Abstract
The Ca2+-binding protein of the EF-hand type, S100B, is abundantly expressed in and secreted by astrocytes, and release of S100B from damaged astrocytes occurs during the course of acute and chronic brain disorders. Thus, the concept has emerged that S100B might act an unconventional cytokine or a damage-associated molecular pattern protein playing a role in the pathophysiology of neurodegenerative disorders and inflammatory brain diseases. S100B proinflammatory effects require relatively high concentrations of the protein, whereas at physiological concentrations S100B exerts trophic effects on neurons. Most if not all of the extracellular (trophic and toxic) effects of S100B in the brain are mediated by the engagement of RAGE (receptor for advanced glycation end products). We show here that high S100B stimulates murine microglia migration in Boyden chambers via RAGE-dependent activation of Src kinase, Ras, PI3K, MEK/ERK1/2, RhoA/ROCK, Rac1/JNK/AP-1, Rac1/NF-κB, and, to a lesser extent, p38 MAPK. Recruitment of the adaptor protein, diaphanous-1, a member of the formin protein family, is also required for S100B/RAGE-induced migration of microglia. The S100B/RAGE-dependent activation of diaphanous-1/Rac1/JNK/AP-1, Ras/Rac1/NF-κB and Src/Ras/PI3K/RhoA/diaphanous-1 results in the up-regulation of expression of the chemokines, CCL3, CCL5, and CXCL12, whose release and activity are required for S100B to stimulate microglia migration. Lastly, RAGE engagement by S100B in microglia results in up-regulation of the chemokine receptors, CCR1 and CCR5. These results suggests that S100B might participate in the pathophysiology of brain inflammatory disorders via RAGE-dependent regulation of several inflammation-related events including activation and migration of microglia.
Keywords: Calcium-binding Proteins, Defensins, Innate Immunity, Neurodegeneration, Signal Transduction, Chemokines, Microglia, Migration, RAGE, S100B
Introduction
S100B is a Ca2+-binding protein of the EF-hand type abundantly expressed in astrocytes (1). S100B has been implicated in the regulation of astrocyte shape, migration, proliferation and differentiation via activation of the Src/PI3K module and PI3K-dependent stimulation of Akt and RhoA activities and hence of the supramolecular organization of F-actin (2). S100B also regulates the state of assembly of microtubules and type III intermediate filaments (3) and the cytosolic Ca2+ concentration (4). In addition to having intracellular regulatory activities, S100B also exerts extracellular effects. Indeed, astrocytes secrete the protein constitutively and to a larger extent under the action of several stimuli including the proinflammatory cytokine, TNF-α (see for review Ref. 1). Moreover, levels of brain S100B are elevated in the aging brain and in several pathological conditions such as Alzheimer disease, brain infarct, epilepsy, and infectious diseases, as well as in Down syndrome (5, 6) in consequence of S100B human gene mapping to chromosome 21q22.3 (7). For example, hypertrophic astrocytes in peri-infarct areas and in neuritic plaques in Alzheimer disease and Down syndrome show elevated expression levels of S100B, and S100B can be detected outside hypertrophic astrocytes (8, 9), pointing to secretion/release of high amounts of the protein under these conditions. Also, old but not young S100B transgenic mice show hypertrophic astrocytes and an enhanced expression of β-amyloid precursor protein in nearby neurons (10). Lastly, S100B TG mice show increased susceptibility to perinatal hypoxia-ischemia (11), and overexpression of S100B has been shown to accelerate Alzheimer disease-like pathology with enhanced astrogliosis and microgliosis (12). Because of these observations, the concept has emerged that S100B, when present in the brain extracellular milieu in relatively high amounts, might act as an unconventional cytokine and/or a damage-associated molecular pattern molecule playing a role in the pathophysiology of neurodegenerative disorders and inflammatory brain diseases (1, 5–13). Indeed, treatment of astrocytes with S100B results in up-regulation of expression of inducible NOS, stimulation of inducible NOS activity, NO release, and NO-dependent killing of astrocytes and co-cultured neurons (14, 15) and in stimulation of release of the inflammatory cytokine IL-1β (16). Neurons and microglia also were shown to be targets of extracellular S100B. Specifically, S100B was shown to cause neuronal death by increasing reactive oxygen species production in neurons (17, 18), to up-regulate inducible NOS expression in and stimulate NO release by microglia in the presence of bacterial endotoxin or interferon-γ (19, 20), to up-regulate the expression in and the release of IL-1β and TNF-α by microglia (14, 21–23), to up-regulate the expression of the proinflammatory enzyme, COX-2, in microglia (22), and to synergize with IL-1β and TNF-α to up-regulate COX-2 expression in microglia (23). Most of these effects were observed at relatively high doses of S100B, pointing to the need of accumulation of the protein in the brain extracellular space for it to act as an inflammatory cytokine/damage-associated molecular pattern factor and/or a neurotoxic factor. Also, S100B was shown to bring about those effects by engaging RAGE (receptor for advanced glycation end products) for the most part (24). RAGE is a multiligand receptor belonging to the immunoglobulin superfamily playing an important role in innate immune response including macrophage migration and activation (25–28). However, at the nanomolar concentrations at which S100B is found in the brain extracellular space under normal physiological conditions and at the very beginning of brain insult (1), S100B was reported to exert trophic effects, protecting neurons against pro-apoptotic stimuli, promoting neurite outgrowth again via RAGE engagement (17, 29–32), and reducing the activation of microglia by neurotoxic agents (33).
Microglia, the brain-resident macrophages, become activated in case of brain injury, thus contributing to the pathophysiology of inflammatory brain diseases and brain damage by releasing NO and inflammatory products such as prostaglandins, cytokines, and chemokines (34–36). However, according to recent views, microglia are active players in brain tissue homeostasis under normal physiological conditions, continuously patrolling the territory, exerting a protective action by virtue of their ability to keep the neuronal and astrocytic extracellular milieu clean, and likely resolving mild degree brain insults (37, 38). Microglia also release chemokines that stimulate the migration and proliferation of cerebellar granule cells and cortical neuron progenitors, thus promoting neurogenesis (38–40).
We show here that: 1) at proinflammatory but not low doses, S100B stimulates microglia migration via RAGE signaling-dependent up-regulation of the expression of CCL3, CCL5, and CXCL12 chemokines and their release, and 2) at low doses S100B, however, can stimulate RAGE-overexpressing microglia migration. These S100B effects require RAGE-dependent activation of Src kinase, Ras, MEK/ERK1/2, PI3K/Akt, Rac1-Cdc42, JNK/activating protein-1 (AP-1),3 nuclear factor κB (NF-κB), the RhoA-associated kinase (ROCK), and, to a lesser extent, p38 MAPK and the presence/activity of diaphanous-1. The present observations support the concept that extracellular S100B act as a damage-associated molecular pattern factor participating in the pathophysiology of brain inflammatory disorders via RAGE-dependent regulation of several inflammation-related events such as activation and migration of microglia.
EXPERIMENTAL PROCEDURES
S100B
Recombinant bovine S100B, which is 97% identical to mouse S100B, was expressed and purified as reported (17, 41) and made free of bacterial endotoxin as described (23). The S100B concentration was calculated using the molecular mass of the S100B dimer, i.e. 21 kDa.
Cell Culture
The murine BV-2 microglial cell line was obtained and characterized as described (20, 42, 43). The cells were cultivated in RPMI 1640 containing 10% heat-inactivated FBS (Hyclone Laboratories, Lagan, UK) supplemented with l-glutamine (4 mm), penicillin (100 units/ml), and streptomycin (0.1 mg/ml) in H2O-saturated 5% CO2 atmosphere at 37 °C. BV-2 microglia were tested periodically and resulted negative for mycoplasma contamination. Primary microglia were isolated from 6-day-old CD rat (Charles River), WT C57BL/6 (Charles River) mouse, and Rage−/− mouse brain and characterized and cultivated as described (44). BV-2 microglia stably transfected with human RAGE cDNA (BV-2/RAGE microglia), human RAGEΔcyto cDNA (BV-2/RAGEΔcyto microglia), or empty vector (BV-2/mock microglia) were obtained as described (45). RAGEΔcyto is a RAGE mutant lacking the cytoplasmic and transducing domain (46, 47). BV-2 microglia express RAGE (45, 46), and BV-2/RAGE microglia express larger amounts of the receptor compared with WT BV-2 microglia, whereas BV-2/RAGEΔcyto microglia express endogenous RAGE plus the signaling-deficient RAGE mutant, RAGEΔcyto (45). BV-2/mock, BV-2/RAGE, and BV-2/RAGEΔcyto microglia were cultivated as above except that gentamicin (5 μg/ml) was used instead of penicillin and streptomycin.
Migration Assay
Migration assays were performed using Boyden chambers (pore size, 8 μm) (Falcon). Cells in DMEM were seeded in the upper chamber, and the insert was placed in the lower chamber of a 24-well dish containing DMEM plus or minus S100B and incubated at 37 °C for 2–6 h according to the manufacturer's instructions. The cells on the upper side of the filters were removed with cotton-tipped swabs, and the filters were fixed in methanol for 2 min and stained with 0.05% crystal violet in PBS for 15 min followed by representative counts of 10 randomly selected microscope fields.
