Abstract
Poly(ADP-ribose) polymerase-1 (PARP-1) modifies various proteins, including itself, with ADP-ribose polymers (automodification). Polymer synthesis is triggered by binding of its zinc finger 1 (Zn1) and 2 (Zn2) to DNA breaks and is followed by inactivation through automodification. The multiple functional domains of PARP-1 appear to regulate activation and automodification-mediated inactivation of PARP-1. However, the roles of these domains in activation-inactivation processes are not well understood. Our results suggest that Zn1, Zn2, and a domain identified in this study, the double-stranded DNA binding (DsDB) domain, are involved in DNA break-dependent activation of PARP-1. We found that binding of the DsDB domain to double-stranded DNA and DNA break recognition by Zn1 and Zn2, whose actual binding targets are likely to be single-stranded DNA, lead to the activation of PARP-1. In turn, the displacement of single- and double-stranded DNA from Zn2 and the DsDB domain caused by ADP-ribose polymer synthesis results in the dissociation of PARP-1 from DNA breaks and thus its inactivation. We also found that the WGR domain is one of the domains involved in the RNA-dependent activation of PARP-1. Furthermore, because zinc finger 3 (Zn3) has the ability to bind to single-stranded RNA, it may have an indirect role in RNA-dependent activation. PARP-1 functional domains, which are involved in oligonucleic acid binding, therefore coordinately regulate PARP-1 activity depending on the status of the neighboring oligonucleic acids. Based on these results, we proposed a model for the regulation of PARP-1 activity.
Keywords: ADP-ribosylation, DNA, DNA-binding Protein, DNA Damage, DNA Enzymes, RNA
Introduction
Poly(ADP-ribose) polymerase-1 (PARP-1)2 is an enzyme that catalyzes post-translational modifications with ADP-ribose polymers (1–4). This modification plays roles in the regulation of various fundamental cellular processes, including DNA repair (5–8), chromatin remodeling (9–11), and transcription (9, 12–14). PARP-1 regulates these processes by modifying proteins and enzymes, including histones and high mobility group protein (15–18), through its interaction with other enzymes, e.g. topoisomerase I and XRCC1 (19–22), by recruiting DNA repair enzymes to DNA damage sites (6–8) and by binding to transcriptional promoters (9, 23).
It has been demonstrated that binding of PARP-1 to double strand DNA breaks (DSB) or single strand DNA breaks (SSB) triggers ADP-ribose synthesis (1, 2). PARP-1 activation also occurs through its binding to the linker DNA of nucleosomes and upon activation of transcription (11, 12, 24, 25). In addition to DNA, PARP-1 is capable of binding to RNA (13, 26). Thus, PARP-1 could have the ability to recognize diverse oligonucleic acid structures. ADP-ribose synthesis leads to PARP-1 inactivation through automodification (27). These complex regulations of PARP-1 activity are carried out by at least six functional domains of PARP-1.
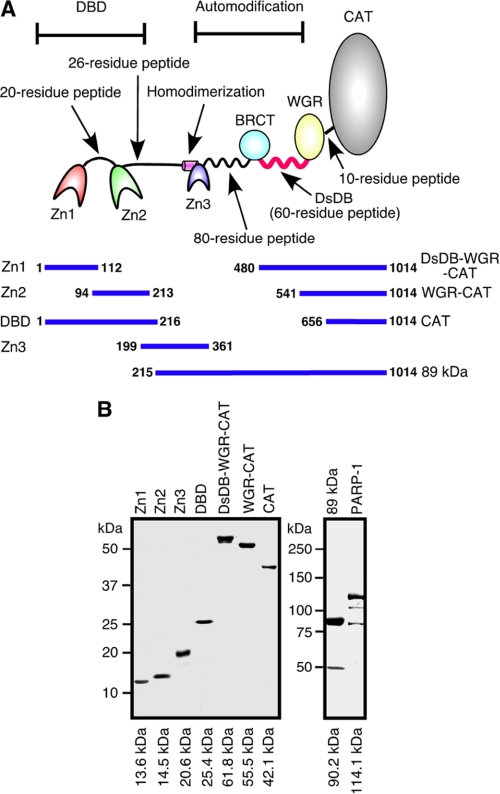
PARP-1 is a 110-kDa enzyme with a modular architecture of multiple functional domains (see Fig. 1A). The most N-terminal end of PARP-1 is the DNA break binding (DBD) domain (28, 29), which contains zinc finger 1 (Zn1), a 20-residue linker peptide, and zinc finger 2 (Zn2), which has over 80% homology with Zn1 (2). Binding of these zinc fingers to DSBs, SSBs and the linker DNA of nucleosomes is essential to activate PARP-1 (1, 2, 11, 30). Although it has been suggested that Zn1 recognizes DSBs and that Zn2 has a binding preference for SSBs, both fingers are required for full activation of PARP-1 (28, 29). It is, however, not well known how Zn1 and Zn2 recognize diverse types of DNA structures. Zn2 is linked to zinc finger 3 (Zn3) by a 26-residue peptide. This zinc finger was identified in recent years as being involved in PARP-1-PARP-1 homodimer formation (31, 32). Zn3 mutants are not activated by DNA breaks, indicating that functional homodimerization of PARP-1 through Zn3 is required for efficient activation of PARP-1 (33, 34). Following Zn3, an 80-residue peptide, which does not form any particular ternary structure, connects Zn3 with a domain called BRCT, involved in the interaction between PARP-1 and other enzymes, including XRCC1 and topoisomerase I (19–21). Between the BRCT and the WGR domain, there is a highly basic 60-residue peptide in which the lysine and glutamic acid residues are known as ADP-ribose polymer attachment sites (33, 35–37). Because this peptide does not form any particular ternary structure either, it is susceptible to protease digestion (35). The precise function of the WGR domain is not known, although a recent report suggests that it is involved in the regulation of the catalytic activity of PARP-1 (33). The C-terminal end of PARP-1 is the catalytic domain (CAT) domain, connected to the WGR domain by a 10-residue peptide. This domain is involved in ADP-ribose synthesis (2).
FIGURE 1.
Purified PARP-1 domains and fragments. A, PARP-1 functional domains are illustrated. B, purified PARP-1 domains and fragments were fractionated on SDS-polyacrylamide gels and visualized by silver staining.
It appears that PARP-1 controls various fundamental cellular processes by coordinating the action of PARP-1 domains. Despite the accumulation of knowledge regarding such domains, the underlying mechanisms involved in PARP-1 activity regulation have not been fully elucidated. We thus performed characterization of Zn1, Zn2, Zn3, and the WGR domain. Furthermore, we have identified the double-stranded DNA binding domain (DsDB), which corresponds to the highly basic 60-residue peptide located between the BRCT and WGR domains. This domain, in its unbound form, inhibited the elongation of ADP-ribose polymers. Binding of the domain to dsDNA released the inhibition, allowing long ADP-ribose polymer formation. Our results suggest that the DsDB domain is one of the key domains involved in regulation of ADP-ribose polymer synthesis. Together with characterization results of the PARP-1 zinc fingers and WGR domain, we proposed a model for PARP-1 activity regulation.
EXPERIMENTAL PROCEDURES
Molecular Cloning and Materials
Sequences corresponding to Zn1 (aa 1–112), Zn2 (aa 94–213), Zn3 (aa 199–361), the DBD domain (aa 1–216), the DsDB-WGR-CAT fragment (aa 480–1014), the WGR-CAT fragment (aa 541–1014), the 89-kDa fragment (aa 215–1014), and the CAT domain (aa 656–1014) were cloned into pET32a. Proteins were expressed in HMS174 (DE3) pLysS and purified using nickel-nitrilotriacetic acid agarose gel chromatography. In addition to His tag, FLAG tag was also attached at the N terminus of Zn1, Zn2, the DsDB-WGR-CAT, and the WGR-CAT fragments. Poly(dA), poly(dC), poly(T), poly(rA), poly(rC), poly(U), poly(dC)-biotin, and poly(U)-biotin of 30 nucleotides in length were purchased from Integrated DNA Technologies Inc. (Coralville, IA).
