Abstract
The nonstructural protein NS1 of influenza A virus blocks the development of host antiviral responses by inhibiting polyadenylation of cellular pre-mRNA. NS1 also promotes the synthesis of viral proteins by stimulating mRNA translation. Here, we show that recombinant NS1 proteins of human pandemic H1N1/2009, avian highly pathogenic H5N1, and low pathogenic H5N2 influenza strains differentially affected these two cellular processes: NS1 of the two avian strains, in contrast to NS1 of H1N1/2009, stimulated translation of reporter mRNA in cell-free translation system; NS1 of H5N1 was an effective inhibitor of cellular pre-mRNA polyadenylation in A549 cells, unlike NS1 of H5N2 and H1N1/2009. We identified key amino acids in NS1 that contribute to its activity in these two basic cellular processes. Thus, we identified strain-specific differences between influenza virus NS1 proteins in pre-mRNA polyadenylation and mRNA translation.
Keywords: Protein-Protein Interactions, RNA, Transcription, Translation, Viral Protein, NS1 Protein, Influenza A Virus, Virus-Host Interaction
Introduction
Influenza A viruses of the Orthomyxoviridae family endemically infect both birds and mammalian species. In human, influenza A viruses replicate in the respiratory tract, whereas in avian species, the primary site of virus replication is the intestinal tract. The site of viral entry is partly defined by the distribution of sialic acid molecules on cell surfaces. These surface molecules are recognized by viral HA and neuraminidase. There are sixteen HA (H1–H16) and nine neuraminidase (N1–N9) subtypes. At least 103 of the possible 144 combinations have been found in wild birds, a primary host of influenza A viruses. Depending on the composition of amino acids in the HA cleavage site avian influenza strains can be classified further as high (HP) or low pathogenic (LP) strains.
Only four influenza A subtypes have been detected in humans. The three pandemics of the 20th century were caused by H1N1 (1918), H2N2 (1957), and H3N2 (1968). The first pandemic in this century was caused by an H1N1/2009 strain of swine origin. Highly pathogenic avian influenza H5N1 has also a pandemic potential (1). Pandemics can occur when novel influenza strains cross the species barrier (2). Pandemic strains can be generated by either reassortment of genomic segments, which take place when more than one strain infect the same cell, or by accumulation of specific mutations (virulence/pathogenicity markers) in the viral genome as a result of the error-prone viral RNA-dependent RNA polymerase activity (3). These two mechanisms are also important for the generation of the influenza A virus diversity.
Influenza A virus replicates for almost 2 days after infection before detection by the immune system (4). This evasion of immune surveillance involves NS1 interference with the expression of key proinflammatory cytokines via interactions with specific cellular factors (5). NS1 interacts with CPSF4 (cleavage and polyadenylation specificity factor 4) to inhibit polyadenylation of cellular pre-mRNA and, thereby, to block host transcription (6). The interaction of NS1 with the cellular translation apparatus stimulates the synthesis of viral proteins (7). The N-terminal 74 amino acids of NS1 form a functional RNA-binding domain (RBD),3 whereas the C-terminal 150 amino acids form an effector domain (ED), which predominantly mediates interactions with cellular proteins (8).
Here, we analyzed recombinant NS1 proteins of human pandemic H1N1/2009 and avian highly pathogenic (HP) H5N1 and low pathogenic (LP) H5N2 strains (see Fig. 1A) for their ability to inhibit pre-mRNA polyadenylation and stimulate mRNA translation and showed that these proteins have strain-specific effects. Our analysis revealed key amino acids that are essential for the differential effects of NS1 on these two cellular processes.