Transfections and Other Treatments of Microglia
Transient transfections were carried out using Lipofectamine 2000 as recommended by the manufacturer. Briefly, microglia cultured in 10% FBS without antibiotics were transfected with expression plasmid N17Rac1, N17Cdc42, N17Ras, or N19RhoA (constitutively inactive forms of Rac1, Cdc42, Ras, and RhoA, respectively), IκBαSR (a nonphosphorylatable form of the NF-κB inhibitor, IκBα) (48), or empty vector. After 24 h, the medium was changed to RPMI 1640, and the cells were detached by agitation and layered on the upper well of Boyden chambers for transmigration assay. Transfection efficiency was estimated by transfecting parallel cells with GFP cDNA. The percentage of GFP-positive cells (20–25%) was determined by fluorescence-activated cell sorter analysis. Parallel cells were analyzed for viability by trypan blue exclusion assay and by a tetrazolium-based (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) colorimetric assay. No significant changes could be registered in the numbers of cells transfected with expression plasmids or empty vector (data not shown). For RNA interference, microglia were transfected with Stealth RNAi Negative Universal Control or diaphanous-1 siRNA (Invitrogen) using Lipofectamine 2000 according to the manufacturer's instructions. After 72 h the cells were cultivated in RPMI 1640 and processed as described in Fig. 3. Where required, the cells were treated for 1 h with PP2 (Calbiochem) (20 μm), SP600125 (Calbiochem) (20 μm), LY294002 (10 μm) (Alexis), NSC23766 (Calbiochem) (50 μm), PD98059 (Calbiochem) (30 μm), Bay 11-7082 (Calbiochem) (5 μm), SB203580 (Calbiochem) (5 μm), Y27632 (Calbiochem) (10 μm), BoxA (HMG Biotech) (200 ng/ml), pertussis toxin (Sigma) (100 ng/ml), RAGE-neutralizing antibody (N16; Santa Cruz Biotechnology, 10 μg/ml) (49), or 10 μg/ml nonimmune IgG in RPMI 1640 before migration assay.
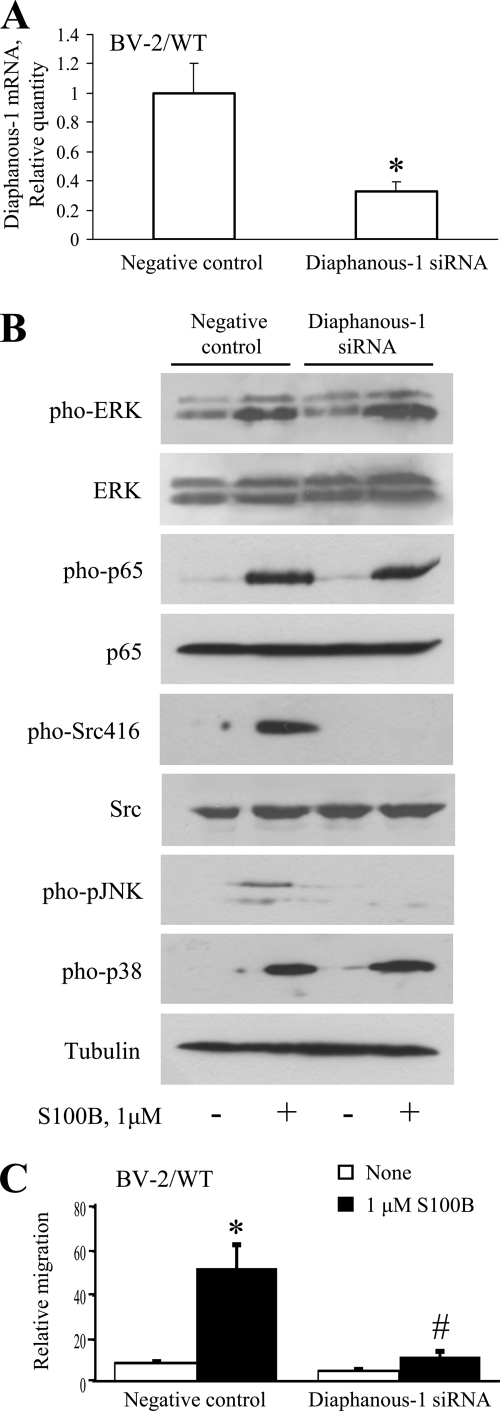
FIGURE 3.
Role of diaphanous-1 in S100B/RAGE-dependent stimulation of microglia migration. A, treatment of microglia with diaphanous-1 siRNA reduces diaphanous-1 expression as investigated by real time PCR. B, knockdown of diaphanous-1 results in reduction of S100B-dependent activation of JNK, but not NF-κB, ERK1/2, or p38 MAPK, and reduction of basal and S100B-dependent activation of Src. The conditions were as described in the legend to Fig. 2A, except that BV-2 microglia were transiently transfected with diaphanous-1 siRNA or nonsilencing siRNA before processing for Western blotting. Shown is one representative experiment of three. C, S100B/RAGE-dependent chemoattraction of microglia is dependent on diaphanous-1 in part. Conditions were as described in the legend to Fig. 2A except that BV-2 microglia were transiently transfected with diaphanous-1 siRNA or nonsilencing siRNA and then transferred to Boyden chambers for migration assay. The results are expressed as the means ± S.D. (n = 3). *, significantly different from control (first columns from left in A and C). #, significantly different from internal control.
Western Blot Analyses
BV-2 microglia (3 × 105) were seeded in 24-multiwell plates and cultivated in the presence or absence of S100B. The cells were then solubilized with 2.5% SDS, 10 mm Tris-HCl, pH 7.4, 0.1 m dithiothreitol, 0.1 mm tosylsulfonyl phenylalanyl chloromethyl ketone protease inhibitor (Roche Applied Science) for Western blot analyses. Phosphorylated Src, JNK, ERK1/2, p38 MAPK, Akt, and p65 NF-κB were detected using a polyclonal anti-phosphorylated Src (Ser-416) (1:1,000; Cell Signaling Technology), JNK (Thr-183/Tyr-185) antibody (1:1,000; Cell Signaling Technology), a polyclonal anti-phosphorylated (Thr-202/Tyr-204) ERK1/2 antibody (1:2,000; Cell Signaling Technology), a polyclonal anti-phosphorylated (Thr-180/Tyr-182) p38 MAPK antibody (1:1,000; Cell Signaling Technology), polyclonal anti-phosphorylated (Ser-473) Akt (1:1000; Cell Signaling Technology), and a polyclonal anti-phosphorylated (Thr-534) p65 NF-κB (1:1,000; Cell Signaling Technology) antibody, respectively. Total Src, ERK1/2, p38 MAPK, Akt, and p65 NF-κB were detected using a polyclonal anti-Src (1:1,000; Cell Signaling Technology), anti-ERK1/2 antibody (1:20,000; Sigma), a polyclonal anti-p38 MAPK antibody (1:2,000; Cell Signaling Technology), polyclonal anti-Akt (1:1000; Cell Signaling Technology), and a polyclonal anti-p65 NF-κB antibody (1:1,000; Santa Cruz Biotechnology), respectively. Analysis of culture medium HMGB1 was performed as described (50). Briefly, culture media were clarified by centrifugation, added with 1/100 volume of 2% sodium deoxycholate, and subjected to precipitation with 1/10 volume of 100% trichloroacetic acid. The resultant pellets were resuspended in Laemmli buffer and titrated with 1 n NaOH to obtain the normal blue color of the sample buffer, boiled for 5 min, and subjected to Western blotting using an anti-HMGB1 antibody (BD PharMingen). A monoclonal anti-α-tubulin (1:10,000; Sigma) was used to monitor protein loading on SDS gels. Peroxidase-conjugated secondary antibodies were from Sigma. Antibodies were diluted in blocking buffer (10 mm Tris-HCl, pH 7.4, 0.1 m NaCl, 5% nonfat dried milk powder, 0.1% Tween 20). The immune reaction was developed by ECL (SuperSignal West Pico; Pierce).
Real Time PCR
BV-2 microglia were incubated with S100B as shown in Figs. 4 and 5. Where appropriate, the cells were pretreated for 1 h with 20 μm PP2, 20 μm SP600125, 10 μm LY294002, 50 μm NSC23766, 30 μm PD98059, 5 μm Bay 11-7082, or 5 μm SB203580. Total cytoplasmic RNA was isolated from BV-2 microglia using the TRIzol reagent method. To detect CCL3, CCL5, CXCL1, CXCL7, and CXCL12 mRNAs, cDNA (0.1 μg/sample) was incubated with primers 5′-TTCTGCTGACAAGCTCACCCT-3′ and 5′-ATGGCGCTGAGAAGACTTGGT-3′ for CCL3, 5′-TTCCCTGTCATCGCTTGCTCT-3′ and 5′-CGGATGGAGATGCCGATTTT-3′ for CCL5, 5′-ATTGTATGGTCAACACGCACG-3′ and 5′-TTTGAACGTCTCTGTCCCGAG-3′ for CXCL1, 5′-GCCCACTTCATAACCTCCA-3′ and 5′-ATCACTTCCACATCAGCACA-3′ for CXCL7, 5′-CAAGGTCGTCGCCGTGCTG-3′ and 5′-GCTCAGGCTGACTGGTTTACCG-3′ for CXCL12, 5′-AGGTGACTGAGGTGATTGCC-3′ and 5′-CTGTGGATGGAGATATAGAACTGG-3′ for CCR1, 5′-CGAGCCCGAACTGTGACTTTTG-3′ and 5′-GTCTTCTTCACCCTCTGGATAGCG-3′ for CCR3, 5′-GGTGGAGGAGCAGGGAGAACGAG-3′ and 5′-CTTTCAGGAACCCAGCGGTGAGAC-3′ for CCR5, and 5′-CCTCTGCCTGGTGACTCTGG-3′ and 5′-AGGAGGAGGTGGAGGGATGG-3′ for diaphanous-1 in a reaction volume of 20 μl containing Real Master Mix and SYBR solution (Eppendorf). The reaction mixtures were incubated in a thermocycler (Stratagene) and analyzed by Multiplex Quantitative PCR System. Housekeeping β-actin mRNA was used as a control (primers 5′-AGCCATGTACGTAGCCATCC-3′ and 5′-CTCTCAGCTGTGGTGGTGAA-3′).