Binding Assay
Single-stranded DNA (ssDNA) and single-stranded RNA (ssRNA) were incubated with [γ-32P]ATP and polynucleotide kinase. ADP-ribose polymers labeled with 32P were prepared by incubation of PARP-1 (100 pmol) and [32P]NAD+ (10 μCi) in 50 μl of reaction mixture containing 1 mm NAD+, 10 mm Tris-HCl, pH 7.5, and 1 mm MgCl2 for 30 min at 30 °C. PARP-1 was then digested by proteinase K, and 32P-labeled ADP-ribose polymers were precipitated with ethanol. Reactions were carried out with 30 pmol of probes and various amounts of PARP-1 domains or fragments in 10-μl buffer aliquots containing 25 mm Tris-HCl, pH 8.0, 50 mm NaCl, and 1 mm MgCl2 for 15 min at 23 °C. Proteins were fractionated on 7% native acrylamide gels. 32P activity was visualized by autoradiography and quantified using a Typhoon 9200 imager (GE Healthcare) or an AlphaImager (Packard).
Surface Plasmon Resonance Biosensor
Carboxyl surface sensor chips were purchased from Reichert Analytical Instruments. To create the nickel-nitrilotriacetic acid surface, chips were washed with 1 mm NaOH. Then, the surface of the chips was soaked with 200 mm 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide and 50 mm N-hydroxysuccinimide for 10 min at room temperature followed by washing with 20 mm sodium acetate. Sensor chips were incubated with 1 mg/ml (S)-N-(5-amino-1-carboxypentyl)iminodiacetic acid hydrate for 30 min at 4 °C. After washing, the surface was treated with 1 m ethanolamine for 10 min at room temperature. Then, the surface was treated with 1 mm NaOH for 10 min and then incubated with 40 mm NiSO4 for 10 min at room temperature. After the surface was rinsed with 150 mm NaCl, the chips were used for analysis. A Reichert SR7000 was employed for analysis after immobilization of Zn1 or Zn2 to the sensor surface by injecting 1 mg/ml Zn1 or Zn2 at a flow rate of 0.005 ml/min. Then, the response was measured by injecting 25, 50, 75, 100, and 200 nm poly(dC) or poly(rC) at a flow rate of 1 ml/min. Binding constants of poly(dC) and poly(rC) to Zn1 or Zn2 were determined by the Prism and Scrubber programs.
Dumbbell DNA (dumbDNA) Preparation
For dumbDNA, the sequence reported by Kulczyk et al. (38) was used. Two DNA stem loops (100 pmol) were ligated by T4 DNA ligase. Then, dumbDNA was fractionated on ethidium bromide-1% agarose gels and extracted. For the preparation of dumbDNA, which lacks DNA loops, 5′-CGACTCTGGCTGGCCGCGACCTCTGTCG-3′ and 5′-CGACAGAGGTCGCGGCCAGCCAGAGTCG-3′ were prepared and annealed.
Double-stranded RNA Preparation
5′-AAUGUUCUCGACAGCAAGUAC-3′ and 5′-GUACTTGCTGTCGAGAACAUU-3′ were annealed and used for assays.
Interaction Assay for dumbDNA or Double-stranded RNA-PARP-1 Domains
dumbDNAs or dsRNA (10 pmol) were incubated with various amounts of PARP-1 domains or fragments in a 20-μl mixture containing 10 mm Tris-HCl, pH 7.5, 100 mm NaCl, and 1 mm MgCl2 for 15 min at 37 °C. Reaction mixtures were fractionated on ethidium bromide-1.5% agarose gel. dumbDNAs or dsRNA were visualized using UV.
Poly(ADP-ribosyl)ation Assay
Poly(ADP-ribosyl)ation assays were carried out with dumbDNAs with PARP-1 domains or fragments in a 10-μl reaction mixture containing 1 μCi of [32P]NAD+, 1 mm NAD+, 10 mm Tris-HCl, pH 7.5, 100 mm NaCl, and 1 mm MgCl2 at 37 °C for 15 min. Proteins were fractionated on an SDS-polyacrylamide gel. Stacking gel was not used. 32P activity was visualized by autoradiography and quantified using a Typhoon 9200 imager.
Pulldown of 32P-labeled ADP-ribose Polymers by Zn1, Zn2, the DsDB-WGR-CAT, and the WGR-CAT Fragment
The binding reaction was carried out as described above with 32P-labeled ADP-ribose polymers and Zn1, Zn2, the DsDB-WGR-CAT, or the WGR-CAT fragment. Then, these fingers or fragments were incubated with the anti-FLAG M2 affinity gel (Sigma-Aldrich). After washing precipitates with 50 mm Tris-HCl, pH 7.5, 100 mm NaCl, 1 mm MgCl2, and 0.1% Nonidet P-40, urea-loading buffer was added. After incubation at 65 °C for 15 min, samples were fractionated on urea-10% or urea-15% polyacrylamide gel, and 32P activity was visualized by autoradiography.
Pulldown of 32P-labeled Oligonucleic Acids with Biotinylated Oligonucleic Acids
Either poly(dC)-biotin or poly(U)-biotin (0.25 pmol) was incubated with 32P-labeled poly(rA), poly(U), or poly(dC) (0.25 pmol) in 10 μl of reaction mixture containing 50 mm Tris-HCl, pH 7.5, 100 mm NaCl, 1 mm MgCl2, 0.1% Nonidet P-40, and various amounts of PARP-1, Zn3, or the DBD domain for 15 min at 37 °C. The reaction mixture was then mixed with 5 μl of avidin-Sepharose for 1 h at room temperature. After washing the Sepharose, urea-loading buffer was added and incubated at 65 °C for 10 min. 32P-labeled oligonucleic acids were fractionated on urea-10% polyacrylamide gel and visualized by autoradiography.
RESULTS
ssDNAs as Potential Binding Targets of Zn1 and Zn2
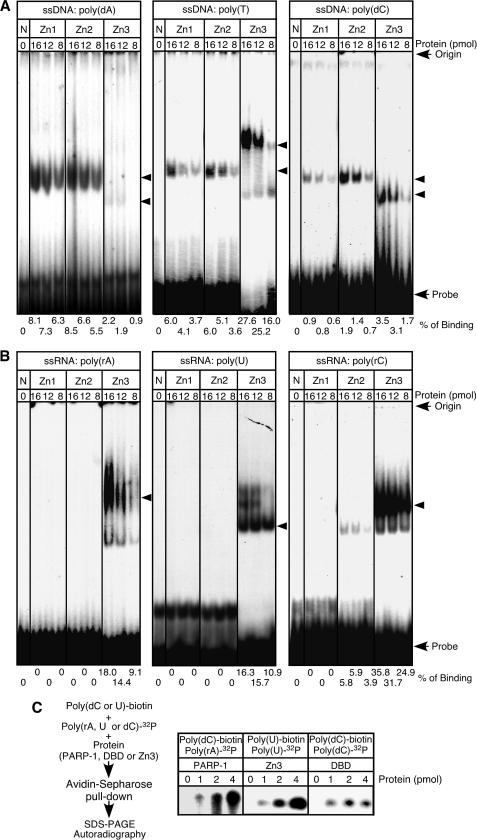
DNA nicks, DNA gaps, and DSBs, including blunt, 3′-protruding, and 5′-protruding ends, are known binding targets of the DBD domain (2, 29, 39, 40). This domain is also capable of binding to DNA stem loops (41, 42), implying that the domain recognizes oligonucleic acid structures that commonly appear in DNA breaks and in DNA stem loops, such as ssDNA. Binding of the DBD domain to DNA breaks is a key step in PARP-1 activation, but its mechanism is not well understood. We thus began this study by characterizing Zn1 and Zn2 binding to ssDNA. When recombinant Zn1 and Zn2 (Fig. 1, A and B) were incubated with 30 nucleotides of synthetic 32P-labeled ssDNAs, i.e. poly(dA), poly(T), and poly(dC) (except poly(dG), which forms G-quadruplexes), discrete retarding bands were formed (Fig. 2A). On the other hand, neither recombinant showed significant binding to poly(rA), poly(U), and poly(rC) (Fig. 2B). We also used Zn3 and found that Zn3 is capable of binding to ssRNA (Fig. 2B). These results suggest that ssDNA is one of the potential binding targets of Zn1 and Zn2.
