Abstract
Accumulating evidence indicates that dysfunction of mitochondria is a common feature of Parkinson disease. Functional loss of a familial Parkinson disease-linked gene, BRPK/PINK1 (PINK1), results in deterioration of mitochondrial functions and eventual neuronal cell death. A mitochondrial chaperone protein has been shown to be a substrate of PINK1 kinase activity. In this study, we demonstrated that PINK1 has another action point in the cytoplasm. Phosphorylation of Akt at Ser-473 was enhanced by overexpression of PINK1, and the Akt activation was crucial for protection of SH-SY5Y cells from various cytotoxic agents, including oxidative stress. Enhanced Akt phosphorylation was not due to activation of phosphatidylinositol 3-kinase but due to activation of mammalian target of rapamycin complex 2 (mTORC2) by PINK1. Rictor, a specific component of mTORC2, was phosphorylated by overexpression of PINK1. Furthermore, overexpression of PINK1 enhanced cell motility. These results indicate that PINK1 exerts its cytoprotective function not only in mitochondria but also in the cytoplasm through activation of mTORC2.
Keywords: Akt PKB, Apoptosis, Cell Migration, Oxidative Stress, Parkinson Disease, TOR Complex (TORC), PINK1, Rapamycin
Introduction
Parkinson disease (PD)2 is a neurodegenerative disorder with progressive loss of dopaminergic neurons, affecting more than 1% of the population older than 65 years. The molecular pathogenesis of PD is not well understood, but identification of genes causatively linked to familial PD has provided insights into the mechanisms. This is rationalized by the fact that symptoms observed in familial PD patients with different genetic abnormalities are very similar to those of sporadic idiopathic PD patients (1, 2). About a dozen genes have so far been identified as PD-linked genes (3), including genes encoding a synaptic protein, α-synuclein (4), an E3 ubiquitin ligase, parkin (5), a putative antioxidant chaperone, DJ-1 (6), a mitochondrial kinase, PINK1 (7, 8), a mitochondrial serine protease, OMI/HTRA2 (9), and leucine-rich repeat kinase 2 (LRRK2 (10)).
Accumulating clinical and experimental evidence indicates that dysfunction of mitochondria is a common feature of PD, leading to reduced complex I activity and overproduction of oxygen radicals (11, 12). Among the genes aforementioned, PINK1 has been attracting particular attention because it is a gene that has a mitochondrial targeting signal. PINK1 was identified independently by our group (8) as a gene differentially expressed in cancer cells with higher and lower metastatic potentials (AF316873 registered on Oct. 26, 2000 as BRPK) and by Unoki and Nakamura (7) as a gene induced by a tumor suppressor gene, PTEN (AB053323 registered on Jan. 8, 2001 as PINK1). Valente et al. (13) first showed the causal linkage between PINK1 and hereditary early onset PD. Several different mutations of PINK1 have thereafter been reported in PD patients of different racial origin.
PINK1 is a serine/threonine-type protein kinase localized primarily in mitochondria (13). Overexpression of PINK1 protects neuron cells against various stresses (13, 14), although down-regulation of PINK1 sensitizes the cells to various stresses (15, 16). Knock-out of the PINK1 gene in mice resulted in a decrease in evoked dopamine release in striata and affected striatal synaptic plasticity (17). Furthermore, neuron type-specific mitochondrial dysfunctions were observed in PINK1-null mice, and these dysfunctions were exacerbated by aging and stresses (18). Pridgeon et al. (19) identified a mitochondrial chaperone, TRAP1/Hsp75, as a substrate of PINK1 kinase and showed that the protective action of PINK1 against oxidative stress depended on phosphorylation of TRAP1/Hsp75. These findings are consistent with the notion that mitochondria are the primary intracellular site for pathogenesis of PD.
On the other hand, PINK1 was reported to be localized also in the cytoplasm (14, 20, 21). The cytoplasmic localization of PINK1 may be affected by N-terminal cleavage at least for overexpressed PINK1 protein (20). Furthermore, cytoplasmically localized PINK1 could protect neurons from a dopaminergic neurotoxin (14). These results prompted us to search for possible cytoplasmic targets of PINK1. We found that phosphorylation of Akt at Ser-473 was enhanced by overexpression of PINK1 and that the Akt phosphorylation was due to activation of mammalian target of rapamycin complex 2 (mTORC2) by PINK1.
EXPERIMENTAL PROCEDURES
Cells, Chemicals, and Antibodies
SH-SY5Y (ECACC, Wiltshire, United Kingdom), PC3, and DU-145 were cultured in D/F medium (Invitrogen) supplemented with 10% fetal bovine serum. Rotenone, cisplatin, hydrogen peroxide solution, tunicamycin, and MG-132 were purchased from Sigma. Inhibitors for EGF receptor (AG1478), PI3K (wortmannin and PI-103), Akt (Akt inhibitor VIII), and mTOR (rapamycin) were purchased from Merck.