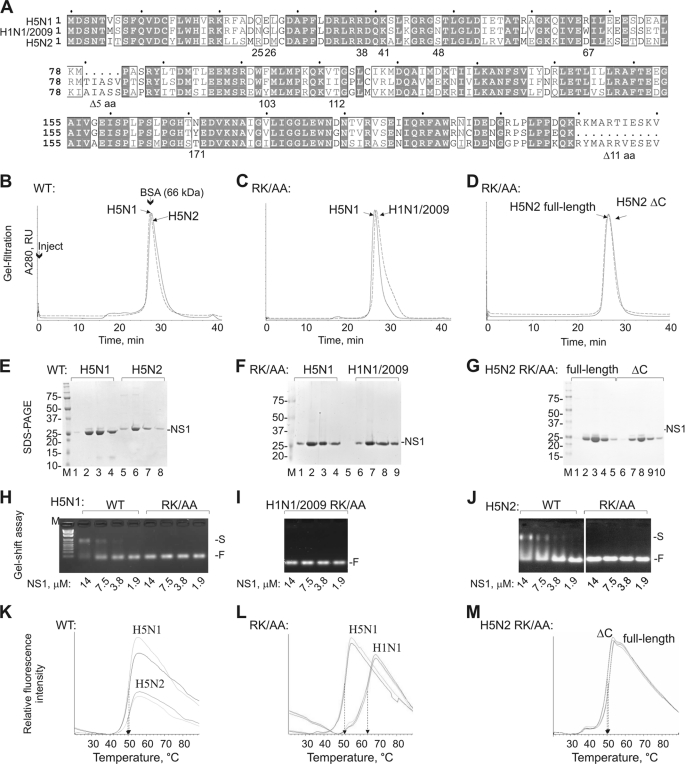
FIGURE 1.
Purified recombinant NS1 proteins of three influenza A viruses possesses different thermal stability. A, sequence alignment of analyzed NS1 proteins, generated using ESPript program (version 2.2). Key residues and two deletions are indicated. B–D, elution profiles of NS1 wild-type and mutants from analytical gel filtration (Superdex-200, 0.5 ml min−1, absorbance at 280 nm). The elution peak of BSA, one of the six calibration standards, is shown. E–G, SDS-PAGE analysis of NS1-containing fractions eluted from gel filtration column. H–J, gel shift assay monitoring binding of wild-type and RK/AA NS1 proteins to tRNAAsp. Positions of free (F) and NS1-bound (S) RNA are marked. NS1 concentrations in the mixtures are indicated. K–M, thermal stability of purified NS1 proteins. Two thermal melting curves for each NS1 proteins are shown. Arrows indicate the Tm, which corresponds to the midpoint of the unfolding transition. RU, relative units; M, marker.
EXPERIMENTAL PROCEDURES
Cloning and Mutagenesis
NS1 genes were derived from pandemic H1N1/2009, HP H5N1, and LP H5N2. The H1N1/2009 was isolated from patients with influenza-like symptoms in Luxembourg in 2009 (GenBankTM accession no. CAZ66453.1).4 The HP H5N1 strain was obtained from farm chicken (GenBankTM accession no. CAQ58520.1; (9)), and the H5N2 strains was from healthy wild waterfowls.5
The nucleotide sequence encoding NS1 protein of A/Luxembourg/43/2009 (H1N1/2009) strain was amplified from cDNA by PCR and cloned into the pET151/D-Topo vector (Invitrogen). The resulting plasmid pET151-NS1-H1N1/2009 encodes a polypeptide with an N-terminal His tag, V5 epitope, and tobacco etch virus protease cleavage site (MHHHHHHGKRIPNPLLGLDSTENLYPQ↓GIDPFT). The nucleotide sequences encoding NS1 proteins of A/chicken/Nigeria/OG10/2007 (HP H5N1) and A/Spur-winged goose/Nigeria/210/2008 (LP H5N2) viruses were amplified from cDNA by PCR and cloned into the pNic28-Bsa4 vector. The resulting plasmids, pNic-NS1-HP H5N1 and pNic-NS1-H5N2, encoded polypeptides with an N-terminal His tag and tobacco etch virus protease cleavage site (MHHHHHHSSGVDLGTENLYFQ↓S). The R38A/K41A mutations (RK/AA) in H1N1/2009, H5N1, and H5N2 NS1, the deletion of C-terminal 11 amino acids in H5N2 NS1, and the deletion of ED in H5N1 NS1 were introduced by site-directed mutagenesis using 5′-phosphorylated oligonucleotides and Phusion DNA polymerase (Finnzymes). The Q25N/E26G, S48N, R67W, or F103Y mutations were introduced into plasmids encoding RK/AA NS1 of H5N1. Mutations N25Q/G26E, N48S, or W67R were introduced into plasmids encoding RK/AA NS1 of H1N1/2009.