FIGURE 2.
Analysis of Zn1, Zn2, and Zn3 binding to ssDNA and ssRNA probes. A, binding assays were carried out with 32P-labeled poly(dA), poly(T), or poly(dC) (30 pmol) and Zn1, Zn2, or Zn3. Retarded bands are indicated by arrowheads. N, no protein. B, instead of ssDNA probes, 32P-labeled poly(rA), poly(U), and poly(rC) (30 pmol) were used. C, biotinylated poly(dC) or poly(U) (0.25 pmol) and 32P-labeled poly(rA), poly(U), or poly(dC) (0.25 pmol) were used. After incubation of a biotinylated and a 32P-labeled oligonucleic acid with PARP-1, Zn3, or the DBD domain, biotinylated oligonucleic acid was pulled down by avidin-Sepharose. 32P-labeled oligonucleic acid was analyzed using urea-10% polyacrylamide gels.
Cross-binding of ssDNA by the DBD Domain
Binding of Zn1 and Zn2 to ssDNA suggests that the DBD domain contains two ssDNA binding sites. To study whether the domain is capable of binding to two ssDNA molecules, we carried out cross-binding assays. As illustrated in Fig. 2C, this assay was designed to pull down 32P-labeled oligonucleic acid, using avidin-Sepharose, when the labeled oligonucleic acid binds to one functional zinc finger and biotinylated oligonucleic acid binds to the other. For example, 32P-labeled poly(rA) was pulled down when PARP-1 was incubated with biotinylated poly(dC), demonstrating the binding of Zn3 to poly(rA) and of Zn1 or Zn2 to poly(dC). 32P-labeled poly(U) is expected to be pulled down with biotinylated poly(U) if Zn3 forms a functional homodimer and if each Zn3 in the homodimer binds ssRNA. Indeed, 32P-labeled poly(U) was pulled down, suggesting that both Zn3 domains are capable of binding to ssRNA. In the case of the DBD domain, 32P-labeled poly(dC) was pulled down by avidin-Sepharose by incubation of the domain with biotinylated poly(dC). Two zinc fingers in the DBD domain can thus indeed independently bind to ssDNA.
Binding Affinity of Zn1 and Zn2 to ssDNA
We then employed surface plasmon resonance biosensor and poly(dC) to investigate whether Zn1 and Zn2 have the necessary affinity to form stable complexes with ssDNA. As summarized in Table 1, the KD of Zn1 was 7.9 × 10−8 M. Although Zn2, which has a secondary role in DNA break binding (33, 35–37), had a lower affinity for poly(dC) than Zn1, the KD of Zn2 was still 1.6 × 10−7 m. Consistent with binding assay results (Fig. 2B), Zn1 and Zn2 showed negligible affinity for poly(rC). It has been reported that the binding affinity of PARP-1 toward DNA breaks is about 2.6 × 10−9 to 1.1 × 10−10 m (43). Although the KD of Zn1 and Zn2 to poly(dC) is about 30–60-fold lower than that of PARP-1 to DNA breaks, these results suggest that Zn1 and Zn2 have sufficient affinity to establish stable bounds with ssDNAs. Thus, ssDNAs, which are formed at DNA break ends and at the loop region of DNA stem loops, can be binding targets of Zn1 and Zn2.
TABLE 1.
Analysis of Zn1 and Zn2 binding to poly(dC) by surface plasmon resonance biosensor
ND, not detected.
| Zn1 |
Zn2 |
|||||
|---|---|---|---|---|---|---|
| Ka | Kd | KD | Ka | Kd | KD | |
| m−1 s−1 | s−1 | m | m−1 s−1 | s−1 | m | |
| poly(dC) | 1.3 × 105 | 0.0108 | 7.9 × 10−8 | 2.1 × 105 | 0.0342 | 1.6 × 10−7 |
| poly(rC) | ND | ND | ND | ND | ND | ND |
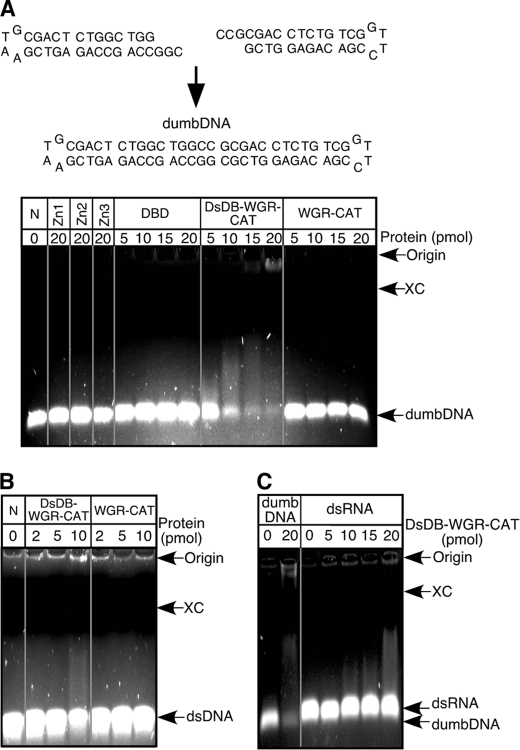
DsDB Domain
Although we have demonstrated that Zn1 and Zn2 bind to ssDNAs, biochemical studies of PARP-1 have indicated that ssDNAs only served as a weak activator of PARP-1 (39). One of the explanations for this lack of full activation of PARP-1 by ssDNAs is that PARP-1 can distinguish whether it binds to free ssDNAs or to ssDNAs formed in dsDNA structures such as DNA breaks. Previously, Ikejima et al. (29) suggested the presence of a dsDNA binding domain in the 89-kDa fragment of PARP-1 that is involved in the activation of PARP-1. Thus, we have studied whether such a domain indeed existed by preparing dsDNA with a minimal length of loop (dumbDNA (38)) (Fig. 3A) and by carrying out DNA mobility assays. Zn1, Zn2, the DBD domain, Zn3, and the WGR-CAT fragment did not show any significant binding to dumbDNA (Fig. 3A). On the other hand, incubation of dumbDNA with the DsDB-WGR-CAT fragment, which contained a highly basic 60-residue peptide between the BRCT and the WGR domains, resulted in the formation of a smear that migrated near the origin. The DsDB-WGR-CAT fragment therefore bound to dumbDNA. Because dumbDNA contained small terminal loops, we then used dumbDNA that lacked these loops. Even in the absence of the loops, the DsDB-WGR-CAT fragment still bound dumbDNA (Fig. 3B). On the other hand, the DsDB-WGR-CAT fragment did not show any specific binding to ssDNA (data not shown), and double-stranded RNA does not appear to be a preferred binding target for the DsDB domain (Fig. 3C). Thus, these results suggest that the dsDNA binding domain indeed exists as Ikejima et al. (29) predicted. We referred to this domain as the DsDB domain.
FIGURE 3.
dsDNA binding domain of PARP-1. A, dumbDNA was prepared by ligating two DNA stem loops and used for DNA mobility assays on ethidium bromide-1.5% agarose gel. The xylene cyanol (XC) marker is seen as a black line. N, no protein. B, dumbDNA, which lacks terminal loops, was incubated with the DsDB-WGR-CAT or WGR-CAT fragment. C, double-stranded RNA was incubated with the DsDB-WGR-CAT fragment and used for RNA mobility assay with ethidium bromide-1.5% agarose gels.