The antibodies used were as follows: antibody against PINK1 (Abcam, Cambridge, MA); antibody against PINK1 (for immunoprecipitation, a polyclonal anti-PINK1 antibody was raised against amino acids 135–155 of human PINK1); antibodies against phospho-Ser-473 Akt, phospho-Thr-308 Akt, PTEN, phospho-Ser-380/Thr-382/383 PTEN, Bad, phospho-Ser-136 Bad, FoxO1, phospho-Thr-24 FoxO1, TSC2, phospho-Thr-1462 TSC2, GSK-3β, phospho-Ser-9 GSK-3β, p70 S6K, phosphor-Thr-389 p70 S6K, mTOR, and raptor, and HRP-labeled anti-mouse and anti-rabbit secondary antibodies (Cell Signaling Technologies, Danvers, MA); antibody against Akt (Merck); antibody against mTOR (N-19, for immunoprecipitation) (Santa Cruz Biotechnology, Santa Cruz, CA); antibodies against rictor, raptor (for immunoprecipitation), and SIN1 (Bethyl Laboratories, Montgomery, TX); antibody against HA tag (Roche Applied Science); antibody against phosphoserine/threonine (Pharmingen); antibody against tubulin (Sigma); and HRP-labeled Trueblot anti-rabbit and anti-goat secondary antibodies (eBioscience, San Diego).
Plasmid and Adenovirus Constructs
Plasmid vectors expressing PINK1 wild-type, G309D variant (22), C-terminal truncated variant W437X (23), and kinase-dead triple mutant (K219A/D362A/D384A(20)) were constructed as HA-tagged forms at the C-terminal end using the pDNR-CMV vector (Clontech). The vectors were converted into adenovirus constructs using an Adeno-X Expression System 2 (Clontech).
RNA Interference
siGENOME SMARTpool siRNA targeting PINK1 (NM_032409), rictor (NM_152756), or raptor (NM_020761) (Thermo Scientific Dharmacon, Lafayette, CO) was transfected into cells using FuGENE-HD (Roche Applied Science). A control siRNA with no known mammalian homology (siGENONE nontargeting siRNA pool 1, Thermo Scientific Dharmacon) was used as negative control.
Western Blot Analysis, Immunoprecipitation, and Kinase Assay
Western blot analysis was performed under conventional conditions after lysing cells with M-PER mammalian protein extraction reagent (Pierce) with 50 mm NaF and 1 mm orthovanadate. For immunoprecipitation, cells were lysed in an ice-cold lysis buffer (40 mm Hepes, pH 7.5, 120 mm NaCl, 1 mm EDTA, 10 mm pyrophosphate, 10 mm glycerophosphate, 50 mm NaF, 1 mm orthovanadate, and 0.3% CHAPS) to prevent decomposition of mTORC2 (30), incubated with 4 μg of respective antibodies for 90 min, and precipitated after another incubation with 30 μl of 50% slurry of Trueblot anti-rabbit or anti-goat IgG beads or monoclonal anti-HA-agarose (Sigma). Immunoprecipitates were washed three times with the lysis buffer before Western blot analysis.
For kinase assays, the immunoprecipitates were further washed with kinase buffers, i.e. 25 mm Hepes, pH 7.5, 100 mm potassium acetate, and 1 mm MgCl2 and incubated with dephosphorylated recombinant Akt substrate (Millipore, Temecula, CA).
Immunostaining
Cells were fixed with 4% paraformaldehyde and permeabilized with 100% ethanol for 1 h at −20 °C. After incubation with the respective first antibodies, the samples were incubated with Alexa Fluor 488 goat anti-rabbit IgG antibody (Invitrogen). Mitochondria were stained with MitoTracker Orange CMTMRos (Invitrogen). The specimens were observed using a confocal laser-scanning microscope (model LSM 510; Carl Zeiss, Jena, Germany).
Assays for Apoptosis
Apoptotic cells were identified after staining with Hoechst 33342 for 30 min (Dojin Laboratories, Kumamoto, Japan). Activation of caspase 3/7 was determined using Caspase-Glo 3/7 assay (Promega).
Invasion Assay
For invasion assay, 50,000 SH-SY5Y cells incubated with adenovirus vectors, with siRNAs, and with inhibitors in advance were inoculated into top wells of Boyden chambers (pore size, 8 μm; BD Biosciences) pre-coated with growth factor-reduced Matrigel (Invitrogen). The filters were stained after incubation for 8 h, and invading cells were counted.
Statistical Analysis
Prior to statistical analysis, each experiment was repeated at least three times. The results are expressed as means ± S.D. For comparison of two groups, Student's t test was used (Fig. 1C). For comparison of more than two groups, analysis of variance was used. If the analysis of variance showed significant difference, the Bonferroni procedure was used as a post hoc test. p values of less than 0.05 were considered statistically significant.