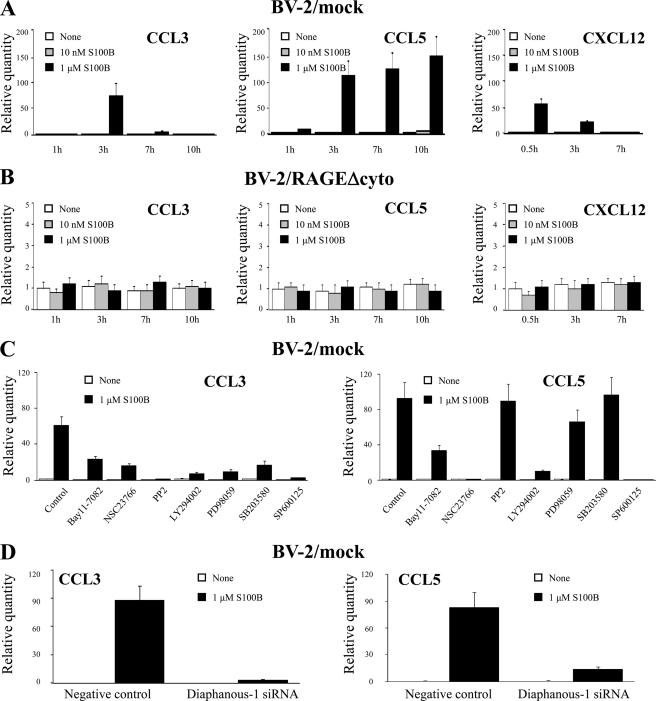
FIGURE 4.
S100B up-regulates the expression of CCL3, CCL5, and CXCL12 chemokines in a RAGE-dependent manner. A, BV-2/mock microglia were treated with increasing concentrations of S100B for the indicated time, and total mRNA was extracted and subjected to real time PCR for quantification of CCL3, CCL5, and CXCL12 mRNAs. B, same as in A except that BV-2/RAGEΔcyto microglia were used. C, same as in A except that BV-2 microglia were pretreated with the indicated inhibitors, and analyses were restricted to CCL3 and CCL5. D, diaphanous-1 siRNA-treated and control BV-2 microglia were analyzed for CCL3 and CCL5 expression by real time PCR. The results are expressed as the means ± S.D. (n = 3).
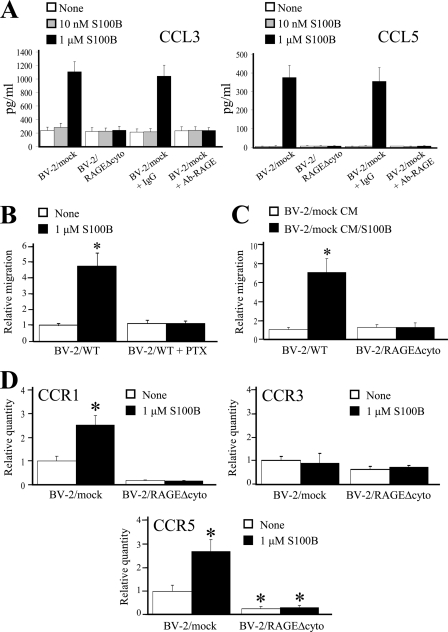
FIGURE 5.
S100B stimulates CCL3 and CCL5 secretion and CCR1 and CCR5 expression in a RAGE-dependent manner. A, BV-2/mock and BV-2/RAGEΔcyto microglia were treated for 6 h with increasing doses of S100B. Culture media were analyzed for CCL3 and CCL5 content by ELISA. B, conditions were as described for Fig. 1A except that BV-2 microglia were pretreated with pertussis toxin (PTX) before migration assay. C, conditioned media from control (CM) and S100B-treated (CM/S100B) BV-2/mock microglia stimulate BV-2/mock but not BV-2/RAGEΔcyto microglia migration. BV-2/mock microglia were treated for 20 h with vehicle or 1 μm S100B. The culture media were collected and added to the lower compartment of Boyden chambers, and BV-2/mock or BV-2/RAGEΔcyto microglia were added to the upper compartment and allowed to migrate for 6 h. D, BV-2/mock and BV-2/RAGEΔcyto microglia were treated for 5 h with vehicle or 1 μm S100B, and CCR1, CCR3, and CCR5 expression levels were measured by real time PCR. The results are expressed as the means ± S.D. (n = 3). *, significantly different from control (first column from left in D).
Determination of Chemokines by ELISA
BV-2 microglia (5 × 105/ml) were cultivated in the presence or absence of S100B. After 6 h, the culture media were taken up, centrifuged, and subjected to ELISA using commercial kits (R & D System) to measure CCL3 and CCL5. Each sample was tested in triplicate.
Immunofluorescence
BV-2 microglia (5 × 105/ml) were cultivated for 3 h in the presence or absence of S100B on glass coverslips and processed for detection of F-actin and microtubules as described (2).
Statistical Analysis
Each experiment was repeated at least three times. Representative experiments are depicted in the figures unless stated otherwise. The data were subjected to analysis of variance with Student-Newman-Keuls post hoc analysis using a statistical software package (GraphPad Prism version 4.00; GraphPad Software, San Diego, CA). Statistical significance was assumed when p < 0.05.
RESULTS
S100B Stimulates Microglia Transmigration in a RAGE-dependent Manner
When assayed at 2 h, S100B stimulated BV-2 microglia transmigration in Boyden chambers at 5 and 10 μm but not at lower concentrations (supplemental Fig. S1A). However, when assayed at 6 h, S100B stimulated BV-2/WT microglia transmigration dose-dependently with maximum stimulation at 1 μm and decreasing effects at higher concentrations (Fig. 1A). Rat primary microglia responded to S100B again with no effects at nanomolar doses and stimulation of transmigration at 1 μm (Fig. 1B). However, adding S100B (0.1–1.0 μm) to the cells in the upper well of Boyden chambers resulted in no changes in S100B-induced microglia transmigration relative to the internal control (i.e. no S100B present in upper wells) (Fig. 1C), suggesting that S100B might not be a chemoattractant toward microglia per se, but rather it might act in an indirect manner.
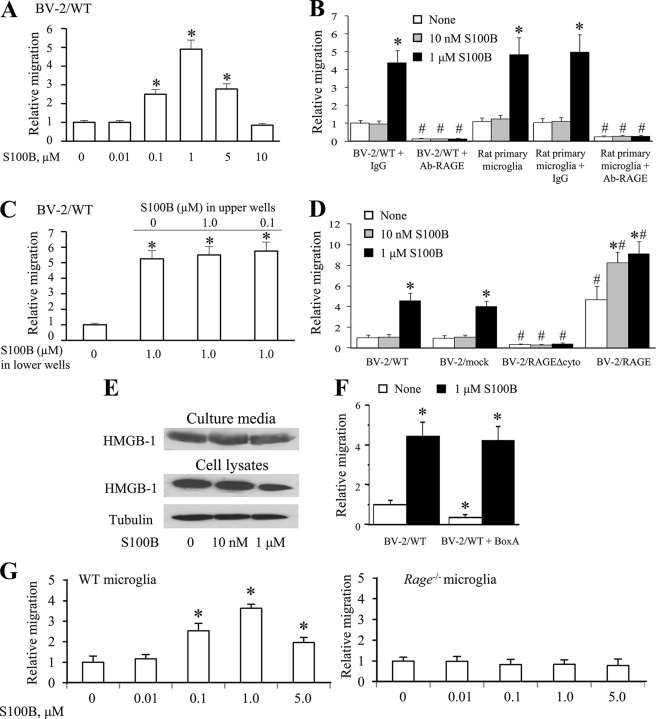
FIGURE 1.
S100B stimulates BV-2 microglia migration in a RAGE-dependent manner. A, migration assays were performed using Boyden chambers. The cells (1 × 105) were seeded onto the top of Transwell® migration chambers and allowed to migrate for 6 h toward S100B or medium alone negative control placed in the lower chamber. B, conditions were as in A except that BV-2 or rat primary microglia were pretreated with nonimmune IgG or a RAGE neutralizing antibody for 60 min and then transferred to the upper chamber. C, conditions were as in A except that S100B (0–1.0 μm) was added to the cells placed on the upper well of Boyden chambers, where indicated. D, conditions were as in A except that WT BV-2 microglia (BV-2/WT), mock-transfected BV-2 microglia (BV-2/mock), BV-2 microglia stably expressing a RAGE mutant lacking the cytoplasmic and transducing RAGE domain (BV-2/RAGEΔcyto), or BV-2 microglia stably overexpressing full-length RAGE (see “Experimental Procedures”) were used. E, BV-2 microglia were cultivated in DMEM for 6 h in the presence of increasing S100B concentrations. The culture media were collected, trichloroacetic acid-precipitated as described under “Experimental Procedures,” and subjected to Western blotting using an anti-HMGB1 antibody. Residual cells were lysed, and cell lysates were probed with anti-HMGB1 antibody. Also shown is a Western blot of tubulin. F, BV-2 microglia were pretreated with BoxA and allowed to migrate for 6 h toward medium alone placed in the lower chamber. The results are expressed as the means ± S.D. (n = 3) (A–D, F, and G). G, mouse WT and Rage−/− microglia were subjected to migration assay as described in A in the presence of increasing S100B concentrations. *, significantly different from control (first columns in A, C, F, and G) or from internal control (first columns from left in each group in B and D). #, significantly different from the corresponding column in the BV-2/WT + IgG group or the primary microglia group in B and in the BV-2/mock group in D.