The coding regions of WT NS1 proteins with the tags and the protease cleavage site were subcloned into the pIRES2-AcGFP vector (to generate pIRES-NS1-H1N1/2009, pIRES-NS1-H5N1, and pIRES-NS1-H5N2). This vector includes SV40 polyadenylation signals downstream of the NS1-AcGFP genes to direct proper processing of the 3′ end of the bicistronic mRNA. The Gallus gallus CPSF4 fragment was inserted into the pNic28-Bsa4 vector to generate pNic-chCPSF4F1–3 plasmid expressing zinc finger domains F1, F2, and F3 of CPSF4 (amino acids 1–114, CPSF4F1–3). All constructs were verified by sequencing.
Protein Expression and Purification
NS1 proteins and their mutants and CPSF4F1–3 were expressed in Escherichia coli and purified. Briefly, bacterial cells were grown at 37 °C in 0.5L of StabySwitch autoinducible media (Eurogentec) until the absorbance at 600 nm reached 1. Flasks were transferred to 17 °C, and cells were grown for 10 h. Cells were collected by centrifugation and resuspended in 20 ml TN buffer (20 mm Tris-HCl, pH 8.6, 100 mm NaCl) containing 10 mm imidazole. The cell suspensions were sonicated and centrifuged at 120,000 × g for 1 h. Supernatants were loaded onto HisTrap columns (GE Healthcare), and proteins were eluted with a linear 10–500 mm gradient of imidazole. The N-terminal tags were cleaved from proteins by an overnight treatment with AcTev protease (Invitrogen). Proteins were diluted with TN buffer and loaded onto preassembled HisTrap and Q Sepharose HP columns (GE Healthcare). Columns were disassembled, and proteins were eluted from quantitative HP column with a linear 0.1–1.0 m NaCl gradient. All proteins were concentrated and purified by gel filtration on Superdex-200 columns (GE Healthcare) pre-equilibrated with TN buffer.
To purify the NS1·CPSF4F1–3 complex, the purified His-tagged CPSF4F1–3 was incubated with untagged NS1 for 30 min at 4 °C in TN buffer containing 20 mm imidazole. 250 μl of Ni-NTA-agarose beads (Qiagen) in TN buffer were added to the proteins, and the mixture was incubated for another 30 min at 4 °C. Beads were washed with TN buffer, and remaining proteins were eluted with high imidazole and resolved by SDS-PAGE.
NS1-interacting partners were also purified from carcinomic human alveolar basal epithelial A549 cells expressing different His-tagged NS1 proteins. Briefly, 5 × 107 A549 cells were transfected with 44 μg of pIRES-NS1-H5N1, pIRES-NS1-H5N2, or pIRES2-AcGFP vector using 132 μl of Lipofectamine LTX (Invitrogen) and 44 μl of Plus reagent (Invitrogen). Twenty four hours post transfection, cells were trypsinized, collected by centrifugation, resuspended in 2 ml of TN buffer containing 10 mm imidazole and sonicated. Lysates were treated or not with RNase A (Sigma-Aldrich; 0,1 mg/ml) for 15 min at 4 °C and centrifuged at 20,000 × g for 20min. 250 μl of Ni-NTA agarose beads in TN buffer were added to the proteins, and the mixture was incubated for another 30 min at +4 °C. Beads were washed with TN buffer containing 10 mm imidazole to remove unbound polypeptides. Remaining proteins were eluted with imidazole gradient and resolved by SDS-PAGE. Proteins were transferred to the Hybond-LFP membrane (GE Healthcare) using a Novex Semi-Dry Blotter (Invitrogen). Membranes were incubated with primary rabbit polyclonal anti-CPSF4 (1:1000, product no. AV40675, Sigma-Aldrich) and then with secondary Cy5-conjugated goat anti-rabbit IgG (1:5000, product no. PA45011, GE Healthcare). His-tagged NS1 proteins were stained with Penta-His Alexa Fluor 647-conjugated antibody (1:1000; catalog no. 35370, Qiagen). Blots were scanned using a Typhoon 9400 or Odyssey imager. The images were analyzed with ImageQuant (GE-Healthcare).
