of vascular smooth muscle cell differentiation. β-Tubulin was used as the loading control. D, quantification of the SM2 myosin heavy chain results from three separate experiments. veh, vehicle; AU, arbitrary units.
Variant Association with Disease May Be Grouped by Downstream Signaling Rather than Position of Variant
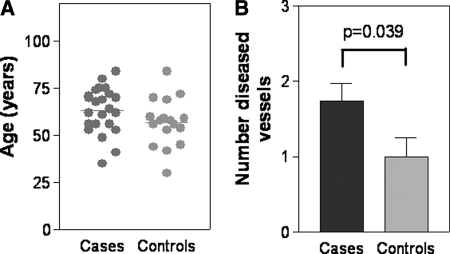
Although COS-1 analyses have provided important insight into the structure-function relationship of the prostacyclin receptor, we must also assess whether such studies can offer useful information/correlation to clinical disease. This is particularly relevant in light of our data demonstrating the role of prostacyclin in coronary vascular smooth muscle cell proliferation and differentiation. As most of our samples were obtained from a high risk cardiology cohort, many of our patients had coronary angiograms available for analysis. Coronary angiograms are currently the gold standard in determining the presence or absence of coronary artery disease. In a case-control study, we initially directly compared patients with silent non-synonymous mutations (no biochemical defect cohort; controls, n = 17) with those with detected biochemical defects upon COS-1 analysis (defect cohort; cases, n = 23). There was no significant age difference between the two groups (controls, 57.0 ± 3.1 years; cases, 63.3 ± 2.5 years; p = 0.12) (Fig. 7A). The body mass index for the cases was 28.3 ± 1.7 and for the controls was 32.1 ± 1.8 (p = 0.15). The cardiovascular risk factors for the cases (n = 23) versus controls (n = 17) were male (74% cases versus 71% controls), hypertension (74% cases versus 60% controls), hyperlipidemia (70% cases versus 65% controls), diabetes (27% cases versus 29% controls), family history (43% cases versus 35% controls), and smoking (18% cases versus 6% controls). Initial χ2 analysis demonstrated that there was a significant association of the functional defect group (cases) with coronary artery obstruction (odds ratio, 4.67 (0.99–22.02); p = 0.04) in comparison with the group with silent mutations. Moreover, upon assessment of disease severity based upon scores from 0 (no vessels significantly occluded) to 3 (all three major coronary vessels occluded), patients with mutations that demonstrated reduced cAMP had significantly more coronary blockages than those who exhibited normal cAMP production (controls, 1.0 ± 0.26 vessels; cases, 1.7 ± 0.23 vessels; p = 0.039) (Fig. 7B). Although the two groups only represented a small handful of mutations, the potential utility and importance of grouping mutations by in vitro analysis appear promising. Variants may be grouped by downstream signaling rather than the position of the variant.
FIGURE 7.
Association with clinical coronary artery disease. A, a scatter plot of the ages of the patients in the group with no functional defects (controls) and the group with detected function defects (cases). The line represents the mean. B, a histogram showing the disease severity in each of the two groups. The number of diseased vessels ranges from 0 to 3. Both the number of patients in each group and the results of statistical analysis (Student's t test) are shown.