In the absence of S100B, the extent of migration of BV-2 microglia overexpressing RAGEΔcyto, a RAGE mutant lacking the cytoplasmic and transducing domain (BV-2/RAGEΔcyto microglia), was significantly lower than that detected using BV-2/mock microglia, whereas the number of migrated RAGE-overexpressing BV-2 microglia (BV-2/RAGE microglia) was more than four times larger than that of BV-2/mock microglia under basal conditions (Fig. 1D). These results suggested that RAGE signaling plays an important role in microglia migration and that factors in the culture medium might stimulate basal microglia migration in a RAGE-mediated manner. One candidate RAGE agonist with the ability to stimulate basal microglia transmigration is high mobility group protein 1 (HMGB1), an established RAGE ligand (51–53) released by activated macrophages (54) and implicated in RAGE-dependent migration of several cell types (55–58). Indeed, the microglia culture medium contained HMGB1 (Fig. 1E), which might explain basal BV-2/mock microglia migration in the present assay and the enhanced basal migration of BV-2/RAGE microglia compared with BV-2/mock microglia. Consistently, treating primary and BV-2 microglia with BoxA, an HMGB1 antagonist (59), resulted in a significant reduction of basal migration (Fig. 1F). However, as mentioned earlier, adding S100B to the lower compartment of Boyden chambers resulted in stimulation of migration of rat primary, BV-2/mock, and BV-2/RAGE but not BV-2/RAGEΔcyto microglia (Fig. 1, B and D), and pretreatment of primary and BV-2 microglia with a RAGE neutralizing antibody resulted in the abrogation of S100B-stimulated migration along with a significant reduction of basal migration (Fig. 1B). These results suggested that the ability of S100B to stimulate microglia migration was dependent on RAGE signaling, and it added to basal, HMGB1-induced migration. Consistently, at 6 h S100B did not stimulate the migration of Rage−/− microglia, whereas the protein efficiently stimulated migration of mouse WT microglia (Fig. 1G). Also, similar to BV-2 microglia, at 2 h S100B (≥5 μm) stimulated mouse WT but not Rage−/− microglia migration (supplemental Fig. S1, B and C). Notably, S100B stimulated microglia migration with the same efficacy irrespective of the absence or presence of BoxA (Fig. 1D), in accordance with the specificity of BoxA blocking effect toward HMGB1 (60). The present results also suggested that a lack of effects of low doses of S100B on microglia migration might be dependent on the inability of S100B to displace RAGE-bound HMGB1, because HMGB1 and S100B both bind to RAGE V domain (51, 62).
Whereas at 1 μm S100B caused an ∼4-fold increase in the number of BV-2/mock migrated cells, the protein caused only an ∼2-fold increase in the migration of BV-2/RAGE cells, compared with controls (Fig. 1A). This might be a consequence of the presence of HMGB1 in the microglia culture medium, which might enhance basal migration of BV-2/RAGE cells. Thus, the stimulatory effect of S100B on microglia migration was dependent on functional RAGE and, to a certain extent, the amount of expressed RAGE.
In addition, low S100B stimulated the migration of RAGE-overexpressing BV-2 microglia (BV-2/RAGE microglia) (Fig. 1, A and C). This suggested that increasing the RAGE abundance might make low S100B add its stimulatory effect on microglia migration to that of HMGB1.
S100B Stimulates Microglia Migration via Multiple Pathways
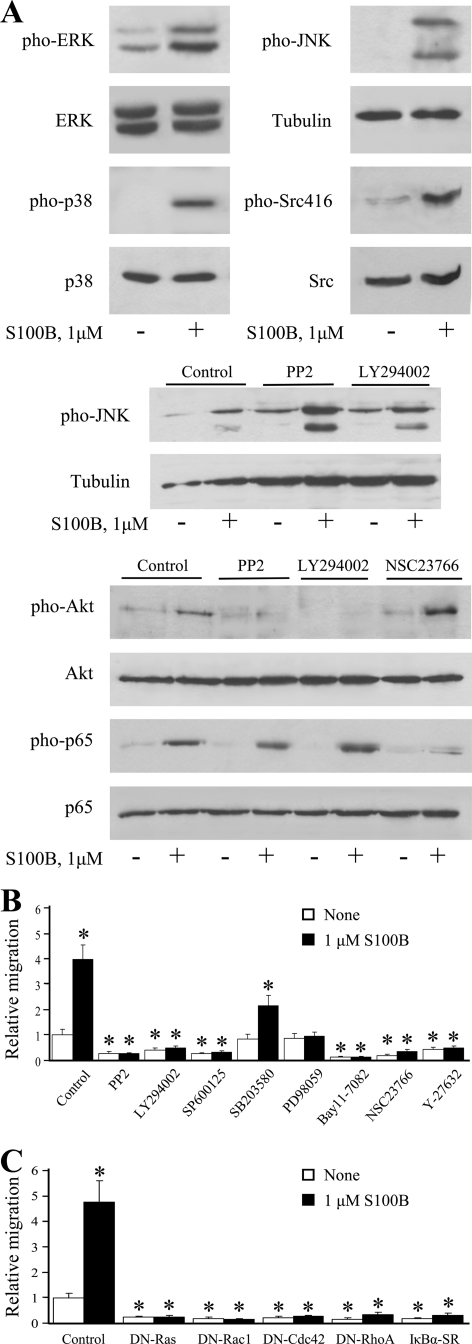
We have previously shown that S100B signals to the small GTPases, Ras, Rac1, and Cdc42 via RAGE ligation in microglia with ensuing activation of the transcription factors NF-κB and AP-1, leading to up-regulation of COX-2, IL-1β, and TNF-α expression (22, 23), and others have reported that RAGE engagement results in activation of Src kinase in monocytes/macrophages and vascular smooth muscle cells (63, 64). At proinflammatory doses S100B enhanced the phosphorylation (activation) levels of ERK1/2, p38 MAPK, JNK, Akt, and Src (Ser-416) kinases and of NF-κB (p65) in microglia (Fig. 2A and supplemental Fig. S2). Inhibition of Src using PP2 and of the Akt upstream activator, PI3K, using LY294002, resulted in reduced ability of S100B to activate Akt while leaving the stimulatory effect of S100B on NF-κB unaffected (Fig. 2A). By contrast, inhibition of Rac1 by NSC23766 significantly reduced S100B-dependent activation of NF-κB (p65) (Fig. 2A) (also see Refs. 22 and 23). This suggested that in addition to signaling to Ras, Rac1, and Cdc42 to activate NF-κB and AP-1 (22, 23), S100B/RAGE activated a Src/PI3K/Akt module in microglia that did not impinge on NF-κB. However, S100B-dependent transmigration of microglia was abrogated when the cells were pretreated with inhibitors of either Src kinase (PP2), JNK (SP600125), PI3K (LY294002), Rac1 (NSC23766), MEK/ERK1/2 (PD98059), or NF-κB (Bay 11-7082) and significantly reduced but not abrogated when the cells were treated with an inhibitor of p38 MAPK (SB203580) (Fig. 2B). Also, treatment of BV-2 microglia with Y27632 (Fig. 2B), a specific inhibitor of the RhoA-associated kinase, ROCK, or transfection with a dominant negative mutant of either Ras, Rac1, Cdc42, or RhoA or transfection with IκBαSR, a nonphosphorylatable mutant of the endogenous NF-κB inhibitor, IκBα, resulted in reduction of basal and S100B-dependent migration (Fig. 2C). However, S100B still stimulated JNK activity in the presence of the Src inhibitor, PP2 (Fig. 2A), suggesting that the stimulatory effect of S100B on microglia migration relied on RAGE-dependent activation of a Ras/Rac1-Cdc42/NF-κB, a Ras/MEK/ERK1/2/NF-κB, a Ras/Rac1-Cdc42/JNK/AP-1, and a Src/Ras/PI3K/RhoA/ROCK pathway, and individual pathways were necessary for S100B/RAGE-stimulated microglia migration. Interestingly, a dramatic reduction of basal and S100B-stimulated microglia migration was observed with 20–25% transfection efficiency using either dominant negative Ras, RhoA, Rac1, or cdc42 or IκBαSR. This suggested that abolition of the activity of any one of these G-proteins or IκBαSR in 20–25% of cells might result in profound alteration of, for example, secretion of factors required for microglia migration (an analysis of which is beyond the scope of the present work). Of note, no toxic effects of transfection that might result in reduction of cell numbers were observed. We reported that transfection (20–25% transfection efficiency) of microglia with the above mutants under the same conditions used in the present work resulted in abrogation of S100B ability to up-regulate COX-2 and to activate JNK and NF-κB, again with no evidence for toxic effects (22).
FIGURE 2.