Analytical Gel Filtration
The oligomeric states of NS1 proteins and its mutants were determined by analytical gel filtration at 20 °C on a Superdex-200 10/30 column (GE Healthcare) in TN buffer at a flow rate of 0.5 ml min−1. The column was calibrated using molecular weight markers for gel filtration chromatography (Sigma-Aldrich).
Thermal Shift Assay
Thermal shift assay was performed with 3 μg of purified NS1 proteins, NS1 mutants, CPSF4F1–3, or its complex with NS1 in TN buffer containing 900× dilution of SYPRO Orange dye (Invitrogen). The assay was carried out in the DNA engine Opticon 2 continuous fluorescence detection system (Bio-Rad). The dye was excited at 490 nm, and the emission light was recorded at 575 nm, whereas the temperature was increased by increments of 0.5 °C per minute from 20 to 90 °C. Control assays were carried out to verify that no fluorescence signal was recorded in the absence of protein. The Tm values were calculated from the maximum value of the negative first derivative of fluorescence intensity versus temperature; this is approximately the midpoint of the unfolding transition. A shift in Tm (i.e. in the presence of mutation) indicated a change in protein stability. An increase in Tm indicated a stabilization of the protein by an increase in structural order and a reduction in conformational flexibility, whereas a decrease in Tm indicates a destabilization. Each reaction was carried out in triplicate.
Gel Shift Assay
Purified NS1 or its mutants were incubated for 15 min on ice with yeast tRNAAsp (10, 11) in 8-μl reaction volumes. Subsequently, 2 μl of the TN buffer containing 10% glycerol was added, and the samples were run in a 1% agarose gel containing 0.1 μg/ml ethidium bromide in Tris-Borate EDTA buffer (5 V/cm for 90 min).
In Vitro Translation Assay
Luciferase T7 control DNA (1 μg, Promega) was linearized with XmnI restriction enzyme (New England Biolabs). Unlabeled or [α-32P]CTP-labeled (PerkinElmer Life Science) capped polyadenylated luciferase mRNA were produced with mMESSAGE mMACHINE kit (Ambion). Luciferase was produced from resulting RNA using nuclease-treated rabbit reticulocyte lysate system (Promega) in the presence or absence of purified NS1 proteins. Translation reactions were carried out in 50 μl containing 35 μl of rabbit reticulocyte lysate, 40 μm amino acid mixture, 80 units of RNAseOut (Invitrogen), and 1 μg of mRNA. Reaction mixtures were incubated at 30 °C. Reactions were stopped by transferring the tubes to 4 °C, luciferase activity was assayed using a luciferase reporter gene assay kit (Roche Applied Science), and luminescence was measured using a Tecan or Paradigm plate reader (Beckman Coulter). Alternatively, luciferase expression was detected by Western blotting. Primary antibody was goat IgG polyclonal anti-luciferase (part no. G745A, Promega), and the secondary antibody was Cy5-conjugated donkey anti-goat IgG (ab6566-100, Abcam). The 32P-labeled reporter mRNA was analyzed by electrophoresis in 1% agarose gel. The gel was dried and analyzed by phosphorimaging (Fuji BAS 1500). The RNA was quantified by ImageJ software.