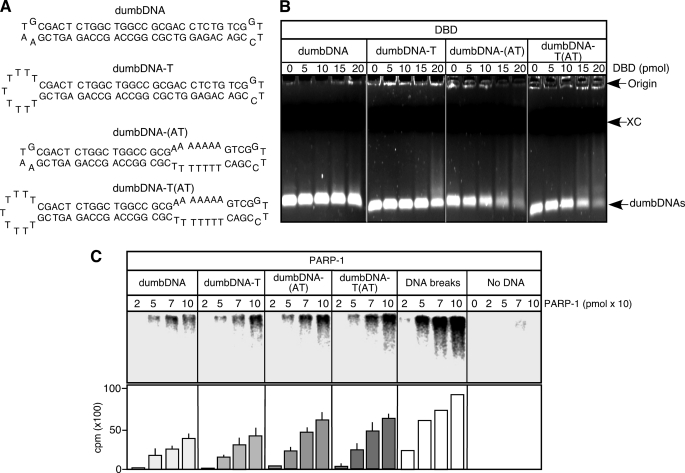
Activation of PARP-1 by dumbDNA-(AT)
Binding of Zn1 and Zn2 of the DBD domain to ssDNA and binding of the DsDB domain to dsDNA imply that a DNA structure composed of a junction between ssDNA and dsDNA could activate PARP-1. Thus, we prepared dumbDNA with a T-loop (dumbDNA-T) and dumbDNA containing an AT-rich region (dumbDNA-(AT)), which can be destabilized at 37 °C, thereby producing a single-stranded region (Fig. 4A). Although the DBD domain did not show significant binding to dumbDNA, it had the ability to bind to dumbDNA-T, dumbDNA-(AT), and dumbDNA-T(AT), which contained both the T-loop and the AT-rich region (Fig. 4B). Consistent with the ability of the DBD domain to bind dumbDNAs, the poly(ADP-ribosyl)ation activity of PARP-1 was promoted, particularly by dumbDNA-(AT) and dumbDNA-T(AT) (Fig. 4C). Although DNA breaks were still the best PARP-1 activators, the promotion of automodified PARP-1 synthesis by these dumbDNAs suggests that activation of PARP-1 occurs through binding of Zn1 and Zn2 to ssDNA and of the DsDB domain to dsDNA at the junction between ssDNA and dsDNA.
FIGURE 4.
Poly(ADP-ribosyl)ation assay with various dumbDNAs. A, various dumbDNAs including ones containing a T-loop (dumbDNA-T), an AT-rich region (dumbDNA-(AT)), or both a T-loop and an AT-rich region (dumbDNA-T(AT)) were prepared. The Tm of the AT-rich region is 24 °C. Thus, the region is expected to be destabilized at 37 °C. B, DNA mobility assays with ethidium bromide-1.5% agarose gels were carried out with the DBD domain. Various dumbDNAs (10 pmol) were used. XC, xylene cyanol. C, poly(ADP-ribosyl)ation assays were carried out with various dumbDNAs (800 fmol) and PARP-1 in the presence of [32P]NAD+. For assays with DNA breaks, DSBs corresponding to 800 fmol were added. Automodified PARP-1 was fractionated with SDS-10% polyacrylamide gels. Labeled PARP-1 was visualized by autoradiography, and 32P activity was quantified by a Typhoon scanner. Standard deviations are shown.
WGR Domain and RNA-dependent Activation of PARP-1
We then characterized the WGR domain, which is located at the N terminus of the DsDB domain and has been suggested to be involved in the regulation of the CAT domain (33). Because this domain contains a WGR consensus peptide sequence that is found in RNA-metabolizing enzymes, we investigated whether the WGR domain has the ability to interact with RNA by employing the WGR-CAT fragment and the CAT domain. Results of binding assays with ssRNA and ssDNA probes have demonstrated that the WGR-CAT fragment is in fact capable of binding to poly(rA), poly(U), and poly(rC), whereas ssDNAs are not preferred binding targets of the WGR-CAT fragment (Fig. 5A). Because the CAT domain alone did not show significant binding to ssRNA, these results suggest that the WGR domain has the ability to bind to ssRNA. Furthermore, as shown in Fig. 5B, incubation of ssRNA with the WGR-CAT fragment led to activation of ADP-ribose polymer synthesis, whereas ssDNA induced less activation than ssRNA, indicating that the CAT domain can be activated through binding of the WGR domain to ssRNA. The WGR domain is thus involved in RNA-dependent activation of PARP-1.
FIGURE 5.
Analysis of the WGR domain binding to ssRNA and poly(ADP-ribosyl)ation of PARP-1 functional domains activated by poly(rA) or dumbDNA. A, binding assays were carried out with either the WGR-CAT fragment or the CAT domain (12 pmol). ssDNA or ssRNA probes labeled with 32P (30 pmol) were used. Retarded bands are indicated by arrowheads. N, no protein. B, poly(ADP-ribosyl)ation assays were carried out with the WGR-CAT fragment in the presence of ssRNA (20 pmol) or ssDNA (20 pmol). C and D, poly(ADP-ribosyl)ation assays were carried out with PARP-1 and the 89-kDa fragment in the presence of poly(rA) (C). Alternatively, assays were carried out with the DsDB-WGR-CAT fragment, the WGR-CAT fragment, and the CAT domain (D). E, poly(ADP-ribosyl)ation assays were carried out with either the WGR-CAT or the DsDB-WGR-CAT fragment (5 pmol) in the presence of various concentrations of dumbDNA.
Negative Regulation of ADP-ribose Polymer Synthesis by the DsDB Domain
As shown in Fig. 5, C and D, poly(rA) activates ADP-ribose polymer synthesis when poly(ADP-ribosyl)ation assays are carried out with PARP-1, the 89-kDa fragment, the DsDB-CAT-WGR fragment, and the WGR-CAT fragment. Typically, automodified PARP-1, the 89-kDa fragment, and the DsDB-WGR-CAT fragment, which migrated around their original molecular masses, were produced due to the attachment of short poly(ADP-ribose) polymers to these proteins (44). On the other hand, removal of the DsDB domain (the WGR-CAT fragment) induced its automodified form to migrate near the origin due to the formation of long ADP-ribose polymers (typically over 50 ADP-ribose residues (44)). The DsDB domain thus inhibited the formation of long ADP-ribose polymers. In the poly(ADP-ribosyl)ation assay with poly(A), the DsDB domain remained unbound to dsDNA. Thus, we then investigated the effect on ADP-ribose polymer synthesis of the DsDB domain binding to dsDNA by employing dumbDNA. As shown in Fig. 5E, in the presence of dumbDNA, an automodified DsDB-WGR-CAT fragment that migrated near the origin was in fact produced, allowing the formation of long ADP-ribose polymers by binding of the DsDB domain to dsDNA. These results suggest that the DsDB domain controls ADP-ribose polymer synthesis; this domain negatively regulates synthesis of ADP-ribose polymers when it does not bind to dsDNA, thereby limiting PARP-1 to the synthesis of only short ADP-ribose polymers, whereas binding of the domain to dsDNA allows PARP-1 to produce long ADP-ribose polymers.