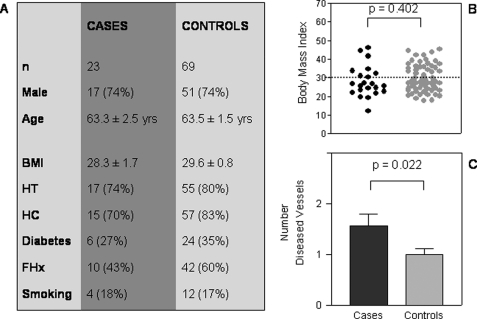
To further reaffirm our hypothesis that reduced cAMP (from hIP dysfunction) is associated with increased coronary disease and is not due to increased cardiovascular risk factors in the cases, we performed a case-control study (1:3) using age- and risk factor-matched controls from our cardiovascular population. A database was established for 5,560 patient angiogram files, and a program was written to select controls based upon age, body mass index, and other cardiovascular risk factors (i.e. family history, hypertension, hypercholesterolemia, diabetes, and smoking status). As each of the controls had to have at least the same risk factors present in the cases, some of the best matches had other risk factors (most often hypertension and/or hypercholesterolemia) in addition to the risk factors in the case subject. Thus, the overall risk factors were higher in the controls versus the cases (Fig. 8A). The age and body mass index were closely matched in the two groups. Scatter plot analysis demonstrated that a significant portion of both control and cases were obese (body mass index >30) (Fig. 8B). The new control patients were additionally genotyped for the prostacyclin receptor. The sequences demonstrated no non-synonymous mutations. Upon assessment of the number of major vessels occluded, there were significantly more vessels occluded in cases than controls (p = 0.022) (Fig. 8C). Patients who had hIP variants with normal cAMP production and function had occlusive disease in 1.0 ± 0.26 vessels (Fig. 7B) in comparison with the 69 control patients (1.0 ± 0.11 vessels) (Fig. 8C). This further supports that (in our high cardiovascular risk population) dysfunctional hIP variants are associated with significantly increased coronary artery occlusion, and there is a lack of association with increased coronary occlusion in those with non-synonymous mutations and normal hIP function.
FIGURE 8.
Case-control study for R212C/R215C/L104R patients and coronary artery disease. A, table demonstrating characteristics of the R212C/R215C/L104R (cases) and risk factor-matched controls (controls) matched in the ratio 1:3. B, graph demonstrating body mass index of cases and controls. A dashed line is drawn at 30, which represents the definition of obesity. Analysis was performed with a Student's t test where p < 0.05 was considered significant. C, the number of obstructed major vessels (0, 1, 2, or 3) between the case and control groups. Analysis was performed with a Student's t test where p < 0.05 was considered significant. BMI, body mass index; HT, hypertension; HC, hypercholesterolemia; FHx, family history.
DISCUSSION
Our goal for this study was to demonstrate that detailed biochemical analysis of rare prostacyclin receptor genetic variants may be useful in identifying patients at significant risk of developing serious cardiovascular disease. This is particularly important and timely with recent demonstration of the critical role that hIP plays in development of cardiovascular disease (27, 34, 35). Characterization of these mutations additionally provides important insights into the amino acid requirements for hIP structure and function and the importance of complementing basic sciences with clinical studies.
Human Prostacyclin Receptor Non-synonymous Polymorphisms
Receptor polymorphisms are emerging as important contributors to the understanding of both disease pathophysiology and therapeutics (36–38). The presence of such variants, especially those that alter ligand binding and G-protein coupling, will have important effects in both pathophysiology and response to receptor-targeted therapy (39). Examples of such variants affecting ligand binding include transmembrane domain variants in rhodopsin (40), the dopamine D4 receptor (41), and the vasopressin V2 receptor (42). Intracellular loop variants in the dopamine D2 receptor (43), the endothelin ETB receptor (44), and the vasopressin V2 receptor (45) exhibit impairment in G-protein coupling. We have previously described and characterized SNPs in the hIP (20). This was followed by extensive sequencing of patient samples to discover novel genetic variants (23). We now report our most recent finding from sequencing a total of 1,761 patient samples from which we have found 18 mutations that lead to a change in amino acid. It was therefore important to examine these variant receptors for functional abnormalities. The specific functional defect may prove to be more important than the site of mutation on the prostacyclin receptor. These prostacyclin receptor mutations may be analogous to cystic fibrosis transmembrane conductance regulator (chloride channel) mutations leading to cystic fibrosis and rhodopsin mutations leading to retinitis pigmentosa in that there are now hundreds of rare mutations for which there are insufficient numbers to prove a direct disease association. However, based upon the functional defect observed for the Δ508 (most common cystic fibrosis genetic defect) and P23H (most common retinitis pigmentosa mutation), it has been shown and generally accepted that a functional defect leads to disease in these rarer mutations (46). This highlights the critical importance for the additional study of the receptors themselves and detailed assessment of the signaling pathways. Patients with similarly dysfunctional mutations may ultimately benefit from the same therapies.