S100B activates ERK1/2, p38 MAPK, JNK, Src (Ser-416), Akt, and NF-κB in microglia, and S100B-induced migration of microglia is differentially regulated by signaling molecules downstream of RAGE. A, BV-2 microglia were treated with 1 μm S100B for 30 min. Where required BV-2 microglia were pretreated with 20 μm PP2 (inhibitor of Src), 10 μm LY294002 (inhibitor of PI3K), or 50 μm NSC23766 (inhibitor of Rac1) before exposure to 1 μm S100B. The cells were harvested and processed for Western blotting to detect phosphorylated Src (Ser-416), ERK1/2, Akt, p38 MAPK, JNK, and NF-κB (p65), as indicated. Shown is one representative experiment of three. B, conditions were as described in the legend to Fig. 1D except that BV-2 microglia were pretreated for 30 min with the indicated inhibitors and then transferred to the upper chambers for migration assay. The results are expressed as the means ± S.D. (n = 3). C, S100B stimulates microglia migration via RAGE-dependent activation of Ras, Rac1, Cdc42, and RhoA. Conditions were as described in the legend to Fig. 1B except that BV-2 microglia were transiently transfected with dominant negative mutant (DN) of Ras, Rac1, Cdc42 or RhoA, IκBα-SR, or empty vector and then transferred to the upper chambers for migration assay. The results are expressed as the means ± S.D. (n = 3). *, significantly different from control (first column from left in A and B).
S100B/RAGE Recruits Diaphanous-1 in Microglia
Recent work indicated that RAGE signals to Rac1 via diaphanous-1 to induce glioma cell migration (65), and diaphanous-1 was proposed to activate Src (66). Thus, we investigated the possibility that S100B-dependent RAGE engagement in microglia might result in recruitment of diaphanous-1 with consequent activation of multiple pathways leading to NF-κB and/or JNK/AP-1 activation and to microglia chemoattraction. Diaphanous-1 is an adaptor protein of the formin family that mediates the effects of RhoA on cell motility and the cytoskeleton (67–69) as well as of Cdc42 and Rac1 signaling (65), and Cdc42 might play an important role in the activation of diaphanous-1 (70). We found that knockdown of diaphanous-1 by RNA interference in microglia (Fig. 3A) resulted in negation of S100B/RAGE-induced Src and JNK activation (Fig. 3B) and microglia transmigration (Fig. 3C), but not of p38 MAPK, ERK1/2, or NF-κB activation (Fig. 3B). These results established that S100B/RAGE-dependent activation of Src and JNK was dependent on diaphanous-1. However, the S100B/RAGE ability to activate NF-κB in microglia proved not crucially dependent on Src or diaphanous-1 activity and was likely dependent on activation of a Ras/Rac1 module as well as a Ras/MEK/ERK1/2 and/or p38 MAPK pathway (22, 23). Alternatively, the reduced activity of JNK that occurred in diaphanous-1 siRNA-treated microglia (Fig. 3B) might result in activation of NF-κB; it is known that NF-κB and JNK reciprocally modulate their activation state (71, 72).
S100B Up-regulates CCL3, CCL5, and CXCL12 Chemokine Expression via RAGE Engagement
Although the bell-shaped pattern of S100B-dependent microglia transmigration (Fig. 1A) was suggestive of a chemoattractant effect of the protein toward microglia, the results in Fig. 1C pointed to an S100B-dependent chemokinetic effect and/or S100B-induced secretion of chemoattractant factors. Thus, we investigated the possibility that S100B might stimulate the expression and release of chemokines. As investigated by real time PCR, we found that S100B up-regulated the expression of the chemokines CCL3 (macrophage inflammatory protein-1a), CCL5 (RANTES), and CXCL12 (Fig. 4A) but not CXCL1 or CXCL7 (data not shown). Specifically, when used at 1 μm, S100B up-regulated CCL3 expression with maximum stimulation at 3 h, significantly reduced effect at 7 and 10 h, and up-regulated CCL5 expression with a small effect at 1 h and a high effect at 3, 7, and 10 h. S100B also up-regulated the expression of CXCL12 at 30 min with declining effect thereafter (Fig. 4A). At higher concentrations also S100B stimulated CCL3, CCL5, and CXCL12 expression (supplemental Fig. S3). Thus, secretion of CCL3, CCL5, and CXCL12 might contribute to the S100B-dependent transmigration of microglia to a large extent. We concentrated on CCL3 and CCL5 in subsequent analyses.
S100B effects on chemokine up-regulation were dependent on RAGE signaling because no effects of S100B on CCL3 and CCL5 expression could be detected in BV-2/RAGEΔcyto microglia (Fig. 4B), in BV-2/WT microglia pretreated with a RAGE neutralizing antibody, or in Rage−/− microglia (data not shown). Pharmacological inhibition of Src, Rac1, PI3K, JNK, MEK/ERK1/2, p38 MAPK, or NF-κB resulted in a significant decrease in S100B-induced expression of CCL3 mRNA (Fig. 4C), although the stimulatory effect of S100B on expression of CCL5 mRNA was decreased by inhibition of Rac1, PI3K, JNK, or NF-κB, but not Src, MEK/ERK1/2, or p38 MAPK (Fig. 4C). However, knockdown of diaphanous-1 resulted in negation of the ability of S100B to up-regulate CCL3 and CCL5 mRNAs (Fig. 4D). Thus, S100B-dependent engagement of RAGE appeared to impact on the expression of the two chemokines via different molecular mechanisms, i.e. activation of a diaphanous-1/Rac1/JNK/AP-1, a Src/Ras/MEK/ERK1/2/NF-κB, a Src/Ras/p38 MAPK/NF-κB, and a Ras/Rac1/NF-κB pathway in the case of CCL3 and activation of a Ras/Rac1/NF-κB and/or JNK/AP-1 and a diaphanous-1/Rac1/NF-κB pathway in the case of CCL5.
S100B Stimulates CCL3 and CCL5 Release via RAGE Engagement
At 1 μm but not at 10 nm, S100B stimulated CCL3 and CCL5 secretion by microglia at 6 h (Fig. 5A), and pre-treatment with a RAGE neutralizing antibody blunted this effect (Fig. 5B). Also, no S100B-dependent stimulation of CCL3 and CCL5 secretion could be observed using BV-2/RAGEΔcyto microglia (Fig. 5A) or Rage−/− microglia (data not shown). Moreover, treatment of microglia with pertussis toxin, an inhibitor of chemokine receptor-associated G proteins, abolished S100B/RAGE-induced microglia migration (Fig. 5B). By contrast, S100B did not stimulate HMGB1 release from microglia at 6 h (Fig. 1C). These results supported the possibility that S100B/RAGE-induced microglia migration might be mediated by secreted chemokines.
We also found that the conditioned medium (20 h) from control BV-2/mock microglia caused a ∼100% increase in migration of BV-2/mock microglia, compared with the control (Fig. 5C), likely because of the presence of basal levels of chemokines (Fig. 4A), whereas the conditioned medium from S100B-treated BV-2/mock microglia caused a ∼700% increase in migration, likely because of the relatively high levels of chemokines released under the action of S100B (Fig. 5C). However, the two conditioned media caused the same, modest (i.e. ∼20%) increase in migration of BV-2/RAGEΔcyto microglia, that is, the migratory performance of BV-2/RAGEΔcyto microglia was not enhanced by either conditioned medium. This suggested that S100B-stimulated RAGE signaling played an important role not only in the activation of signaling pathways leading to the up-regulation of the expression of certain chemokines; it might also impact on the extent of expression of chemokine receptors and/or the molecular machinery responsible for microglia locomotion. This latter possibility was supported by the observation that whereas inhibition of RAGE function resulted in a significantly reduced basal migration (Fig. 1, A and B), treatment with pertussis toxin did not (Fig. 5B).
S100B Stimulates CCR1 and CCR5 Expression in Microglia via RAGE Engagement
In addition to up-regulating CCL3 and CCL5 expression (Fig. 5A), S100B also up-regulated the expression of the CCL3 and CCL5 receptors, CCR1 and CCR5, with no effects on CCR3, as measured at 5 h (Fig. 5D). Notably, basal expression levels of CCR1 and CCR5 in BV-2/RAGEΔcyto microglia were ∼75% smaller than in BV-2/mock microglia, and S100B did not change them (Fig. 5D). These results suggested that: 1) RAGE signaling was required for induction of CCR1 and CCR5 in microglia, S100B enhancing the effect of RAGE; and 2) lack of stimulation of BV-2/RAGEΔcyto microglia migration upon treatment with BV-2/mock microglia conditioned media (Fig. 5A) was dependent on defective expression of the two chemokine receptors, in part.