Displacement of ssDNA and dsDNA from Zn2 and the DsDB Domain, Respectively, by ADP-ribose Polymers
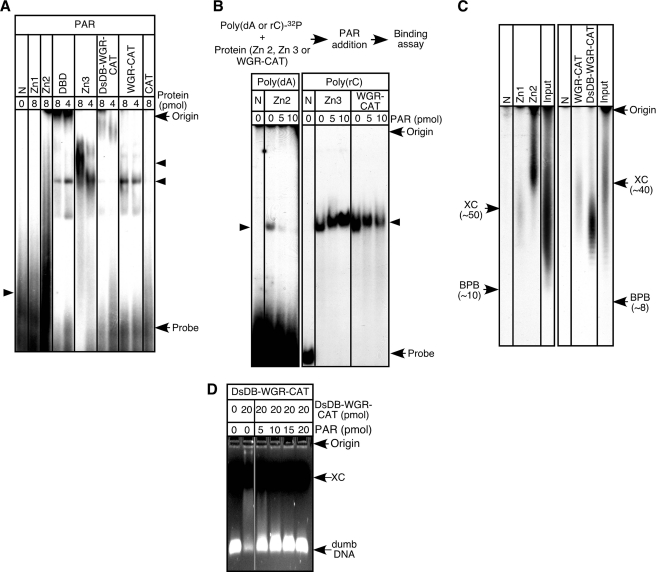
The last step in the PARP-1 activation-inactivation processes is dissociation of automodified PARP-1 from DNA breaks (27). The mechanism of such dissociation is not well known. Because our current results suggest that PARP-1 is retained at DNA breaks through binding of its functional domains to ssDNA and dsDNA, we have studied the effect of ADP-ribose polymers on the binding of Zn1, Zn2, Zn3, the DsDB domain, and the WGR domain to their target oligonucleic acids. We first tested whether PARP-1 functional domains bind to ADP-ribose polymers. Results shown in Fig. 6A indicate that Zn2, Zn3, the DsDB domain, and the WGR domain can indeed bind to ADP-ribose polymers. Therefore, we have carried out displacement assays with these functional domains. In the case of Zn2, poly(dA)-Zn2 complexes were preformed, and then ADP-ribose polymers were added to the reaction (Fig. 6B). Such an addition led to the dissolution of the preformed complexes (Fig. 6B), suggesting that ADP-ribose polymers displace poly(dA) from Zn2. Because Zn2 binds to ADP-ribose polymers that are more than 50 residues in length (Fig. 6C), this dissociation could occur only following long ADP-ribose polymer formation. Preformed complexes of Zn3 or the WGR-CAT fragment with poly(rC) were not well dissolved by the polymers (Fig. 6B). Thus, binding of Zn3 or the WGR domain is unlikely affected by ADP-ribose polymer formation. In the case of the DsDB domain, preformed dsDNA-DsDB domain complexes were dissolved by the addition of ADP-ribose polymers (Fig. 6D). Distinct from Zn2, however, the DsDB domain has a preference for ADP-ribose polymers of around 30 ADP-ribose residues in length (Fig. 6C). These results demonstrate that one of the mechanisms involved in the dissociation of PARP-1 from DNA breaks is the displacement of ssDNA and dsDNA from Zn2 and the DsDB domain, respectively, by ADP-ribose polymers.
FIGURE 6.
Binding of ADP-ribose polymer to PARP-1 functional domains. A, binding assays were carried out with 32P-labeled ADP-ribose polymers (30 pmol) and PARP-1 domains. N, no protein; PAR, ADP-ribose polymers. B, Zn2 (12 pmol) was preincubated with 32P-labeled poly(dA) (30 pmol) for 15 min at room temperature. Alternatively, Zn3 and the WGR-CAT fragment (12 pmol) were incubated with 32P-labeled poly(rC) (30 pmol). Then, ADP-ribose polymers were added. The resulting samples were analyzed by 7.5%-native acrylamide gel electrophoresis. C, binding assays were carried out with 32P-labeled ADP-ribose polymers (30 pmol) with either FLAG-tagged Zn1 or FLAG-tagged Zn2 (20 pmol). Then, these fingers were pulled down by anti-FLAG M2 affinity gel. After washing of the precipitates, zinc fingers were denatured, and 32P-labeled ADP-ribose polymers were analyzed using urea-10% polyacrylamide gels. When FLAG-tagged WGR-CAT or DsDB-WGR-CAT fragment was used, 20 pmol of poly(dC) was included in the reaction mixture, and fractionation of 32P-labeled ADP-ribose polymers was carried out by using urea-15% polyacrylamide gels. For urea-10% polyacrylamide gels, xylene cyanol (XC) and bromphenol blue (BPB) migrated around 50 and 10 in length of ADP-ribose residues. For urea-15% polyacrylamide gels, xylene cyanol and bromphenol blue migrated around 40 and 8 in length of ADP-ribose residues. D, the DsDB-WGR-CAT fragment was incubated with dumbDNA (10 pmol) for 15 min at 37 °C, and then ADP-ribose polymers were added. After a 15-min incubation at 37 °C, DNA mobility assays were carried out on ethidium bromide-1.5% agarose gels.
DISCUSSION
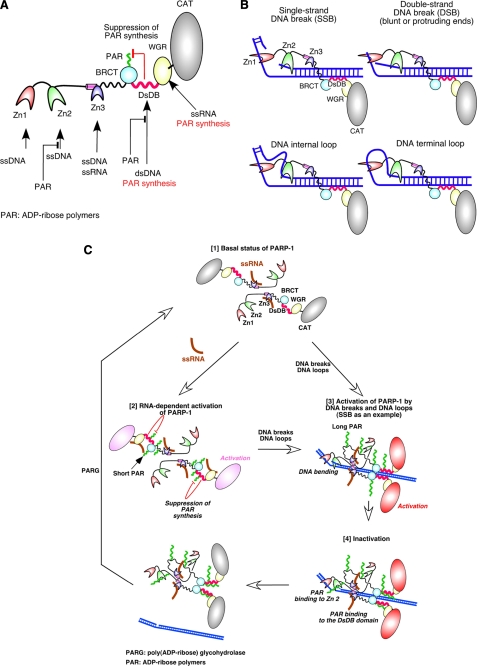
PARP-1 is one of the most highly investigated enzymes (4, 5, 9, 31–33, 40, 45). Despite that fact, the underlying molecular mechanisms that control PARP-1 activity have not been fully elucidated due to the lack of critical information regarding the characteristics of PARP-1 domains. We thus performed the current study to obtain such information and, as summarized in Fig. 7A, our results suggest that: 1) Zn1 and Zn2 have the ability to bind to ssDNA; 2) the DsDB domain is involved dsDNA binding; 3) the unbound form of the DsDB domain inhibits the formation of long ADP-ribose polymer synthesis; 4) Zn1, Zn2, and the DsDB domain play a role in DNA break- and DNA loop-dependent activation of PARP-1; 5) the WGR domain has the ability to bind to ssRNA, leading to RNA-dependent activation of PARP-1; and 6) ADP-ribose polymers displace ssDNA and dsDNA from Zn2 and the DsDB domain, respectively. Based on these results and on reports published by others, we are proposing two models, i.e. models for Zn1 and Zn2 binding to DNA breaks and DNA loops and for PARP-1 activity regulation.
FIGURE 7.
A model for the binding of the DBD domain to DNA breaks and DNA loops and the regulatory mechanisms of PARP-1 activity. A, a summary of our results is shown. Zn1 and Zn2 bind to ssDNA. Zn3 has the ability to bind to ssRNA in addition to ssDNA. The DsDB domain is involved in recognition of dsDNA. When the domain does not bind to dsDNA, it suppresses ADP-ribose polymer synthesis. By binding of this domain to dsDNA, the suppression is removed. The WGR domain binds to ssRNA, resulting in activation of the CAT domain. Upon the formation of ADP-ribose polymers, ssDNA and dsDNA are displaced from Zn2 and the DsDB domain, respectively, by the polymers. B, a model for the DBD domain binding to DNA breaks and DNA loops is illustrated. Zn1 binds to one DNA strand, and Zn2 holds the complementary strand of SSBs. Because Zn1 and Zn2 are connected by a peptide of only 20 residues, binding of both fingers to DNA strands could bend DNA. In a similar manner, Zn1 and Zn2 can bind to DSBs, DNA internal loops, and DNA terminal loops. C [1], in the basal status, poly(ADP-ribosyl)ation activity of PARP-1 is suppressed by other PARP-1 domains. The DsDB domain is one of such domains, which has role in inhibition of long ADP-ribose polymer formation. Two PARP-1 molecules are illustrated. [2], binding of ssRNA to the WGR domain activates the CAT domain. However, only short polymers are produced due to the suppression of ADP-ribose polymer synthesis by the DsDB domain. [3], when Zn1 and Zn2 bind to a SSB, PARP-1 forms a functional homodimer. PARP-1 is aligned with DNA strand through binding of the DsDB domain to dsDNA, leading to the activation of the CAT domain. Binding of the DsDB domain to dsDNA releases the suppression of ADP-ribose polymer formation, allowing the CAT domain to produce long ADP-ribose polymers. PARP-1 could bind to DSBs and to DNA loops in a similar manner to as to SSBs. [4], ADP-ribose polymers then displace ssDNA and dsDNA from Zn2 and the DsDB domain, respectively, resulting in dissociation of PARP-1 from DNA breaks. ADP-ribose polymers are then degraded by poly(ADP-ribose) glycohydrolase (PARG).