New Insights into hIP Structure and Function
We have recently made a number of contributions to current knowledge on the structure and function of the hIP, including a putative agonist-binding pocket for the hIP (29), conformational changes leading to downstream receptor signaling (13, 14), and dual disulfide bonds in the hIP (47). Studies using both natural (polymorphisms) and unnatural (site-directed) mutations will provide further critical information on the binding and activation of the hIP. Both L104R and M113T have identified critical residues required to maintain structural integrity, the replacement of which leads to severe protein misfolding. This knowledge may assist in the rational design of stable, orally bioavailable prostacyclin analogues for combating cardiovascular disease. Indeed, the presence of the mutations may require the design of differently structured analogues to combat prostacyclin-related diseases. Due to the 100% conservation across prostanoid receptors for L104R and M113T, these principles will likely extend to the whole family of prostanoid and prostanoid-related G-protein-coupled receptors.
A further area that remains unexplored is the detailed analysis of the non-synonymous mutations on agonist-dependent internalization and trafficking as has recently been elegantly described (48). Indeed, our saturation binding experiments and confocal microscopy suggest that some of these mutations may have defects in such transcellular movements. Further detailed studies, particularly under native conditions, would be of great importance in correlating in vitro function with clinical disease.
Receptor Structure and Function under Native Conditions
Although COS-1 detected functional defects are important to finding correlations to clinical disease, there additionally may be mutations that exhibit defects only under conditions of stress. Although many genetic variants may be silent under normal physiological conditions, underlying functional abnormalities can manifest only in a diseased state (49, 50). We have determined previously that an R212H mutation (expressed in COS-1 cells) leads to defective ligand binding and activation, particularly when exposed to stress conditions such as acidosis, e.g. pH 6.8 (20). During pathological stress such as respiratory or cardiac failure (where severe acidosis may provoke defective prostacyclin binding), the added receptor defect may promote vasoconstriction and/or thrombosis, warranting urgent correction of pH to restore proper receptor function and prevent catastrophic ischemia. Thus, despite the utility of COS-1 analysis, parallel analysis of patient tissues ex vivo may provide additional essential information.
Limitations
In addition to limitations of our in vitro testing and in vivo correlations as described above, the current study (although based upon the analysis of 1,761 patients) is relatively small, particularly when mutations of such low prevalence are being studied, and many of our mutations are not represented. It is, therefore, mandatory that further confirmation is required on a larger scale. Following our group of patients over many years, particularly those with the highly conserved mutations and currently no angiographic results, will provide further information.
All patients studied except for one R212C were heterozygote. This strongly supports a dominant negative effect conferred by the mutant receptor probably through a heterodimerization mechanism as suggested recently (51). With larger clinical studies, more dramatic effects conferred by homozygote mutations may be uncovered. Alternatively knock-in mouse studies may be informative.
The molecular modeling presented in this study is based upon the crystal structure of rhodopsin using multiple structural and energy minimization algorithms. Although we recognize that it remains a crude model at best, based upon extensive mutagenesis and complimentary predictions, we believe that it is reasonably accurate. Crystallization studies in addition to NMR studies will be the ultimate proof of accuracy. However, although the structural mechanism of specific mutations may be important in designing drugs for therapy, resultant downstream signaling defects are likely the most important for disease correlation.
Conclusions
We report the most comprehensive biochemical study of hIP non-synonymous polymorphisms to date, extending fundamental structure/function to clinical observations. This has provided significant insights into critical residues required for hIP and prostanoid receptor structure and function. We have additionally highlighted the importance of biochemical characterization as only functional defects correlated with the severity of clinical disease as observed through coronary angiography. Such pooling of biochemically characterized variants focusing on functional signaling defects rather than the site of the mutation may ultimately be useful for correlation of function with clinical disease for rare variants.