Reverse Transcription and Real-time Quantitative PCR
One million of A459 cells were transfected with pIRES-NS1-H1N1/2009, pIRES-NS1-H5N1, pIRES-NS1-H5N1 (F103Y), pIRES-NS1-H5N2, or pIRES2-AcGFP as described above. The GFP-expressing cells were counted using Cellometer vision instrument (Nexcelom Bioscience). Total RNA was extracted from the cells with RNeasy Mini kit (Qiagen). cDNA synthesis was performed with random primers (Invitrogen) or dT20 primer (Eurogentec) and SuperScript III reverse transcriptase (Invitrogen). Polyadenylated and total RNA encoding β-actin and peptidylprolyl isomerase A were quantified. Briefly, real-time PCRs were done on DNA engine Opticon 2 continuous fluorescence detection system (Bio-Rad) using Platinum TaqDNA polymerase (Invitrogen), SYBR Green (Cambrex), and a set of specific primers (5′-GGCCACGGCTGCTTC-3′ and 5′-GTTGGCGTACAGGTCTTTGC-3′ for β-actin and 5′-TCCTGGCATCTTGTCCATG-3′ and 5′-CCATCCAACCACTCAGTCTTG-3′ for peptidylprolyl isomerase A). The copy numbers of total and polyadenylated β-actin and peptidylprolyl isomerase A RNAs were calculated using standard curve analysis as in (12). Each reaction was carried out in triplicate. The ratios of the averaged copy numbers of dT20 and random primer reverse-transcribed RNA of control transfected cells (x̄dT20(neg)/x̄R(neg)) were divided by the dT20/random ratio for NS1 expressing cells (x̄dT20/x̄R). The change (F) in RNA polyadenylation was expressed using Equation 1.
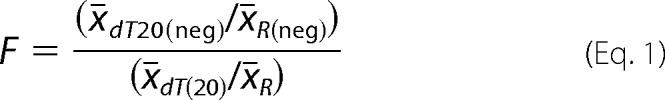 |
RESULTS
NS1 Protein of H1N1/2009 Possesses Higher Thermal Stability than NS1 Proteins of Avian H5N1 and H5N2
In contrast to RBD and ED, which can be purified individually, full-length NS1 proteins are known to readily aggregate and precipitate at the concentrations required for biochemical studies. Surprisingly, we found that the full-length NS1 proteins of HP A/chicken/Nigeria/OG10/2007 (H5N1) and LP A/Spur-winged-goose/Nigeria/210/2008 (H5N2) strains could be expressed in E. coli and purified to homogeneity using a combination of affinity, anion-exchange, and size-exclusion chromatography (Fig. 1, B and E). In contrast, NS1 of pandemic H1N1/2009 strains precipitated after the last chromatographic step.
It has been shown that R38A and K41A mutations increase NS1 solubility (8). Therefore, RK/AA mutations were introduced into NS1 of pandemic H1N1/2009, and soluble protein was obtained after last purification step (Fig. 1, C and F). These substitutions abrogated nucleic acid binding by NS1 as demonstrated by gel shift assays (Fig. 1, H–J). The NS1 proteins of the three analyzed strains, including their RK/AA mutants, were purified as dimers, with an apparent molecular mass of ∼60 kDa. (The predicted mass for an NS1 dimer is 52 kDa.)
The stability of the dimeric NS1 was probed by thermal denaturation assay. The NS1 of the pandemic H1N1/2009 had a significantly higher melting temperature than the H5N1 and H5N2 NS1 (Tm = 65 versus 51.0 and 51.5 °C; Fig. 1, K and L). The increased thermal stability of H1N1/2009 NS1 was not caused by the RK/AA mutations or C-terminal deletion, which is naturally present in H1N1/2009 NS1, as the same mutations introduced into the H5N2 NS1, did not affect temperature-dependent unfolding of the protein (Fig. 1, D, G, and M). Altogether, these results show that purified NS1 proteins of human pandemic H1N1/2009 and avian HP H5N1 and LP H5N2 strains have similar oligomeric status but differ in their thermal stability.