Model for Zn1 and Zn2 Binding to DNA Breaks and DNA Loops
The DBD domain binds to a variety of DNA breaks, including blunt ends, 5′-protruding and 3′-protruding ends, DNA nicks, DNA gaps, DNA stem loops, and dsDNA at the linker region of nucleosomes (2, 11, 24, 29, 39–41). Recognition and binding of such diverse types of DNA structures are carried out by Zn1 and Zn2, although the underlying mechanism of this binding remains unclear. Because we found that Zn1 and Zn2 have the ability to bind to ssDNA (Fig. 2), we propose the ssDNA binding model. As illustrated in Fig. 7B (SSB), when Zn1 binds to one DNA strand at a SSB, the second zinc finger, Zn2, can bind to the complementary DNA strand. As Zn1 is tandemly connected to Zn2 by only about 2 nm of peptide (20 peptide residues, corresponding to about 5 bp), binding of Zn1 and Zn2 to DNA strands in the manner illustrated in Fig. 7B would create DNA bending (40). Binding of Zn1 and Zn2 to DSBs could occur in a similar manner as binding to SSBs (Fig. 7B). Furthermore, this model can predict that the DBD domain has a preference for either 3′-protruding or 5′-protruding ends as Zn1 could more efficiently recognize protruded ssDNA from DSB ends. Indeed, it has been reported that 3′-protruding ends serve as better activators of PARP-1 (39) and that the DBD domain has a higher binding affinity toward 3′-protruding ends than blunt or 5-protruding ends (40). Thus, 3′-ssDNA created at the DSB ends could be binding targets of Zn1. These ssDNA binding models are also consistent with the notion that the DSB domain recognizes a junction between ssDNA and dsDNA (Fig. 4) (42, 46). Any DNA containing such a junction could be a binding target for the DBD domain. ssDNA at DNA stem loops and internal loops may thus serve as binding targets for Zn1 and Zn2 (Fig. 7B).
Model for PARP-1 Activity Regulation, Basal Status
It has been reported that PARP-1 forms functional homodimers (47). Several domains, including the DBD and DsDB domain, have been identified as homodimerization domains by biochemical investigations (48). However, structural analysis of Zn3 reveals that the zinc ribbon fold of Zn3 is potentially involved in PARP-1-PARP-1 homodimerization (31, 32), although a recent report suggests that such homodimerization occurs in crystal lattice but not in solution (34). Langelier et al. (34) thus suggest that homodimerization of PARP-1 through the zinc ribbon fold occurs upon PARP-1 binding to DNA breaks or when concentration of PARP-1 is high enough to allow the formation of Zn3 dimers. Pion et al. (40) indeed found that the DBD domain is able to bind to two SSBs. However, the DBD domain that was used in their study did not contain Zn3. Thus, it is not yet clear whether multiple PARP-1 domains are involved in PARP-1-PARP-1 homodimerization. In our model, we illustrated two PARP-1 molecules in a head-to-tail arrangement by a manner proposed by Langelier et al. (31) (Fig. 7C, [1]) because cross-binding of two ssRNA molecules by Zn3 suggests the formation of Zn3-Zn3 homodimers (Fig. 2). However, the two PARP-1 molecules are separated as there is no conclusive evidence for the existence of preformed PARP-1-PARP-1 homodimers.
In the basal status, catalytic activity of PARP-1 is tightly regulated as it only shows extremely weak poly(ADP-ribosyl)ation activity in the absence of its activators (e.g. Fig. 5C, lane 1). It appears that one or more of the PARP-1 domains thus directly or indirectly suppress ADP-ribose polymer formation. The DsDB domain, which was identified in this study, could be involved in such suppression by inhibiting the synthesis of ADP-ribose polymers (Fig. 7C, [1]). The WGR domain could also be involved in the suppression as it has a role in the regulation of PARP-1 catalytic activity (33).
Other than its involvement in PARP-1-PARP-1 homodimerization and chromatin compaction (31, 32, 34), the functional roles of the Zn3 finger in the basal status of PARP-1 are not yet clear. However, it is plausible that Zn3 has a role in the retention of PARP-1 on nascent RNA as Zn3 can bind to ssRNA (Fig. 2). Furthermore, Fakan et al. (26) previously observed that PARP-1 binds to RNA stem loops, Tulin and Spradling (25) have reported accumulation of PARP-1 to Drosophila puffs upon activation of transcription, and it has been demonstrated that PARP-1 is concentrated to transcriptionally active nucleoli (49, 50). In addition, the KD of PARP-1 to RNA stem loop is 1.0 × 10−10 m (13), which allows the formation of highly stable complexes with RNA. Thus, in its basal status, a fraction of PARP-1 in the nucleus may accumulate at actively transcribed genes through Zn3 binding to nascent RNA.
RNA-dependent Activation of PARP-1
Binding of the WGR domain to ssRNA (Fig. 5) leads to RNA-dependent activation of PARP-1 (Fig. 7C, [2]). This activation unlikely causes the formation of heavily automodified PARP-1 as the DsDB domain could suppress long ADP-ribose polymer formation (Fig. 5). The functional role of this weak poly(ADP-ribosyl)ation of PARP-1 is, however, not well understood. Perhaps this activation is required so that PARP-1 can reach a form that can be efficiently automodified by ADP-ribose polymers upon its activation by DNA breaks or DNA loops. Indeed, the first ADP-ribose residue needs to be attached to glutamic acid, aspartic acid, or lysine, which are ADP-ribose polymer attachment sites (33, 35–37). This weak activation may thus be involved in PARP-1 priming. Alternatively, RNA-dependent activation of PARP-1 could have a role in the regulation of nascent RNA elongation as suppression of RNA synthesis occurs upon binding of PARP-1 to RNA stem loops (13). Such suppression is related to the negative transcription elongation factors, negative elongation factor (NELF) and 5,6-dichloro-1-β-d-ribofuranosylbenzimidazole sensitivity inducing factor (DSIF), which are now known as critical transcriptional regulators involved in divergent transcription (51, 52). The functional role of this activation still remains to be elucidated, and the role of Zn3, which has the ability to bind to ssRNA in the RNA-dependent activation, is still not clear. However, it is evident that this activation plays critical roles in nascent RNA synthesis as reports suggest the presence of a link between transcription and PARP-1 (14, 25).
Activation of PARP-1 by DNA Breaks and DNA Loops
Following the models shown in Fig. 7B, we illustrated that Zn1 and Zn2 bind to ssDNAs at a DNA breaks site, that the DsDB domain recognizes dsDNA, and that PARP-1 forms a homodimer through the zinc ribbon fold (34) (Fig. 7C, [3], illustration is based on PARP-1 binding to SSB). Suppression of ADP-ribose polymer synthesis by the DsDB domain is released by its binding to dsDNA (Fig. 5E), allowing automodification of PARP-1 with long ADP-ribose polymers. Because the homodimerization of PARP-1 brings the CAT domain close to the automodification site (34), the efficiency of PARP-1 automodification can be significantly promoted (47). In our model, we illustrated that the DsDB domain of the second PARP-1 also binds to dsDNA and that the two PARP-1 molecules interact through the BRCT domain, which has been suggested to have a role in PARP-1-PARP-1 homodimer formation (21), whereas the second pair of zinc fingers remains unbound. Although it is not known whether this pair of zinc finger has functional roles, it may be involved in cross-binding of two DSB ends if a PARP-1-PARP-1 homodimer is formed at the DSB (53, 54). Audebert et al. and Wang et al. (53, 54) in fact suggest that the efficiency of DSB repair is promoted through homodimerization of PARP-1.