This work was supported, in whole or in part, by National Institutes of Health Grant RO1 HL074190 from the NHLBI (to J. H.). This work was also supported by grants from the American Heart Association (to J. H. and J. S.).
- SNP
- single nucleotide polymorphism
- IP
- prostacyclin receptor
- hIP
- human prostacyclin receptor
- COX-2
- cyclooxygenase-2
- TM
- transmembrane
- TMI–VII
- transmembrane α-helices 1–7.
REFERENCES
- 1. Kathiresan S., Melander O., Anevski D., Guiducci C., Burtt N. P., Roos C., Hirschhorn J. N., Berglund G., Hedblad B., Groop L., Altshuler D. M., Newton-Cheh C., Orho-Melander M. (2008) N. Engl. J. Med. 358, 1240–1249 [DOI] [PubMed] [Google Scholar]
- 2. Kimchi-Sarfaty C., Marple A. H., Shinar S., Kimchi A. M., Scavo D., Roma M. I., Kim I. W., Jones A., Arora M., Gribar J., Gurwitz D., Gottesman M. M. (2007) Pharmacogenomics 8, 29–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Abraham J., Earl H. M., Pharoah P. D., Caldas C. (2006) Biochim. Biophys. Acta 1766, 168–183 [DOI] [PubMed] [Google Scholar]
- 4. Dudbridge F., Koeleman B. P. (2004) Am. J. Hum. Genet. 75, 424–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Stoneking M. (2001) Nature 409, 821–822 [DOI] [PubMed] [Google Scholar]
- 6. Glynn R. J., Ridker P. M., Goldhaber S. Z., Zee R. Y., Buring J. E. (2007) Circulation 116, 1497–1503 [DOI] [PubMed] [Google Scholar]
- 7. Trompet S., Pons D., De Craen A. J., Slagboom P., Shepherd J., Blauw G. J., Murphy M. B., Cobbe S. M., Bollen E. L., Buckley B. M., Ford I., Hyland M., Gaw A., Macfarlane P. W., Packard C. J., Norrie J., Perry I. J., Stott D. J., Sweeney B. J., Twomey C., Westendorp R. G., Jukema J. W. (2007) Ann. N.Y. Acad. Sci. 1100, 189–198 [DOI] [PubMed] [Google Scholar]
- 8. Berndt S. I., Platz E. A., Fallin M. D., Thuita L. W., Hoffman S. C., Helzlsouer K. J. (2007) Int. J. Cancer 120, 1548–1554 [DOI] [PubMed] [Google Scholar]
- 9. Ogawa Y., Tanaka I., Inoue M., Yoshitake Y., Isse N., Nakagawa O., Usui T., Itoh H., Yoshimasa T., Narumiya S., Nakao K. (1995) Genomics 27, 142–148 [DOI] [PubMed] [Google Scholar]
- 10. Boie Y., Rushmore T. H., Darmon-Goodwin A., Grygorczyk R., Slipetz D. M., Metters K. M., Abramovitz M. (1994) J. Biol. Chem. 269, 12173–12178 [PubMed] [Google Scholar]
- 11. Narumiya S., Sugimoto Y., Ushikubi F. (1999) Physiol. Rev. 79, 1193–1226 [DOI] [PubMed] [Google Scholar]
- 12. Wise H., Jones R. L. (1996) Trends Pharmacol. Sci. 17, 17–21 [DOI] [PubMed] [Google Scholar]
- 13. Stitham J., Stojanovic A., Ross L. A., Blount A. C., Jr., Hwa J. (2004) Biochemistry 43, 8974–8986 [DOI] [PubMed] [Google Scholar]
- 14. Stitham J., Martin K. A., Hwa J. (2002) Mol. Pharmacol. 61, 1202–1210 [DOI] [PubMed] [Google Scholar]