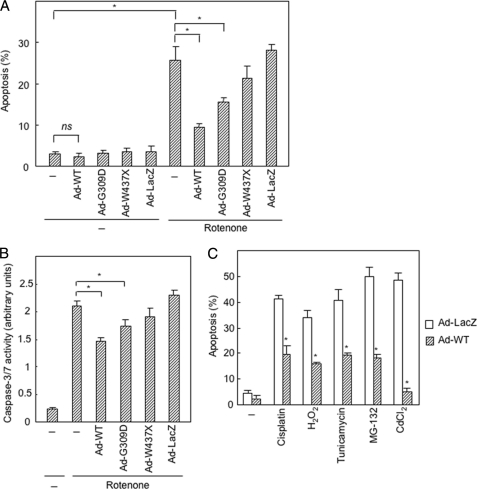
FIGURE 1.
Suppression of apoptosis by overexpression of PINK1 using an adenovirus vector. A, overexpression of PINK1 protects SH-SY5Y cells from apoptosis induced by rotenone. Adenovirus vectors carrying wild-type (WT) and PD-linked variants of PINK1 or LacZ (5 m.o.i.) were infected to cells 48 h prior to treatment with rotenone (1 μm, 24 h). Apoptotic cells were identified after staining with Hoechst 33342. *, p < 0.05; ns, not significant. B, overexpression of PINK1 blocks activation of caspase 3/7 induced by rotenone. Adenovirus vectors (5 m.o.i.) were infected to cells 48 h prior to treatment with rotenone (1 μm, 24 h). *, p < 0.05. C, overexpression of wild-type PINK1 (5 m.o.i.) protects SH-SY5Y cells from apoptosis induced by cisplatin (30 μm), H2O2 (200 μm), tunicamycin (40 μm), MG-132 (0.5 μm), and CdCl2 (40 μm). The experiment was performed under conditions similar to those described in A. Statistical analysis of the data were performed by Student's t test. *, significantly different from the Ad-LacZ-infected cells (p < 0.05).
RESULTS
Overexpression of PINK1 Confers Resistance to Induced Cell Death in SH-SY5Y Cells by Activating Akt
First, we examined the effect of overexpression of PINK1 on cell survival. Infection of SH-SY5Y cells with an adenovirus vector expressing wild-type PINK1 reduced apoptotic cell rate induced by an inhibitor for respiratory complex I, rotenone (1 μm, 24 h), from 26 to 10% (Fig. 1A). Two PD-linked PINK1 variants, G309D and W437X (22, 23), showed lower levels of protective function, although the control vector carrying LacZ showed no effect. Caspase-3/7 activation induced by rotenone was also suppressed by wild-type PINK1 (Fig. 1B). Overexpression of wild-type PINK1 conferred upon SH-SY5Y cells resistance to other apoptosis-inducing agents such as cisplatin (30 μm), H2O2 (200 μm), tunicamycin (40 μm), MG-132 (0.5 μm), and CdCl2 (40 μm) (Fig. 1C). The protective effect of PINK1 was also observed in a prostate cancer cell line, PC3 (supplemental Fig. 1), and two other cancer cell lines, HCT-116 and HeLa (data not shown).
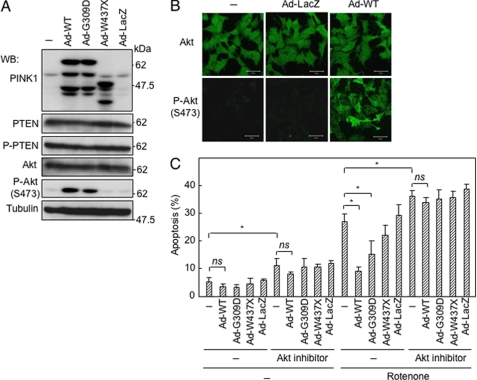
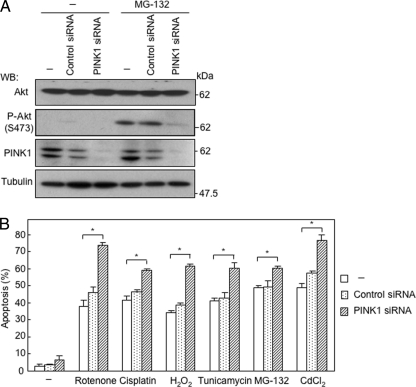
To identify a possible target protein(s) in the cytoplasm, we screened phosphorylation levels of PTEN-related proteins, because PINK1 is induced by overexpression of PTEN (7). As shown in Fig. 2A, phosphorylation of Akt at Ser-473 was markedly enhanced by overexpression of wild-type PINK1 in SH-SY5Y cells, although the amount of Akt protein remained unchanged. A higher phosphorylation level of Akt was also observed in cells with overexpression of the G309D variant but not in cells with overexpression of either the W437X variant or the kinase-dead mutant of PINK1 (Fig. 2A and supplemental Fig. 2A). These results were confirmed by immunostaining for phosphorylated Akt (Fig. 2B). Pretreatment of SH-SY5Y cells with an Akt inhibitor resulted in abrogation of the protective effect of overexpression of PINK1 against rotenone-induced cell death (Fig. 2C), indicating phosphorylation of Akt is critical for the protective function of PINK1. Down-regulation of endogenous PINK1 by siRNA abrogated stress-induced phosphorylation of Akt in SH-SY5Y cells (Fig. 3A). Down-regulation of endogenous PINK1 reduced basal phosphorylation level of Akt, whereas overexpression of PINK1 induced phosphorylation of Akt (supplemental Fig. 2B). In accordance with this, application of PINK1 siRNA sensitized SH-SY5Y cells to induction of apoptosis by cisplatin, rotenone, H2O2, tunicamycin, MG-132, and CdCl2 (Fig. 3B). Although Akt was co-immunoprecipitated with overexpressed PINK1 (supplemental Fig. 3), recombinant PINK1 did not phosphorylate Akt in vitro under the various conditions examined. We therefore searched for a target molecule of PINK1 among the upstream proteins of Akt.