H5N1 and H5N2 but Not H1N1/2009 NS1 Facilitate Translation of Reporter mRNA in Vitro
NS1 proteins orchestrate multiple cellular processes. In particular, NS1 enhances the translation of reporter mRNA in vivo (7, 13). To separate the effect of NS1 on translation from its functions in other cellular processes, purified recombinant NS1 proteins were tested in an in vitro translation reaction based on rabbit reticulocyte lysate containing capped polyadenylated mRNA encoding firefly luciferase. Luminescence was used as readout.
The wild-type NS1 protein of H5N1 stimulated expression of reporter mRNA compared with the control reaction, resulting in a 6-fold increase in luciferase activity within 2 h of incubation (Fig. 2A). We also tested whether the RK/AA mutation may affect NS1-stimulated translation. The RK/AA mutant of H5N1 NS1 stimulated the synthesis of luciferase to similar level as wild-type protein (Fig. 2A). Thus, the RNA-binding residues Arg38 and Lys41 are not involved in the stimulation of translation.
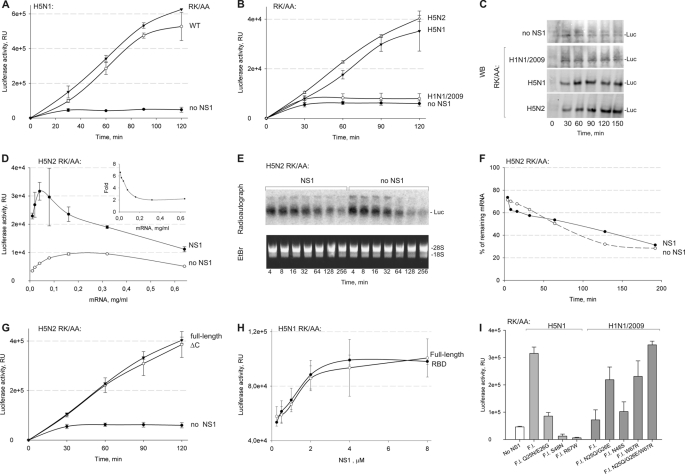
FIGURE 2.
Differential effects of NS1 proteins on translation. A, wild-type H5N1 NS1 and its RK/AA mutant (4 μm) stimulate translation of capped luciferase mRNA in rabbit reticulocyte lysates to a similar extent. B, H5N1 and H5N2 NS1 stimulate translation, whereas H1N1/2009 NS1 does not. Translation reactions were stopped at indicated time points, and luciferase (Luc) activity was measured by enzymatic assays. Control reactions were performed without NS1. All the reactions were performed in triplicate. C, Western blot (WB) showing expression of reporter protein in B. D, effect of mRNA concentration on basal and NS1-stimulated translation. The inset shows the fold increase in translation by RK/AA NS1 of H5N2 (4 μm). E, autoradiograph showing degradation of 32P-labeled reporter mRNA (final concentration of 0.04 mg/ml) and agarose gel showing ribosomal RNA in translation reactions with and without RK/AA NS1 of H5N2 (4 μm). F, quantification of reporter mRNA in translation reactions. G, deletion of the 11 C-terminal amino acids in H5N2 NS1 does not abolish translation stimulation of capped luciferase mRNA. H, RBD of H5N1 NS1 is sufficient to stimulate translation of capped reporter mRNA. Luciferase activity was measured after 1 h of incubation. I, effect of mutations introduced into RK/AA mutant of H5N1 NS1 or H1N1/2009 (4 μm) on translation stimulation. Luciferase activity was measured after 1h of incubation. F.l., full-length. All reactions were performed in triplicate (G–I). RU, relative units.