- 15. Murata T., Ushikubi F., Matsuoka T., Hirata M., Yamasaki A., Sugimoto Y., Ichikawa A., Aze Y., Tanaka T., Yoshida N., Ueno A., Oh-ishi S., Narumiya S. (1997) Nature 388, 678–682 [DOI] [PubMed] [Google Scholar]
- 16. Cheng Y., Austin S. C., Rocca B., Koller B. H., Coffman T. M., Grosser T., Lawson J. A., FitzGerald G. A. (2002) Science 296, 539–541 [DOI] [PubMed] [Google Scholar]
- 17. Xiao C. Y., Hara A., Yuhki K., Fujino T., Ma H., Okada Y., Takahata O., Yamada T., Murata T., Narumiya S., Ushikubi F. (2001) Circulation 104, 2210–2215 [DOI] [PubMed] [Google Scholar]
- 18. Egan K. M., Lawson J. A., Fries S., Koller B., Rader D. J., Smyth E. M., Fitzgerald G. A. (2004) Science 306, 1954–1957 [DOI] [PubMed] [Google Scholar]
- 19. Fitzgerald G. A. (2004) N. Engl. J. Med. 351, 1709–1711 [DOI] [PubMed] [Google Scholar]
- 20. Stitham J., Stojanovic A., Hwa J. (2002) J. Biol. Chem. 277, 15439–15444 [DOI] [PubMed] [Google Scholar]
- 21. Saito S., Iida A., Sekine A., Kawauchi S., Higuchi S., Ogawa C., Nakamura Y. (2003) J. Hum. Genet. 48, 461–468 [DOI] [PubMed] [Google Scholar]
- 22. Stitham J., Arehart E., Gleim S. R., Li N., Douville K., Hwa J. (2007) Br. J. Pharmacol. 152, 513–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Stitham J., Arehart E. J., Gleim S., Douville K., MacKenzie T., Hwa J. (2007) Gene 396, 180–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Stojanovic A., Stitham J., Hwa J. (2004) J. Biol. Chem. 279, 35932–35941 [DOI] [PubMed] [Google Scholar]
- 25. Stojanovic A., Hwang I., Khorana H. G., Hwa J. (2003) J. Biol. Chem. 278, 39020–39028 [DOI] [PubMed] [Google Scholar]
- 26. Guex N., Peitsch M. C. (1997) Electrophoresis 18, 2714–2723 [DOI] [PubMed] [Google Scholar]
- 27. Arehart E., Stitham J., Asselbergs F. W., Douville K., MacKenzie T., Fetalvero K. M., Gleim S., Kasza Z., Rao Y., Martel L., Segel S., Robb J., Kaplan A., Simons M., Powell R. J., Moore J. H., Rimm E. B., Martin K. A., Hwa J. (2008) Circ. Res. 102, 986–993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Stitham J., Arehart E. J., Gleim S. R., Douville K. L., Hwa J. (2007) Prostaglandins Other Lipid Mediat. 82, 95–108 [DOI] [PubMed] [Google Scholar]
- 29. Stitham J., Stojanovic A., Merenick B. L., O'Hara K. A., Hwa J. (2003) J. Biol. Chem. 278, 4250–4257 [DOI] [PubMed] [Google Scholar]
- 30. Farrens D. L., Altenbach C., Yang K., Hubbell W. L., Khorana H. G. (1996) Science 274, 768–770 [DOI] [PubMed] [Google Scholar]
- 31. Patrignani P., Di Febbo C., Tacconelli S., Douville K., Guglielmi M. D., Horvath R. J., Ding M., Sierra K., Stitham J., Gleim S., Baccante G., Moretta V., Di Francesco L., Capone M. L., Porreca E., Hwa J. (2008) Pharmacogenet. Genomics 18, 611–620 [DOI] [PubMed] [Google Scholar]
- 32. Rosolowsky M., Campbell W. B. (1993) Am. J. Physiol. 264, H327–H335 [DOI] [PubMed] [Google Scholar]