A similar model could be applied for PARP-1 activation by its binding to DNA loops or DNA structure containing a junction of ssDNA and dsDNA, e.g. internal loops. Activation of PARP-1 by this type of DNAs is likely equivalent to the DNA damage-independent PARP-1 activation (11), which occurs upon binding of PARP-1 to the linker DNA of nucleosomes and perhaps at transcription promoter.
It has been demonstrated that PARP-1 is recruited to transcriptional promoters through interaction with transcription co-activators (14), e.g. NF-κB (48). Although it is not known whether recruited PARP-1 is indeed activated, recent studies suggest that the transcription of a subset of genes is promoted, whereas others are suppressed by ADP-ribose polymer formation (24, 55), thereby indicating that DNA structures that can activate PARP-1 are indeed formed at transcriptional promoters.
Such structures could be produced at other regions of chromatin as PARP-1 can be activated by nucleosome linker DNA, leading to chromatin remodeling (11). A recent report in fact suggests that a chromatin-remodeling factor, ALC1, which has a role in transcription and DNA repair, is recruited to the remodeling site through its binding to ADP-ribose polymers (56). This observation also implies that chromatin remodeling per se occurs as a downstream event of PARP-1 activation, thereby suggesting that DNA structures that activate PARP-1 are produced by other mechanisms. Although it has been demonstrated that activation of PARP-1 by DNA breaks triggers DNA damage-dependent chromatin remodeling through recruitment of ALC1 to DNA damage sites (10), how DNA structures, which are involved in DNA break-independent activation of PARP-1, are formed is not known. Perhaps such structures could be formed or exposed following the progression of RNA polymerases as accumulation of PARP-1 to actively transcribed regions of chromatin has been reported (25). Once PARP-1 is activated, histones, in addition to PARP-1 itself, are poly(ADP-ribosyl)ated (57). ADP-ribose polymer binding factors, e.g. ALC1 (10, 56), and DNA repair factors, XRCC1 (7, 58) and aprataxin and PNK-like factor (APLF) (8), could thus be recruited to the polymers produced on PARP-1 or histones. If these factors bind to automodified PARP-1, they could be brought away from chromatin-remodeling sites upon dissociation of automodified PARP-1 from DNA. If binding of these factors to ADP-ribose polymers formed on histones occurs, they could be able to remain at the site. Nevertheless, it is not known how these factors exert their function after their binding to ADP-ribose polymers and how PARP-1, particularly homodimerized PARP-1, plays a regulatory role in transcription and DNA repair in the chromatin context.
Dissociation of Automodified PARP-1 from DNA Breaks
Automodified PARP-1 then dissociates from DNA breaks or DNA loops (27). Displacement of PARP-1 domains from ssDNA and dsDNA could explain the mechanism of such dissociation (Fig. 7C, [4]). Our results suggest that binding of ADP-ribose polymers to the DsDB domain leads to displacement of the domain from dsDNA (Fig. 6). Zn2 is also displaced from ssDNA by the polymers. Because the DsDB domain binds to shorter lengths of ADP-ribose polymers (∼30 ADP-ribose residues) than Zn2 (longer than 50 ADP-ribose residues), dissociation of the DsDB domain from dsDNA might occur prior to the dissociation of Zn2 from ssDNA. Although it has been assumed that electric repulsion between DNA and ADP-ribose polymer is involved in the dissociation (27, 59), the dissociation mechanism of automodified PARP-1 from DNA beaks or DNA loops could thus be explained by our model. Then, ADP-ribose polymers are degraded by poly(ADP-ribose) glycohydrolase, bringing PARP-1 back to its basal status.
In this study, we have investigated the mechanisms of PARP-1 activation and found that five out of seven PARP-1 functional domains are involved in oligonucleic acid binding. This suggests that PARP-1 initiates ADP-ribose polymer synthesis depending on the status of neighboring oligonucleic acids. Although PARP-1 is an enzyme that catalyzes post-translational protein modification by ADP-ribose polymers, the primary roles of PARP-1 may be more related to its ability to survey unique oligonucleic acid structures to regulate various fundamental cellular processes, including DNA repair, transcription, and chromatin remodeling (4–14, 60).
Acknowledgments
We thank S. Sato and M. E. Mirault for critical reading and J. Nieminen for text editing. We also thank members of the Poirier group for various discussions.
This work was supported by grants from the Canadian Institutes of Health Research.
- PARP-1
- poly(ADP-ribose) polymerase-1
- DSB
- double strand DNA break
- SSB
- single strand DNA break
- DBD
- DNA break binding
- CAT
- catalytic domain
- DsDB
- double-stranded DNA binding
- dumbDNA
- dumbbell DNA
- aa
- amino acids.
REFERENCES
- 1. Althaus F. R., Richter C. (1987) ADP-ribosylation of Proteins: Enzymology and Biological Significance, Springer-Verlag, Berlin: [PubMed] [Google Scholar]
- 2. de Murcia G., Ménissier-de Murcia J., Schreiber V. (1991) Bioessays 13, 455–462 [DOI] [PubMed] [Google Scholar]
- 3. Lindahl T., Satoh M. S., Poirier G. G., Klungland A. (1995) Trends Biochem. Sci. 20, 405–411 [DOI] [PubMed] [Google Scholar]
- 4. Schreiber V., Dantzer F., Ame J. C., de Murcia G. (2006) Nat. Rev. Mol. Cell Biol. 7, 517–528 [DOI] [PubMed] [Google Scholar]
- 5. Woodhouse B. C., Dianov G. L. (2008) DNA Repair 7, 1077–1086 [DOI] [PubMed] [Google Scholar]
- 6. Kanno S., Kuzuoka H., Sasao S., Hong Z., Lan L., Nakajima S., Yasui A. (2007) EMBO J. 26, 2094–2103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Heale J. T., Ball A. R., Jr., Schmiesing J. A., Kim J. S., Kong X., Zhou S., Hudson D. F., Earnshaw W. C., Yokomori K. (2006) Mol. Cell 21, 837–848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ahel I., Ahel D., Matsusaka T., Clark A. J., Pines J., Boulton S. J., West S. C. (2008) Nature 451, 81–85 [DOI] [PubMed] [Google Scholar]
- 9. Kraus W. L. (2008) Curr. Opin. Cell. Biol. 20, 294–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ahel D., Horejsí Z., Wiechens N., Polo S. E., Garcia-Wilson E., Ahel I., Flynn H., Skehel M., West S. C., Jackson S. P., Owen-Hughes T., Boulton S. J. (2009) Science 325, 1240–1243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kim M. Y., Mauro S., Gévry N., Lis J. T., Kraus W. L. (2004) Cell 119, 803–814 [DOI] [PubMed] [Google Scholar]
- 12. Ju B. G., Solum D., Song E. J., Lee K. J., Rose D. W., Glass C. K., Rosenfeld M. G. (2004) Cell 119, 815–829 [DOI] [PubMed] [Google Scholar]