FIGURE 2.
Suppression of apoptosis by overexpression of PINK1 is mediated by activation of Akt. A, effect of overexpression of PINK1 variants on PTEN and Akt. SH-SY5Y cells were infected with indicated constructs using adenovirus vectors (5 m.o.i.) 48 h before harvesting cells for Western blot (WB) analysis. P-, phosphorylated. B, immunostaining for Ser-473-phosphorylated Akt in SH-SY5Y cells infected with indicated constructs using adenovirus vectors (5 m.o.i.) for 48 h. C, Akt inhibitor abrogated PINK1-induced resistance to apoptosis. Akt inhibitor VIII (10 μm) was applied to SH-SY5Y cells 1 h prior to infection with adenovirus vectors carrying various PINK1 variants. Forty eight hours later, rotenone (1 μm) was added, and cells were stained with Hoechst 33342 after incubation for a further 24 h. *, p < 0.05; ns, not significant.
FIGURE 3.
Knockdown of PINK1 reduces phosphorylation level of Akt and sensitizes SH-SY5Y cells to various apoptotic agents. A, suppression of proteasome inhibitor-induced phosphorylation of Akt by knockdown of PINK1. SH-SY5Y cells were transfected with indicated siRNAs 60 h prior to treatment with MG-132 (0.5 μm, 12 h). WB, Western blot. B, sensitization of SH-SY5Y cells to various apoptotic agents by knockdown of PINK1. Cisplatin (30 μm), H2O2 (200 μm), tunicamycin (40 μm), MG-132 (0.5 μm), and CdCl2 (40 μm) were applied to cells for 24 h. *, p < 0.05.
EGF Receptor-PI3K Pathway Is Not Involved in Activation of Akt by Overexpression of PINK1
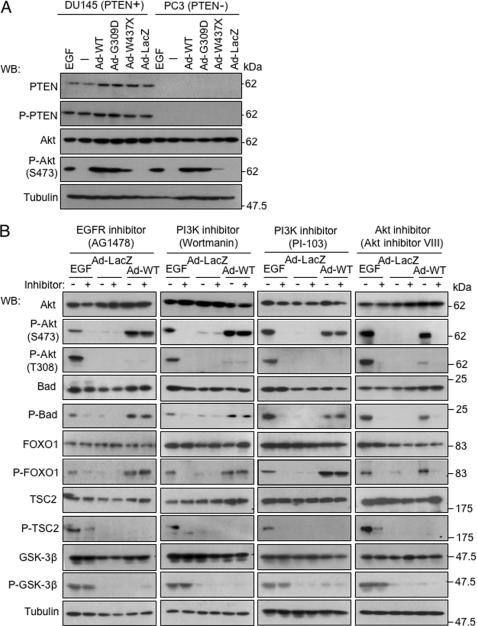
A major upstream mechanism for Akt activation is the EGF receptor-PI3K pathway. However, phosphorylation of Akt was enhanced by PINK1 in PTEN-deficient PC3 cells as well as PTEN-competent DU145 cells (Fig. 4A). Inhibitors of the EGF receptor (AG1478, 10 μm) and PI3K (1 μm wortmannin and 0.1 μm PI-103) did not affect phosphorylation of Akt induced by overexpression of PINK1, in contrast to abrogation of EGF-induced phosphorylation of Akt (Fig. 4B). Intriguingly, PINK1-mediated phosphorylation of Akt specifically occurred at Ser-473 but not at Thr-308. We also found that overexpression of PINK1 was associated with enhanced phosphorylation of only a subset of Akt targets, including Bad and FOXO1, although other targets of Akt, TSC2 and GSK3β, were not affected (Fig. 4B). These results indicate that the EGF receptor-PI3K pathway does not mediate phosphorylation of Akt by PINK1.
FIGURE 4.