- 33. Martin K. A., Rzucidlo E. M., Merenick B. L., Fingar D. C., Brown D. J., Wagner R. J., Powell R. J. (2004) Am. J. Physiol. Cell Physiol. 286, C507–C517 [DOI] [PubMed] [Google Scholar]
- 34. Arehart E., Gleim S., Kasza Z., Fetalvero K. M., Martin K. A., Hwa J. (2007) Curr. Med. Chem. 14, 2161–2169 [DOI] [PubMed] [Google Scholar]
- 35. Fetalvero K. M., Martin K. A., Hwa J. (2007) Prostaglandins Other Lipid Mediat. 82, 109–118 [DOI] [PubMed] [Google Scholar]
- 36. Liggett S. B. (1997) Am. J. Respir. Crit. Care Med. 156, S156–S162 [DOI] [PubMed] [Google Scholar]
- 37. Bengtsson K., Melander O., Orho-Melander M., Lindblad U., Ranstam J., Råstam L., Groop L. (2001) Circulation 104, 187–190 [DOI] [PubMed] [Google Scholar]
- 38. Hiratsuka M., Mizugaki M. (2001) Mol. Genet. Metab. 73, 298–305 [DOI] [PubMed] [Google Scholar]
- 39. Rana B. K., Shiina T., Insel P. A. (2001) Annu. Rev. Pharmacol. Toxicol. 41, 593–624 [DOI] [PubMed] [Google Scholar]
- 40. Hwa J., Garriga P., Liu X., Khorana H. G. (1997) Proc. Natl. Acad. Sci. U.S.A. 94, 10571–10576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Liu I. S., Seeman P., Sanyal S., Ulpian C., Rodgers-Johnson P. E., Serjeant G. R., Van Tol H. H. (1996) Am. J. Med. Genet. 61, 277–282 [DOI] [PubMed] [Google Scholar]
- 42. Oksche A., Rosenthal W. (1998) J. Mol. Med. 76, 326–337 [DOI] [PubMed] [Google Scholar]
- 43. Cravchik A., Sibley D. R., Gejman P. V. (1996) J. Biol. Chem. 271, 26013–26017 [DOI] [PubMed] [Google Scholar]
- 44. Tanaka H., Moroi K., Iwai J., Takahashi H., Ohnuma N., Hori S., Takimoto M., Nishiyama M., Masaki T., Yanagisawa M., Sekiya S., Kimura S. (1998) J. Biol. Chem. 273, 11378–11383 [DOI] [PubMed] [Google Scholar]
- 45. Rosenthal W., Seibold A., Antaramian A., Gilbert S., Birnbaumer M., Bichet D. G., Arthus M. F., Lonergan M. (1994) Cell. Mol. Biol. 40, 429–436 [PubMed] [Google Scholar]
- 46. Stojanovic A., Hwa J. (2002) Receptors Channels 8, 33–50 [PubMed] [Google Scholar]
- 47. Stitham J., Gleim S. R., Douville K., Arehart E., Hwa J. (2006) J. Biol. Chem. 281, 37227–37236 [DOI] [PubMed] [Google Scholar]
- 48. O'Keeffe M. B., Reid H. M., Kinsella B. T. (2008) Biochim. Biophys. Acta 1783, 1914–1928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Liggett S. B., Wagoner L. E., Craft L. L., Hornung R. W., Hoit B. D., McIntosh T. C., Walsh R. A. (1998) J. Clin. Investig. 102, 1534–1539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Taylor D. R., Drazen J. M., Herbison G. P., Yandava C. N., Hancox R. J., Town G. I. (2000) Thorax 55, 762–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ibrahim S., Tetruashvily M., Frey A. J., Wilson S. J., Stitham J., Hwa J., Smyth E. M. (2010) Arterioscler. Thromb. Vasc. Biol. 30, 1802–1809 [DOI] [PMC free article] [PubMed] [Google Scholar]