- 13. Parent M., Yung T. M., Rancourt A., Ho E. L., Vispé S., Suzuki-Matsuda F., Uehara A., Wada T., Handa H., Satoh M. S. (2005) J. Biol. Chem. 280, 448–457 [DOI] [PubMed] [Google Scholar]
- 14. Kraus W. L., Lis J. T. (2003) Cell 113, 677–683 [DOI] [PubMed] [Google Scholar]
- 15. Tanuma S., Yagi T., Johnson G. S. (1985) Arch. Biochem. Biophys. 237, 38–42 [DOI] [PubMed] [Google Scholar]
- 16. Ueda K., Omachi A., Kawaichi M., Hayaishi O. (1975) Proc. Natl. Acad. Sci. U.S.A. 72, 205–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Adamietz P., Rudolph A. (1984) J. Biol. Chem. 259, 6841–6846 [PubMed] [Google Scholar]
- 18. Messner S., Altmeyer M., Zhao H., Pozivil A., Roschitzki B., Gehrig P., Rutishauser D., Huang D., Caflisch A., Hottiger M. O. (2010) Nucleic Acids Res. 38, 6350–6362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bauer P. I., Chen H. J., Kenesi E., Kenessey I., Buki K. G., Kirsten E., Hakam A., Hwang J. I., Kun E. (2001) FEBS Lett. 506, 239–242 [DOI] [PubMed] [Google Scholar]
- 20. Yung T. M., Sato S., Satoh M. S. (2004) J. Biol. Chem. 279, 39686–39696 [DOI] [PubMed] [Google Scholar]
- 21. Masson M., Niedergang C., Schreiber V., Muller S., Menissier-de Murcia J., de Murcia G. (1998) Mol. Cell. Biol. 18, 3563–3571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Caldecott K. W., Aoufouchi S., Johnson P., Shall S. (1996) Nucleic Acids Res. 24, 4387–4394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Krishnakumar R., Gamble M. J., Frizzell K. M., Berrocal J. G., Kininis M., Kraus W. L. (2008) Science 319, 819–821 [DOI] [PubMed] [Google Scholar]
- 24. Krishnakumar R., Kraus W. L. (2010) Mol. Cell 39, 736–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tulin A., Spradling A. (2003) Science 299, 560–562 [DOI] [PubMed] [Google Scholar]
- 26. Fakan S., Leduc Y., Lamarre D., Brunet G., Poirier G. G. (1988) Exp. Cell Res. 179, 517–526 [DOI] [PubMed] [Google Scholar]
- 27. Zahradka P., Ebisuzaki K. (1982) Eur. J. Biochem. 127, 579–585 [PubMed] [Google Scholar]
- 28. Gradwohl G., Ménissier de Murcia J. M., Molinete M., Simonin F., Koken M., Hoeijmakers J. H., de Murcia G. (1990) Proc. Natl. Acad. Sci. U.S.A. 87, 2990–2994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ikejima M., Noguchi S., Yamashita R., Ogura T., Sugimura T., Gill D. M., Miwa M. (1990) J. Biol. Chem. 265, 21907–21913 [PubMed] [Google Scholar]
- 30. Wacker D. A., Ruhl D. D., Balagamwala E. H., Hope K. M., Zhang T., Kraus W. L. (2007) Mol. Cell. Biol. 27, 7475–7485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Langelier M. F., Servent K. M., Rogers E. E., Pascal J. M. (2008) J. Biol. Chem. 283, 4105–4114 [DOI] [PubMed] [Google Scholar]
- 32. Tao Z., Gao P., Hoffman D. W., Liu H. W. (2008) Biochemistry 47, 5804–5813 [DOI] [PubMed] [Google Scholar]
- 33. Altmeyer M., Messner S., Hassa P. O., Fey M., Hottiger M. O. (2009) Nucleic Acids Res. 37, 3723–3738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Langelier M. F., Ruhl D. D., Planck J. L., Kraus W. L., Pascal J. M. (2010) J. Biol. Chem. 285, 18877–18887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kameshita I., Matsuda Z., Taniguchi T., Shizuta Y. (1984) J. Biol. Chem. 259, 4770–4776 [PubMed] [Google Scholar]
- 36. Ogata N., Ueda K., Kagamiyama H., Hayaishi O. (1980) J. Biol. Chem. 255, 7616–7620 [PubMed] [Google Scholar]
- 37. Tao Z., Gao P., Liu H. W. (2009) J. Am. Chem. Soc. 131, 14258–14260 [DOI] [PubMed] [Google Scholar]
- 38. Kulczyk A. W., Yang J. C., Neuhaus D. (2004) J. Mol. Biol. 341, 723–738 [DOI] [PubMed] [Google Scholar]
- 39. Benjamin R. C., Gill D. M. (1980) J. Biol. Chem. 255, 10502–10508 [PubMed] [Google Scholar]
- 40. Pion E., Ullmann G. M., Amé J. C., Gérard D., de Murcia G., Bombarda E. (2005) Biochemistry 44, 14670–14681 [DOI] [PubMed] [Google Scholar]
- 41. Lonskaya I., Potaman V. N., Shlyakhtenko L. S., Oussatcheva E. A., Lyubchenko Y. L., Soldatenkov V. A. (2005) J. Biol. Chem. 280, 17076–17083 [DOI] [PubMed] [Google Scholar]
- 42. Jorgensen T. J., Chen K., Chasovskikh S., Roy R., Dritschilo A., Uren A. (2009) J. Mol. Recognit. 22, 446–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. D'Silva I., Pelletier J. D., Lagueux J., D'Amours D., Chaudhry M. A., Weinfeld M., Lees-Miller S. P., Poirier G. G. (1999) Biochim. Biophys. Acta 1430, 119–126 [DOI] [PubMed] [Google Scholar]
- 44. Satoh M. S., Poirier G. G., Lindahl T. (1994) Biochemistry 33, 7099–7106 [DOI] [PubMed] [Google Scholar]
- 45. Soldatenkov V. A., Potaman V. N. (2004) Curr. Drug Targets 5, 357–365 [DOI] [PubMed] [Google Scholar]
- 46. Pion E., Bombarda E., Stiegler P., Ullmann G. M., Mély Y., de Murcia G., Gérard D. (2003) Biochemistry 42, 12409–12417 [DOI] [PubMed] [Google Scholar]
- 47. Mendoza-Alvarez H., Alvarez-Gonzalez R. (1993) J. Biol. Chem. 268, 22575–22580 [PubMed] [Google Scholar]
- 48. Hassa P. O., Haenni S. S., Buerki C., Meier N. I., Lane W. S., Owen H., Gersbach M., Imhof R., Hottiger M. O. (2005) J. Biol. Chem. 280, 40450–40464 [DOI] [PubMed] [Google Scholar]
- 49. Meder V. S., Boeglin M., de Murcia G., Schreiber V. (2005) J. Cell Sci. 118, 211–222 [DOI] [PubMed] [Google Scholar]
- 50. Rancourt A., Satoh M. S. (2009) DNA Repair (Amst) 8, 286–297 [DOI] [PubMed] [Google Scholar]
- 51. Komori T., Inukai N., Yamada T., Yamaguchi Y., Handa H. (2009) Genes Cells 14, 343–354 [DOI] [PubMed] [Google Scholar]
- 52. Zlatanova J., Bishop T. C., Victor J. M., Jackson V., van Holde K. (2009) Structure 17, 160–171 [DOI] [PubMed] [Google Scholar]
- 53. Audebert M., Salles B., Calsou P. (2004) J. Biol. Chem. 279, 55117–55126 [DOI] [PubMed] [Google Scholar]
- 54. Wang M., Wu W., Wu W., Rosidi B., Zhang L., Wang H., Iliakis G. (2006) Nucleic Acids Res. 34, 6170–6182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Frizzell K. M., Gamble M. J., Berrocal J. G., Zhang T., Krishnakumar R., Cen Y., Sauve A. A., Kraus W. L. (2009) J. Biol. Chem. 284, 33926–33938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Gottschalk A. J., Timinszky G., Kong S. E., Jin J., Cai Y., Swanson S. K., Washburn M. P., Florens L., Ladurner A. G., Conaway J. W., Conaway R. C. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 13770–13774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Poirier G. G., de Murcia G., Jongstra-Bilen J., Niedergang C., Mandel P. (1982) Proc. Natl. Acad. Sci. U.S.A. 79, 3423–3427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. El-Khamisy S. F., Masutani M., Suzuki H., Caldecott K. W. (2003) Nucleic Acids Res. 31, 5526–5533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Satoh M. S., Lindahl T. (1992) Nature 356, 356–358 [DOI] [PubMed] [Google Scholar]
- 60. Frizzell K. M., Kraus W. L. (2009) Breast Cancer Res. 11, 111. [DOI] [PMC free article] [PubMed] [Google Scholar]