EGF receptor-PI3K pathway is not involved in activation of Akt by overexpression of PINK1. A, activation of Akt by overexpression of PINK1 was not affected by the presence or absence of PTEN protein. PTEN-proficient DU145 cells and PTEN-deficient PC3 cells were harvested 48 h after infection with adenovirus vectors (10 m.o.i.) or after incubation with EGF (100 ng/ml) for 5 min. B, inhibitors for EGF receptor and PI3K did not affect phosphorylation of Akt and its substrates by overexpression of PINK1, in contrast to phosphorylation of Akt by EGF. The EGF receptor inhibitor AG1478 (10 μm), PI3K inhibitor wortmannin (1 μm), PI3K inhibitor PI-103 (0.1 μm), or Akt inhibitor VIII (10 μm) was applied to SH-SY5Y cells 1 h prior to application of adenovirus vectors (5 m.o.i., 48 h) or EGF (100 ng/ml, 5 min) WB, Western blot.
mTORC2 Is a Target Molecule of PINK1
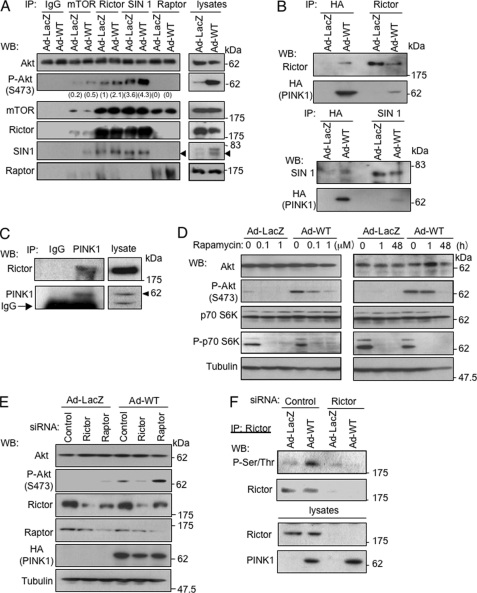
An alternative candidate pathway for activation of Akt is via mTORC2 (24, 25). mTORC2 shares mTOR and mLST8 with mTORC1 and has unique elements such as rictor, SIN1, and Protor (26, 27). Immunoprecipitates with an antibody against mTOR, rictor, or SIN1 but not an mTORC1 component, raptor, phosphorylated Akt at Ser-473 in vitro (Fig. 5A). This phosphorylation was enhanced by overexpression of wild-type PINK1. Rictor and SIN1 were co-precipitated with PINK1 (Fig. 5B). Endogenous PINK1 was also associated with rictor (Fig. 5C). Induced phosphorylation of Akt at Ser-473 was abrogated by prolonged treatment with rapamycin (48 h) but not short term treatment (1 h) (Fig. 5D). Rapamycin is known to inhibit not only mTORC1 but also mTORC2 under certain experimental settings (28). Under short term treatment of rapamycin, only phosphorylation of p70 S6K, a mTORC1 target, was abrogated. siRNAs against rictor and raptor partially down-regulated the corresponding proteins (Fig. 5E). The down-regulation of rictor but not that of raptor resulted in abrogation of the enhancement of phosphorylation of Akt induced by overexpression of PINK1 in SH-SY5Y cells. The phosphorylated form of rictor was detected in SH-SY5Y cells infected with Ad-PINK1, whereas the phosphorylation of rictor was abrogated by down-regulation of rictor (Fig. 5F). These results indicate that PINK1 induces phosphorylation of rictor and thereby activates mTORC2.
FIGURE 5.
PINK1 enhances phosphorylation level of Akt via activation of mTORC2. A, phosphorylation of Akt by immunoprecipitates (IP) in vitro. SH-SY5Y cells were harvested after incubation with Ad-PINK1 (WT) or Ad-LacZ (5 m.o.i.) for 48 h. Immunoprecipitates with designated antibodies were used for the kinase assay using recombinant Akt protein as a substrate in vitro. Rictor and SIN1 are known to be components of mTORC2, and raptor is a component of mTORC1. Phosphorylated Akt was densitometrically quantified. Arrowheads indicate immunoprecipitated SIN1. B, binding of PINK1 to mTORC2 components. SH-SY5Y cells cultured with Ad-PINK1 (WT) or Ad-LacZ for 48 h were immunoprecipitated with designated antibodies and analyzed by Western blot (WB) analysis. C, endogenous PINK1 interacts with rictor in SH-SY5Y cells. Cell lysates were subjected to immunoprecipitation with anti-PINK1 antibody or control rabbit IgG, followed by Western blot analysis. Arrowheads indicate immunoprecipitated PINK1. D, rapamycin dose- and time-dependently inhibited phosphorylation of Akt by Ad-PINK1 (WT) in SH-SY5Y cells. Left panel, SH-SY5Y cells were incubated with indicated adenovirus vectors (5 m.o.i.). Rapamycin (1 μm) was added 1 h prior to the adenovirus infection. Right panel, cells were incubated with adenovirus vectors in the presence of rapamycin (1 μm) for either a period over 48 h or a period of the last 1 h. E, knockdown of rictor suppressed phosphorylation of Akt induced by overexpression of PINK1. siRNAs were transfected into SH-SY5Y cells 24 h prior to infection with Ad-PINK1(WT) for 48 h. F, overexpression of PINK1 resulted in phosphorylation of rictor in cells. SH-SY5Y cells were incubated with indicated siRNAs 24 h prior to infection of adenovirus vectors (5 m.o.i., 48 h). Cell extracts were subjected to immunoprecipitation with anti-rictor antibody, followed by Western blot analysis.