S100B Causes Cytoskeleton Rearrangements in Microglia in a RAGE-dependent Manner
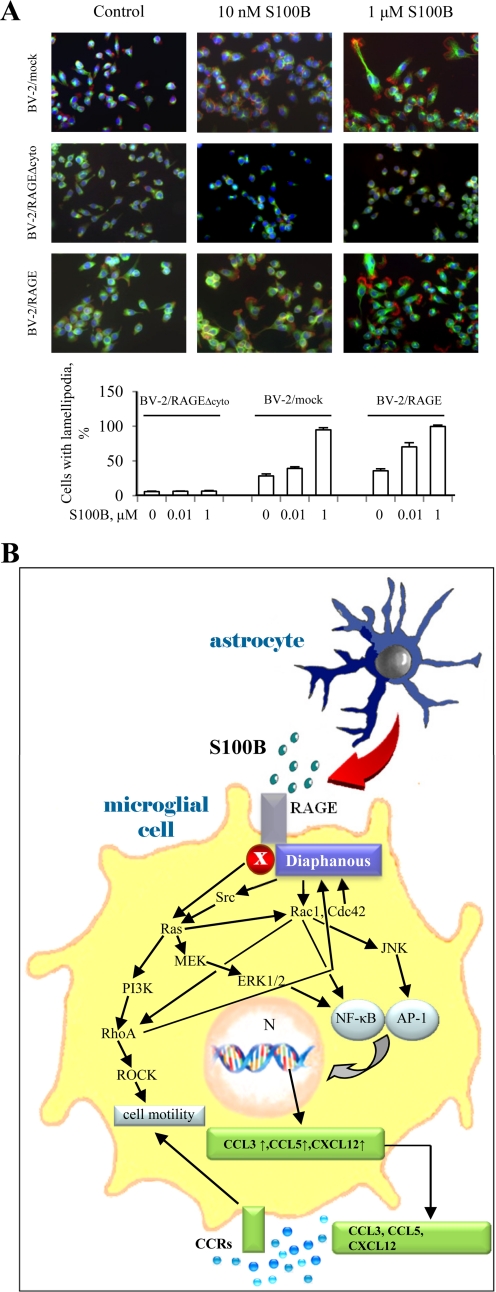
Whereas the few BV-2/RAGEΔcyto microglial cells found on the inferior side of the Boyden chamber filter exhibited a round and/or flat morphology regardless of the absence or presence of S100B in the bottom well, BV-2/mock microglia and, to a larger extent, BV-2/RAGE microglia that had transmigrated in the presence of S100B exhibited cell processes that are typical of highly activated microglia (data not shown). To investigate this point in more detail, we exposed the three BV-2 microglial clones used in the present study directly to increasing doses of S100B and analyzed them by rhodamine-phalloidin staining. This procedure allows for the visualization of lamellipodia and the organization of F-actin, a cytoskeleton component that, when assembled into stress fibers, drives cell locomotion (73–75). Lamellipodia formation at one cell side, which depends on Rac1 activation and is indicative of migration (73–75), was detected rarely in BV-2/RAGEΔcyto microglia, irrespective of the absence or presence of S100B up to 1 μm; only extremely short cell protrusions were detected in a small percentage (∼6%) of the cells (Fig. 6). By contrast and in agreement with the migration results in Boyden chambers (Fig. 1B), BV-2/mock microglia showed lamellipodia in ∼28, ∼40, and ∼95% of the cells in the absence of S100B and in the presence of 10 nm and 1 μm S100B, respectively (Fig. 6A). Lastly, BV-2/RAGE microglia showed lamellipodia in ∼35, ∼70, and 100% of the cells in the absence of S100B and in the presence of 10 nm and 1 μm S100B, respectively (Fig. 6A). Because these morphological changes were detected as early as 3 h after exposure of microglia to S100B, it is possible that they reflected the S100B-stimulated ability of RAGE to enhance Rac1 ability to induce lamellipodia formation and RhoA/ROCK-dependent stimulation of actomyosin contraction (and, hence, cell migration) in addition to the S100B/RAGE ability to stimulate Rac1 signaling-dependent chemokine expression. It is known that RAGE can signal to Rac1 in several cell types (22, 47, 49, 65) and that RAGE signaling to Rac1 is required for RAGE-mediated C6 glioma cell migration (65). Thus, the S100B-stimulated ability of RAGE to activate Rac1 might serve a dual function in microglia, producing the cytoskeleton rearrangement required for cell shape changes during locomotion and inducing chemokine expression and release.
FIGURE 6.
A, S100B induces shape changes in BV-2 microglia in a RAGE-dependent manner. BV-2/mock, BV-2/RAGEΔcyto, and BV-2/RAGE microglia were cultivated on glass coverslips, treated for 3 h with increasing concentrations of S100B, and fixed. The cells were subjected to immunofluorescence using a monoclonal anti-tubulin antibody (green) and then treated with rhodamine-phalloidin to stain F-actin (red). The nuclei were counterstained with DAPI (blue). Shown is one representative field for each condition. A quantitative analysis is also shown. B, schematic representation of the molecular mechanism whereby S100B stimulates microglia migration via RAGE engagement. Through multiple pathways S100B/RAGE stimulates the expression (arrow) and release of chemokines that in turn chemoattract microglia. In addition, S100B activates RAGE/diaphanous-1/Ras/PI3K/RhoA/ROCK and RAGE/diaphanous-1/Cdc42-Rac1 pathways that cause the cytoskeleton rearrangement and cell shape changes required for microglia motility.
DISCUSSION
We have shown that S100B stimulates microglia transmigration in Boyden chambers in a RAGE- and dose-dependent manner. S100B enhanced the migration of primary microglia, BV-2 microglia, and BV-2 microglia overexpressing RAGE but not BV-2 microglia overexpressing a RAGE mutant lacking the cytoplasmic and transducing domain, microglia pretreated with a RAGE neutralizing antibody, or Rage−/− microglia. The stimulatory effect on microglia migration was detected using S100B at proinflammatory doses (e.g. 1 μm) like those shown to be present in the extracellular milieu in case of brain damage (8, 9). No effects of the protein at nanomolar concentrations were detected unless the amount of expressed RAGE was increased, in which case nanomolar doses of S100B efficiently stimulated microglia migration. These latter findings might be explained by the observation that RAGE undergoes ligand-induced oligomerization, which appears to be required for RAGE signaling (62, 76), or that RAGE ligands stabilize naturally occurring RAGE oligomers, an event also proposed to be necessary for RAGE signaling (78); it is possible that increasing the amount of expressed RAGE might either favor (low) S100B-dependent RAGE oligomerization and signaling or increase the probability that preformed RAGE oligomers become stabilized thereby signaling. Overall, the fact that relatively high concentrations of S100B are required for RAGE-dependent stimulation of microglia migration suggests that the ability of RAGE to activate downstream signaling pathways up-regulating chemokine expression might be strongly dependent on a high extent of ligand-induced RAGE oligomerization.
RAGE engagement in several cell types including monocytes/macrophages/microglia results in the activation of several downstream signaling pathways, ultimately leading to NF-κB- and AP-1-dependent transcription of pro-inflammatory genes (22, 23, 26, 27, 79). Intermediates linking S100B/RAGE to NF-κB and AP-1 in microglia include Ras, Rac1, and Cdc42 (22, 23). In the present work, we have shown that S100B/RAGE also activates Src kinase in microglia, and inhibition of either Src, Ras, Rac1, Cdc42, PI3K, JNK, MEK/ERK1/2, p38 MAPK, or NF-κB results in reduction of S100B/RAGE-induced microglia transmigration. Likewise, knockdown of the adaptor protein, diaphanous-1, which was shown to mediate RAGE-dependent activation of Rac1 and Cdc42 in C6 glioma cell migration (65) and to act upstream of Src (66), results in reduced S100B/RAGE-dependent microglia transmigration. However, inhibition of Rac1 but not Src resulted in reduced S100B/RAGE-dependent JNK/AP-1 and NF-κB activation, and knockdown of diaphanous-1 resulted in reduced S100B/RAGE-dependent JNK/AP-1 but not NF-κB activation. Thus, we propose that S100B/RAGE-dependent transmigration of microglia relies on stimulation of diaphanous-1/Rac1-Cdc42/JNK/AP-1, Ras/Rac1-Cdc42/NF-κB, and Src/Ras/MEK/ERK1/2/NF-κB (Fig. 6B). We additionally show that S100B/RAGE signals to a Src/Ras/PI3K module to activate RhoA/ROCK, which is responsible for actomyosin contraction during cell locomotion (73–75). Also, because diaphanous-1 is a RhoA effector (68–70), it is possible that S100B/RAGE/diaphanous-1/Src/PI3K-dependent activation of RhoA translates into a reinforcement of Rac1-Cdc42 signaling via diaphanous-1, with Rac1 in turn potentiating RhoA signaling (80) and Cdc42 potentiating diaphanous-1 activity (70) in a feed-forward loop. However, diaphanous-1 might not be the sole intermediate linking S100B/RAGE to Src and Rac1-Cdc42 because inhibition of either Ras, Rac1, or Cdc42 resulted in negation of S100B-dependent activation of NF-κB (22, 23), whereas inhibition of Src or knockdown of diaphanous-1 did not. Thus, another as yet unknown intermediate might link S100B/RAGE to Ras/Rac1 with ensuing NF-κB activation (Fig. 6B).
Our results suggest that: 1) S100B causes RAGE-dependent up-regulation and secretion of CCL3, CCL5, and CXCL12 chemokines; 2) chemokine release and activity are responsible for S100B/RAGE-dependent transmigration of microglia; 3) S100B also causes RAGE-dependent induction of the chemokine receptors CCR1 and CCR5; and 4) S100B-dependent RAGE activation, however, also impacts on the molecular machinery responsible for microglia migration. This is the first evidence presented that S100B can induce chemokine expression in and release by inflammatory cells and chemokine receptor expression. Rac1 activation appears to play a pivotal role in S100B/RAGE-induced microglia migration and signaling to NF-κB and JNK/AP-1, thereby up-regulating chemokine expression and inducing the cytoskeleton changes responsible for lamellipodia formation.
It should be pointed out that RAGE is expressed at relatively low levels in microglia under normal physiological conditions, becoming up-regulated in the course of brain disorders (81). Thus, our results suggest that by (over)expressing RAGE, activated microglia might become sensitive to concentrations of S100B that would be otherwise neuroprotective (17, 29–32), therefore switching a neurotrophic factor into a proinflammatory factor. Because S100B accumulates in the brain extracellular space in case of damage (8, 9), increases in the S100B local concentration might contribute to amplify and/or propagate the inflammatory response by activating microglia and stimulating their migration. However, activation of microglia might not be necessarily detrimental; microglia also help to clear cellular debris and harmful products (e.g. β-amyloid deposits) and are proposed to resolve mild brain insults without mounting a clinically observable inflammatory response (37, 38). Thus, S100B-dependent stimulation of microglia migration might be part of a wide array of regulatory effects of S100B on microglia including but probably not restricted to exacerbation of inflammation. In addition, the low levels of expression of RAGE in neurons under normal physiological conditions (81) might make it possible that basal levels of extracellular S100B may exert RAGE-dependent trophic effects on neurons (1).
Both BV-2 and primary microglia express RAGE (24, 82), indicating that the immortalization of the BV-2 microglial cell line and the procedure of isolation of primary microglia, respectively, caused RAGE to become expressed to a significant level. Thus, both, BV-2 and primary microglia can be viewed as activated microglia, as also supported by the findings that they express basal levels of cytokines and COX-2 (22, 23) and chemokines (this report) and are able to phagocytose (20, 61, 83, 84). In principle, the low expression level of RAGE in microglia under normal physiological conditions might make it possible that (low) S100B may have a role in microglia patrolling activity (38). Our observation that at nonproinflammatory doses S100B can induce RAGE-dependent changes in microglial shape that are typical of migrating cells (Fig. 6A) supports this notion. Also, it is tempting to speculate that at proinflammatory doses S100B, by inducing CCL3 expression in microglia, not only might participate in stimulation of microglia migration to the site of damage; it might increase the transmigration of blood-borne inflammatory cells across brain capillaries (77).
In conclusion, the present results suggest that extracellular S100B might participate in inflammatory processes in the brain by enhancing microglia activation (via induction of cytokine and COX-2 expression and cytokine release) and by stimulating microglia migration (via up-regulation of chemokine expression and stimulation of chemokine release) through RAGE engagement. RAGE expression appears to be an obligate step in this process because in the absence of functional RAGE no S100B-dependent activation and attraction of microglia could be documented. Up to nanomolar concentrations, however, S100B exerts protective effects on neurons, and again, RAGE expression appears to be an obligate step in this process (17), and it might participate in microglia-based brain homeostasis, thus highlighting the Janus face of S100B (1, 5). Future analyses should focus on the timing of (over)expression of RAGE in neurons and microglia in the insulted brain and establish the minimal level of RAGE expression in each one of these cell types required for S100B neuroprotective and neurotoxic effects.
Supplementary Material
Acknowledgments
We thank Linda J. Van Eldik (Chicago, IL) for providing the bovine S100B cDNA, Xiang-Dong Ren (Stony Brook, NY) for providing the N19RhoA expression vector, Pier Lorenzo Puri (La Jolla, CA) for providing the IκBα-SR expression vector, Heikki Rauvala (Helsinki, Finland) for providing the RAGE, RAGEΔcyto, N17Rac1, N17Cdc42, and N17Ras expression vectors, and Angelika Bierhaus (Heidelberg, Germany) for providing Rage−/− mice.
This work was supported by Associazione Italiana per la Ricerca sul Cancro Project 6021 and Fondazione Cassa di Risparmio di Perugia Grants 2007.0218.020 and 2009.020.0021 (to R. D.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S3.
- AP-1
- activating protein-1
- ROCK
- RhoA-associated kinase
- HMGB1
- high mobility group protein 1.
REFERENCES
- 1. Donato R., Sorci G., Riuzzi F., Arcuri C., Bianchi R., Brozzi F., Tubaro C., Giambanco I. (2009) Biochim. Biophys. Acta 1793, 1008–1022 [DOI] [PubMed] [Google Scholar]
- 2. Brozzi F., Arcuri C., Giambanco I., Donato R. (2009) J. Biol. Chem. 284, 8797–8811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sorci G., Agneletti A. L., Donato R. (2000) Neuroscience 99, 773–783 [DOI] [PubMed] [Google Scholar]
- 4. Xiong Z., O'Hanlon D., Becker L. E., Roder J., MacDonald J. F., Marks A. (2000) Exp. Cell Res. 257, 281–289 [DOI] [PubMed] [Google Scholar]
- 5. Van Eldik L. J., Wainwright M. S. (2003) Restor. Neurol. Neurosci. 21, 97–108 [PubMed] [Google Scholar]
- 6. Mrak R. E., Griffin W. S. (2004) J. Neuropathol. Exp. Neurol. 63, 679–685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Allore R., O'Hanlon D., Price R., Neilson K., Willard H. F., Cox D. R., Marks A., Dunn R. J. (1988) Science 239, 1311–1313 [DOI] [PubMed] [Google Scholar]
- 8. Matsui T., Mori T., Tateishi N., Kagamiishi Y., Satoh S., Katsube N., Morikawa E., Morimoto T., Ikuta F., Asano T. (2002) J. Cereb. Blood Flow Metab. 22, 711–722 [DOI] [PubMed] [Google Scholar]
- 9. Mrak R. E., Sheng J. G., Griffin W. S. (1995) Hum. Pathol. 26, 816–823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sheng J. G., Mrak R. E., Bales K. R., Cordell B., Paul S. M., Jones R. A., Woodward S., Zhou X. Q., McGinness J. M., Griffin W. S. (2000) J. Neurochem. 74, 295–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wainwright M. S., Craft J. M., Griffin W. S., Marks A., Pineda J., Padgett K. R., Van Eldik L. J. (2004) Ann. Neurol. 56, 61–67 [DOI] [PubMed] [Google Scholar]
- 12. Mori T., Koyama N., Arendash G. W., Horikoshi-Sakuraba Y., Tan J., Town T. (2010) Glia 58, 300–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Srikrishna G., Freeze H. H. (2009) Neoplasia 11, 615–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hu J., Castets F., Guevara J. L., Van Eldik L. J. (1996) J. Biol. Chem. 271, 2543–2547 [DOI] [PubMed] [Google Scholar]
- 15. Hu J., Ferreira A., Van Eldik L. J. (1997) J. Neurochem. 69, 2294–2301 [DOI] [PubMed] [Google Scholar]
- 16. Koppal T., Lam A. G., Guo L., Van Eldik L. J. (2001) Neurochem. Int. 39, 401–407 [DOI] [PubMed] [Google Scholar]
- 17. Huttunen H. J., Kuja-Panula J., Sorci G., Agneletti A. L., Donato R., Rauvala H. (2000) J. Biol. Chem. 275, 40096–40105 [DOI] [PubMed] [Google Scholar]
- 18. Esposito G., Imitola J., Lu J., De Filippis D., Scuderi C., Ganesh V. S., Folkerth R., Hecht J., Shin S., Iuvone T., Chesnut J., Steardo L., Sheen V. (2008) Hum. Mol. Genet. 17, 440–457 [DOI] [PubMed] [Google Scholar]
- 19. Petrova T. V., Hu J., Van Eldik L. J. (2000) Brain Res. 853, 74–80 [DOI] [PubMed] [Google Scholar]
- 20. Adami C., Sorci G., Blasi E., Agneletti A. L., Bistoni F., Donato R. (2001) Glia 33, 131–142 [PubMed] [Google Scholar]
- 21. Kim S. H., Smith C. J., Van Eldik L. J. (2004) Neurobiol. Aging 25, 431–439 [DOI] [PubMed] [Google Scholar]
- 22. Bianchi R., Adami C., Giambanco I., Donato R. (2007) J. Leukocyte Biol. 81, 108–118 [DOI] [PubMed] [Google Scholar]
- 23. Bianchi R., Giambanco I., Donato R. (2010) Neurobiol. Aging 31, 665–677 [DOI] [PubMed] [Google Scholar]
- 24. Donato R. (2007) Curr. Mol. Med. 7, 711–724 [DOI] [PubMed] [Google Scholar]
- 25. Schmidt A. M., Yan S. D., Brett J., Mora R., Nowygrod R., Stern D. (1993) J. Clin. Invest. 91, 2155–2168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Schmidt A. M., Yan S. D., Yan S. F., Stern D. M. (2001) J. Clin. Invest. 108, 949–955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bierhaus A., Humpert P. M., Morcos M., Wendt T., Chavakis T., Arnold B., Stern D. M., Nawroth P. P. (2005) J. Mol. Med. 83, 876–886 [DOI] [PubMed] [Google Scholar]
- 28. Sparvero L. J., Asafu-Adjei D., Kang R., Tang D., Amin N., Im J., Rutledge R., Lin B., Amoscato A. A., Zeh H. J., Lotze M. T. (2009) J. Transl. Med. 17, 7–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ahlemeyer B., Beier H., Semkova I., Schaper C., Krieglstein J. (2000) Brain Res. 858, 121–128 [DOI] [PubMed] [Google Scholar]
- 30. Businaro R., Leone S., Fabrizi C., Sorci G., Donato R., Lauro G. M., Fumagalli L. (2006) J. Neurosci. Res. 83, 897–906 [DOI] [PubMed] [Google Scholar]
- 31. Kögel D., Peters M., König H. G., Hashemi S. M., Bui N. T., Arolt V., Rothermundt M., Prehn J. H. (2004) Neuroscience 127, 913–920 [DOI] [PubMed] [Google Scholar]