PINK1 Stimulates Cell Motility
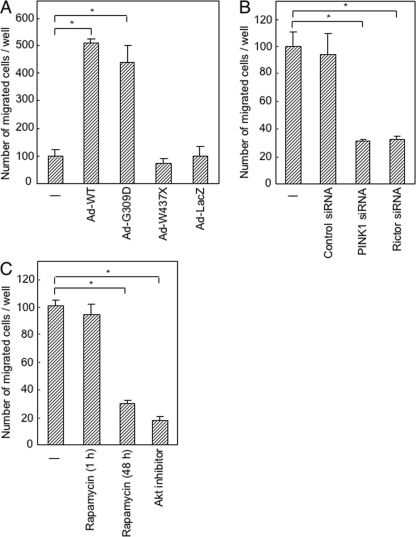
mTORC2 has been shown to regulate cytoskeletal organization (29, 30). If it is true that PINK1 activates mTORC2, overexpression of PINK1 may affect cell motility. Overexpression of wild-type PINK1 significantly increased the number of SH-SY5Y cells that transversed a Matrigel-coated membrane in a Boyden chamber (Fig. 6A). Overexpression of the G309D variant but not the W437X variant increased the number of migrated cells. Down-regulation of PINK1 or rictor by siRNA reduced the number of migrated cells (Fig. 6B). Prolonged treatment with rapamycin (48 h) or Akt inhibitor also reduced the number of migrated cells (Fig. 6C). These results indicate that PINK1 stimulates cell motility through activation of mTORC2.
FIGURE 6.
Stimulation of cellular motility by PINK1. A–C, invasion assay of SH-SY5Y cells incubated with indicated adenovirus vectors for 48 h (A), with indicated siRNAs for 72 h (B), and with indicated inhibitors for 1 h or 48 h (C). *, p < 0.05.
DISCUSSION
In this study, we demonstrated that PINK1 enhanced the phosphorylation level of Akt via activation of mTORC2. Little is known about upstream regulators of mTORC2, and to our knowledge PINK1 is the first candidate of proximate upstream regulators of mTORC2. Frias et al. (31) showed that insulin stimulates kinase activity of mTORC2 containing two specific SIN1 isoforms to phosphorylate Akt Ser-473 in vitro. It remains to be clarified whether insulin affects the function of PINK1 in certain types of cells.
mTOR plays a pivotal role in cells, integrating diverse extra- and intracellular signals and regulating cell growth, metabolism, survival, and motility. It includes two structurally and functionally distinct complexes, mTORC1 and mTORC2. Dysregulation of the coordination among two complexes and their intimate functional interplayer Akt leads to human malignancies, metabolic disorders, and inflammatory diseases (32, 33). mTORC2 shares mTOR and mLST8 with mTORC1 and has unique elements such as rictor, SIN1, and Protor (26, 27). Cells derived from mice lacking mTORC2 components showed largely diminished phosphorylation of Akt at Ser-473, resulting in partial deterioration of kinase activity of Akt (34, 35). Our results showing that overexpression of PINK1 affected phosphorylation level of Akt at Ser-473 (Figs. 2, 4, and 5) are in accordance with these observations.
PINK1 has a mitochondrial targeting signal and is primarily localized in mitochondria, but it is known to be present also in the cytoplasm (supplemental Fig. 4) (14, 20, 21, 36). Our findings showed that cytoplasmic localization of PINK1 has clear functional significance as indicated by Haque et al. (14). How PINK1 is translocated from mitochondria to the cytoplasm is not well understood. Beilina et al. (20) showed that N-terminal cleavage plays some roles in the translocation at least in the case of overexpressed PINK1 protein. Disruption of mitochondrial membrane potential resulted in accumulation of uncleaved PINK1 in mitochondria (37). As Dagda et al. (36) recently demonstrated, PINK1 regulates mitochondrial turnover in cooperation with Parkin.
We cloned PINK1 (BRPK) by a two-step screening strategy based on differential gene expression during placenta formation and between cancers with different metastatic potential (8). Expression of PINK1 was higher in cancer cell lines with higher metastatic potential than in cancer cell lines with lower metastatic potential. This is in line with the observations in this study that overexpression of PINK1 stimulated invasive behavior of SH-SY5Y cells assayed in vitro (Fig. 6). mTORC2 activity assayed using immunoprecipitates has been shown to be elevated in glioma cell lines and primary tumor cells compared with that in normal brain tissue (38). In addition, forced expression of rictor promoted anchorage-independent growth and motility of glioma cells. It would be interesting to determine whether and how PINK1 is involved in altered regulation of mTORC2 in various human cancers.