- 32. Pichiule P., Chavez J. C., Schmidt A. M., Vannucci S. J. (2007) J. Biol. Chem. 282, 36330–36340 [DOI] [PubMed] [Google Scholar]
- 33. Reali C., Scintu F., Pillai R., Donato R., Michetti F., Sogos V. (2005) J. Neurosci. Res. 81, 677–686 [DOI] [PubMed] [Google Scholar]
- 34. Block M. L., Hong J. S. (2005) Prog. Neurobiol. 76, 77–98 [DOI] [PubMed] [Google Scholar]
- 35. Kim S. U., de Vellis J. (2005) J. Neurosci. Res. 81, 302–313 [DOI] [PubMed] [Google Scholar]
- 36. Town T., Nikolic V., Tan J. (2005) J. Neuroinflamm. 2, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Glezer I., Simard A. R., Rivest S. (2007) Neuroscience 147, 867–883 [DOI] [PubMed] [Google Scholar]
- 38. Hanisch U. K., Kettenmann H. (2007) Nat. Neurosci. 10, 1387–1394 [DOI] [PubMed] [Google Scholar]
- 39. Whitney N. P., Eidem T. M., Peng H., Huang Y., Zheng J. C. (2009) J. Neurochem. 108, 1343–1359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Das S., Basu A. (2008) J. Neurosci. Res. 86, 1199–1208 [DOI] [PubMed] [Google Scholar]
- 41. Donato R. (1988) J. Biol. Chem. 263, 106–110 [PubMed] [Google Scholar]
- 42. Blasi E., Barluzzi R., Bocchini V., Mazzolla R., Bistoni F. (1990) J. Neuroimmunol. 27, 229–237 [DOI] [PubMed] [Google Scholar]
- 43. Bocchini V., Mazzolla R., Barluzzi R., Blasi E., Sick P., Kettenmann H. (1992) J. Neurosci. Res. 31, 616–621 [DOI] [PubMed] [Google Scholar]
- 44. Levi G., Patrizio M., Bernardo A., Petrucci T. C., Agresti C. (1993) Proc. Natl. Acad. Sci. U.S.A. 90, 1541–1545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Adami C., Bianchi R., Pula G., Donato R. (2004) Biochim. Biophys. Acta 1742, 169–177 [DOI] [PubMed] [Google Scholar]
- 46. Hofmann M. A., Drury S., Fu C., Qu W., Taguchi A., Lu Y., Avila C., Kambham N., Bierhaus A., Nawroth P., Neurath M. F., Slattery T., Beach D., McClary J., Nagashima M., Morser J., Stern D., Schmidt A. M. (1999) Cell 97, 889–901 [DOI] [PubMed] [Google Scholar]
- 47. Huttunen H. J., Fages C., Rauvala H. (1999) J. Biol. Chem. 274, 19919–19924 [DOI] [PubMed] [Google Scholar]
- 48. Batra R. K., Guttridge D. C., Brenner D. A., Dubinett S. M., Baldwin A. S., Boucher R. C. (1999) Am. J. Respir. Cell Mol. Biol. 21, 238–245 [DOI] [PubMed] [Google Scholar]
- 49. Sorci G., Riuzzi F., Arcuri C., Giambanco I., Donato R. (2004) Mol. Cell. Biol. 24, 4880–4894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Riuzzi F., Sorci G., Donato R. (2007) Am. J. Pathol. 171, 947–961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hori O., Brett J., Slattery T., Cao R., Zhang J., Chen J. X., Nagashima M., Lundh E. R., Vijay S., Nitecki D., Morser J., Stern D., Schmidt A. M. (1995) J. Biol. Chem. 270, 25752–25761 [DOI] [PubMed] [Google Scholar]
- 52. Bianchi M. E., Manfredi A. A. (2007) Immunol. Rev. 220, 35–46 [DOI] [PubMed] [Google Scholar]
- 53. Rauvala H., Rouhiainen A. (2007) Curr. Mol. Med. 7, 725–734 [DOI] [PubMed] [Google Scholar]
- 54. Wang H., Bloom O., Zhang M., Vishnubhakat J. M., Ombrellino M., Che J., Frazier A., Yang H., Ivanova S., Borovikova L., Manogue K. R., Faist E., Abraham E., Andersson J., Andersson U., Molina P. E., Abumrad N. N., Sama A., Tracey K. J. (1999) Science 285, 248–251 [DOI] [PubMed] [Google Scholar]
- 55. Degryse B., Bonaldi T., Scaffidi P., Müller S., Resnati M., Sanvito F., Arrigoni G., Bianchi M. E. (2001) J. Cell Biol. 152, 1197–1206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Rouhiainen A., Kuja-Panula J., Wilkman E., Pakkanen J., Stenfors J., Tuominen R. K., Lepäntalo M., Carpén O., Parkkinen J., Rauvala H. (2004) Blood 104, 1174–1182 [DOI] [PubMed] [Google Scholar]
- 57. Palumbo R., Galvez B. G., Pusterla T., De Marchis F., Cossu G., Marcu K. B., Bianchi M. E. (2007) J. Cell Biol. 179, 33–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Yang D., Chen Q., Yang H., Tracey K. J., Bustin M., Oppenheim J. J. (2007) J. Leukoc. Biol. 81, 59–66 [DOI] [PubMed] [Google Scholar]
- 59. Andersson U., Erlandsson-Harris H., Yang H., Tracey K. J. (2002) J. Leukocyte Biol. 72, 1084–1091 [PubMed] [Google Scholar]
- 60. Rauvala H., Rouhiainen A. (2010) Biochim. Biophys. Acta 1799, 164–170 [DOI] [PubMed] [Google Scholar]
- 61. de Jong E. K., de Haas A. H., Brouwer N., van Weering H. R., Hensens M., Bechmann I., Pratley P., Wesseling E., Boddeke H. W., Biber K. (2008) J. Neurochem. 105, 1726–17236 [DOI] [PubMed] [Google Scholar]
- 62. Ostendorp T., Leclerc E., Galichet A., Koch M., Demling N., Weigle B., Heizmann C. W., Kroneck P. M., Fritz G. (2007) EMBO J. 26, 3868–3878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Xu D., Kyriakis J. M. (2003) J. Biol. Chem. 278, 39349–39355 [DOI] [PubMed] [Google Scholar]
- 64. Reddy M. A., Li S. L., Sahar S., Kim Y. S., Xu Z. G., Lanting L., Natarajan R. (2006) J. Biol. Chem. 281, 13685–13693 [DOI] [PubMed] [Google Scholar]
- 65. Hudson B. I., Kalea A. Z., Del Mar Arriero M., Harja E., Boulanger E., D'Agati V., Schmidt A. M. (2008) J. Biol. Chem. 283, 34457–34468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Tominaga T., Sahai E., Chardin P., McCormick F., Courtneidge S. A., Alberts A. S. (2000) Mol. Cell 5, 13–25 [DOI] [PubMed] [Google Scholar]
- 67. Watanabe N., Madaule P., Reid T., Ishizaki T., Watanabe G., Kakizuka A., Saito Y., Nakao K., Jockusch B. M., Narumiya S. (1997) EMBO J. 16, 3044–3056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Wasserman S. (1998) Trends Cell Biol. 8, 111–115 [DOI] [PubMed] [Google Scholar]
- 69. Wallar B. J., Alberts A. S. (2003) Trends Cell Biol. 13, 435–446 [DOI] [PubMed] [Google Scholar]
- 70. Seth A., Otomo C., Rosen M. K. (2006) J. Cell Biol. 174, 701–713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Tang G., Minemoto Y., Dibling B., Purcell N. H., Li Z., Karin M., Lin A. (2001) Nature 414, 313–317 [DOI] [PubMed] [Google Scholar]
- 72. Polk W. W., Ellis M. E., Kushleika J. V., Simmonds P. L., Woods J. S. (2007) Am. J. Physiol. Cell Physiol. 293, C1160–C1170 [DOI] [PubMed] [Google Scholar]
- 73. Fukata M., Nakagawa M., Kaibuchi K. (2003) Curr. Opin. Cell Biol. 15, 590–597 [DOI] [PubMed] [Google Scholar]
- 74. Raftopoulou M., Hall A. (2004) Dev. Biol. 265, 23–32 [DOI] [PubMed] [Google Scholar]
- 75. Jaffe A. B., Hall A. (2005) Annu. Rev. Cell Dev. Biol. 21, 247–269 [DOI] [PubMed] [Google Scholar]
- 76. Dattilo B. M., Fritz G., Leclerc E., Kooi C. W., Heizmann C. W., Chazin W. J. (2007) Biochemistry 46, 6957–6970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Zozulya A. L., Reinke E., Baiu D. C., Karman J., Sandor M., Fabry Z. (2007) J. Immunol. 178, 520–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Xie J., Reverdatto S., Frolov A., Hoffmann R., Burz D. S., Shekhtman A. (2008) J. Biol. Chem. 283, 27255–27269 [DOI] [PubMed] [Google Scholar]
- 79. Okamoto T., Yamagishi S., Inagaki Y., Amano S., Koga K., Abe R., Takeuchi M., Ohno S., Yoshimura A., Makita Z. (2002) FASEB J. 16, 1928–1930 [DOI] [PubMed] [Google Scholar]
- 80. Ridley A. J., Paterson H. F., Johnston C. L., Diekmann D., Hall A. (1992) Cell 70, 401–410 [DOI] [PubMed] [Google Scholar]
- 81. Lue L. F., Walker D. G., Brachova L., Beach T. G., Rogers J., Schmidt A. M., Stern D. M., Yan S. D. (2001) Exp. Neurol. 171, 29–45 [DOI] [PubMed] [Google Scholar]
- 82. Chen X., Walker D. G., Schmidt A. M., Arancio O., Lue L. F., Yan S. D. (2007) Curr. Mol. Med. 7, 735–742 [DOI] [PubMed] [Google Scholar]
- 83. Blasi E., Barluzzi R., Mazzolla R., Tancini B., Saleppico S., Puliti M., Pitzurra L., Bistoni F. (1995) J. Neuroimmunol. 58, 111–116 [DOI] [PubMed] [Google Scholar]
- 84. Kopec K. K., Carroll R. T. (1998) J. Neurochem. 71, 2123–2131 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.