Activation of Akt is relevant to survival of dopaminergic neurons (39). Transduction of constitutively active Akt using an adeno-associated virus vector protected and preserved both dopaminergic cells and axonal projections in a highly destructive mouse model of PD (40). Yang et al. (41) observed impairment of PI3K/Akt signaling in Drosophila PD models in which PD-linked genes, DJ-1 and parkin, were down-regulated. In this context, our finding of a new cytosolic pathway from PINK1 provides an important line of exploration that should lead to a promising strategy for therapeutic intervention of PD.
Supplementary Material
Acknowledgments
We thank Associate Professor Masato Asanuma and Research Associate Ikuko Miyazaki of Okayama University for critical discussion and technical assistance.
This work was supported in part by Grant 20015031 from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (to N. H.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1–4.
- PD
- Parkinson disease
- m.o.i.
- multiplicity of infection
- mTOR
- mammalian target of rapamycin.
REFERENCES
- 1. Hardy J., Cai H., Cookson M. R., Gwinn-Hardy K., Singleton A. (2006) Ann. Neurol. 60, 389–398 [DOI] [PubMed] [Google Scholar]
- 2. Klein C., Schlossmacher M. G. (2006) Nat. Clin. Pract. Neurol. 2, 136–146 [DOI] [PubMed] [Google Scholar]
- 3. Biskup S., Gerlach M., Kupsch A., Reichmann H., Riederer P., Vieregge P., Wüllner U., Gasser T. (2008) J. Neurol. 255, Suppl. 5, 8–17 [DOI] [PubMed] [Google Scholar]
- 4. Polymeropoulos M. H., Lavedan C., Leroy E., Ide S. E., Dehejia A., Dutra A., Pike B., Root H., Rubenstein J., Boyer R., Stenroos E. S., Chandrasekharappa S., Athanassiadou A., Papapetropoulos T., Johnson W. G., Lazzarini A. M., Duvoisin R. C., Di Iorio G., Golbe L. I., Nussbaum R. L. (1997) Science 276, 2045–2047 [DOI] [PubMed] [Google Scholar]
- 5. Kitada T., Asakawa S., Hattori N., Matsumine H., Yamamura Y., Minoshima S., Yokochi M., Mizuno Y., Shimizu N. (1998) Nature 392, 605–608 [DOI] [PubMed] [Google Scholar]
- 6. Bonifati V., Rizzu P., van Baren M. J., Schaap O., Breedveld G. J., Krieger E., Dekker M. C., Squitieri F., Ibanez P., Joosse M., van Dongen J. W., Vanacore N., van Swieten J. C., Brice A., Meco G., van Duijn C. M., Oostra B. A., Heutink P. (2003) Science 299, 256–259 [DOI] [PubMed] [Google Scholar]
- 7. Unoki M., Nakamura Y. (2001) Oncogene 20, 4457–4465 [DOI] [PubMed] [Google Scholar]
- 8. Nakajima A., Kataoka K., Hong M., Sakaguchi M., Huh N. H. (2003) Cancer Lett. 201, 195–201 [DOI] [PubMed] [Google Scholar]
- 9. Strauss K. M., Martins L. M., Plun-Favreau H., Marx F. P., Kautzmann S., Berg D., Gasser T., Wszolek Z., Müller T., Bornemann A., Wolburg H., Downward J., Riess O., Schulz J. B., Krüger R. (2005) Hum. Mol. Genet. 14, 2099–2111 [DOI] [PubMed] [Google Scholar]
- 10. Zimprich A., Biskup S., Leitner P., Lichtner P., Farrer M., Lincoln S., Kachergus J., Hulihan M., Uitti R. J., Calne D. B., Stoessl A. J., Pfeiffer R. F., Patenge N., Carbajal I. C., Vieregge P., Asmus F., Müller-Myhsok B., Dickson D. W., Meitinger T., Strom T. M., Wszolek Z. K., Gasser T. (2004) Neuron 44, 601–607 [DOI] [PubMed] [Google Scholar]
- 11. Schapira A. H., Cooper J. M., Dexter D., Jenner P., Clark J. B., Marsden C. D. (1989) Lancet 1, 1269. [DOI] [PubMed] [Google Scholar]
- 12. Greenamyre J. T., Sherer T. B., Betarbet R., Panov A. V. (2001) IUBMB Life 52, 135–141 [DOI] [PubMed] [Google Scholar]
- 13. Valente E. M., Salvi S., Ialongo T., Marongiu R., Elia A. E., Caputo V., Romito L., Albanese A., Dallapiccola B., Bentivoglio A. R. (2004) Ann. Neurol. 56, 336–341 [DOI] [PubMed] [Google Scholar]
- 14. Haque M. E., Thomas K. J., D'Souza C., Callaghan S., Kitada T., Slack R. S., Fraser P., Cookson M. R., Tandon A., Park D. S. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 1716–1721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Deng H., Jankovic J., Guo Y., Xie W., Le W. (2005) Biochem. Biophys. Res. Commun. 337, 1133–1138 [DOI] [PubMed] [Google Scholar]
- 16. MacKeigan J. P., Murphy L. O., Blenis J. (2005) Nat. Cell Biol. 7, 591–600 [DOI] [PubMed] [Google Scholar]
- 17. Kitada T., Pisani A., Porter D. R., Yamaguchi H., Tscherter A., Martella G., Bonsi P., Zhang C., Pothos E. N., Shen J. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 11441–11446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gautier C. A., Kitada T., Shen J. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 11364–11369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pridgeon J. W., Olzmann J. A., Chin L. S., Li L. (2007) PLoS Biol. 5, e172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Beilina A., Van Der Brug M., Ahmad R., Kesavapany S., Miller D. W., Petsko G. A., Cookson M. R. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 5703–5708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Takatori S., Ito G., Iwatsubo T. (2008) Neurosci. Lett. 430, 13–17 [DOI] [PubMed] [Google Scholar]
- 22. Kessler K. R., Hamscho N., Morales B., Menzel C., Barrero F., Vives F., Gispert S., Auburger G. (2005) J. Neural. Transm. 112, 1345–1353 [DOI] [PubMed] [Google Scholar]
- 23. Criscuolo C., Volpe G., De Rosa A., Varrone A., Marongiu R., Mancini P., Salvatore E., Dallapiccola B., Filla A., Valente E. M., De Michele G. (2006) Movement Disord. 21, 1265–1267 [DOI] [PubMed] [Google Scholar]
- 24. Sarbassov D. D., Guertin D. A., Ali S. M., Sabatini D. M. (2005) Science 307, 1098–1101 [DOI] [PubMed] [Google Scholar]
- 25. Hresko R. C., Mueckler M. (2005) J. Biol. Chem. 280, 40406–40416 [DOI] [PubMed] [Google Scholar]
- 26. Jacinto E., Facchinetti V., Liu D., Soto N., Wei S., Jung S. Y., Huang Q., Qin J., Su B. (2006) Cell 127, 125–137 [DOI] [PubMed] [Google Scholar]
- 27. Pearce L. R., Huang X., Boudeau J., Pawłowski R., Wullschleger S., Deak M., Ibrahim A. F., Gourlay R., Magnuson M. A., Alessi D. R. (2007) Biochem. J. 405, 513–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sarbassov D. D., Ali S. M., Sengupta S., Sheen J. H., Hsu P. P., Bagley A. F., Markhard A. L., Sabatini D. M. (2006) Mol. Cell 22, 159–168 [DOI] [PubMed] [Google Scholar]
- 29. Jacinto E., Loewith R., Schmidt A., Lin S., Rüegg M. A., Hall A., Hall M. N. (2004) Nat. Cell Biol. 6, 1122–1128 [DOI] [PubMed] [Google Scholar]
- 30. Sarbassov D. D., Ali S. M., Kim D. H., Guertin D. A., Latek R. R., Erdjument-Bromage H., Tempst P., Sabatini D. M. (2004) Curr. Biol. 14, 1296–1302 [DOI] [PubMed] [Google Scholar]
- 31. Frias M. A., Thoreen C. C., Jaffe J. D., Schroder W., Sculley T., Carr S. A., Sabatini D. M. (2006) Curr. Biol. 16, 1865–1870 [DOI] [PubMed] [Google Scholar]
- 32. Wullschleger S., Loewith R., Hall M. N. (2006) Cell 124, 471–484 [DOI] [PubMed] [Google Scholar]
- 33. Bhaskar P. T., Hay N. (2007) Dev. Cell 12, 487–502 [DOI] [PubMed] [Google Scholar]
- 34. Shiota C., Woo J. T., Lindner J., Shelton K. D., Magnuson M. A. (2006) Dev. Cell 11, 583–589 [DOI] [PubMed] [Google Scholar]
- 35. Guertin D. A., Stevens D. M., Thoreen C. C., Burds A. A., Kalaany N. Y., Moffat J., Brown M., Fitzgerald K. J., Sabatini D. M. (2006) Dev. Cell 11, 859–871 [DOI] [PubMed] [Google Scholar]
- 36. Dagda R. K., Cherra S. J., 3rd, Kulich S. M., Tandon A., Park D., Chu C. T. (2009) J. Biol. Chem. 284, 13843–13855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lin W., Kang U. J. (2008) J. Neurochem. 106, 464–474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Masri J., Bernath A., Martin J., Jo O. D., Vartanian R., Funk A., Gera J. (2007) Cancer Res. 67, 11712–11720 [DOI] [PubMed] [Google Scholar]
- 39. Farkas L. M., Krieglstein K. (2002) J. Neural. Transm. 109, 267–277 [DOI] [PubMed] [Google Scholar]
- 40. Ries V., Henchcliffe C., Kareva T., Rzhetskaya M., Bland R., During M. J., Kholodilov N., Burke R. E. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 18757–18762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yang Y., Gehrke S., Haque M. E., Imai Y., Kosek J., Yang L., Beal M. F., Nishimura I., Wakamatsu K., Ito S., Takahashi R., Lu B. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 13670–13675 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.