This observation allowed us to directly compare effects of the NS1 RK/AA mutants of the three viruses on their efficiency to stimulate translation. Although RK/AA NS1 of both avian strains stimulated translation of reporter mRNA, NS1 of the human pandemic H1N1/2009 strain was almost inactive in this assay (Fig. 2B). To exclude the possibility that NS1 proteins act post-translationally, the production of reporter protein was monitored by Western blot (Fig. 2C). This confirmed that only NS1 proteins of avian but not of human pandemic strain efficiently stimulated translation of a reporter mRNA.
We next examined the effect of the NS1 on the stability of reporter RNA during translation. For this, the optimal concentration of reporter mRNA to be added to the translation reaction was determined (Fig. 2D). The 32P-labeled reporter mRNA at final concentration of 0.04 mg/ml was added to the translation reactions containing no or RK/AA NS1 of H5N2. The reactions were stopped after different time intervals, and reporter mRNA in each reaction was analyzed by electrophoresis in agarose gel and quantified (Fig. 2, E and F). Comparison of luciferase mRNA in the translation reactions with and without NS1 showed that degradation of reporter mRNA during translation was independent on NS1. Thus, NS1 had no effect on the relative stability of reporter mRNA in a cell-free translation system.
To map the region on NS1 that is essential for stimulation of translation, two deletion mutants were tested in the reporter assay. Deletion of 11 C-terminal amino acids in H5N2 NS1 (residues 219–230, ΔC), which are also naturally present in the wild-type H1N1/2009 NS1 and deletion of the entire NS1 ED in H5N1 NS1 (residues 75–225) did not affect translational stimulation (Fig. 2, H and G). These results demonstrate that the RBD (residues 1–74) of NS1 is sufficient for the translation stimulation in rabbit reticulocyte lysates.
Because NS1 of H1N1/2009 was almost inactive in the translation reaction, we hypothesized that amino acids that differ between the human pandemic H1N1/2009 and avian strains within the RBD are likely to play a particular role. Sequence comparison of the three NS1 proteins revealed that NS1 of H1N1/2009 possesses four unique amino acids in its RBD that are different from H5N1 and H5N2 NS1 (residues 25, 26, 48, and 67; Fig. 1A). The side chains of these residues are solvent exposed according to available structures of NS1 RBD, indicating that they are most probably implicated in interactions with cellular factors (see Fig. 4B). Therefore, we introduced Q25N/E26G, S48N, or R67W mutations corresponding to H1N1 NS1 into an H5N1 NS1 RK/AA background (loss of function) and N25Q/G26E, N48S, and W67R mutations corresponding to H5N1 NS1 into an H1N1/2009 RK/AA background (gain of function). Proteins were purified and tested in the in vitro translation assay. We found that whereas mutations corresponding to H1N1 diminished the ability of the H5N1 protein to stimulate translation, substitutions corresponding to H5N1 in H1N1/2009 background increased the ability to stimulate translation (Fig. 2I). Therefore, amino acids in positions 25/26, 48, and 67 of NS1 are essential for the stimulation of translation.
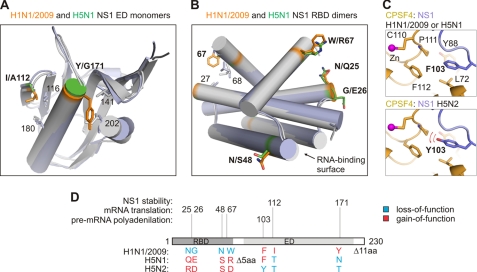
FIGURE 4.
Key amino acids of influenza A virus NS1 protein implicated in protein stability, cellular pre-mRNA polyadenylation, and mRNA translation. A, ribbon representation of aligned ED monomers of NS1 carrying H1N1/2009 or H5N1-type of residues at positions 112 and 171 (PDB codes 3M5R and 3F5T). B, ribbon representation of aligned RBD dimers of NS1 carrying H1N1/2009 or H5N1-type of residues at positions 25, 26, 48, and 67 (PDB codes 3M8A and 2ZKO). C, ribbon representation of the NS1 ED·CPSF4F2F3 complex focusing on a residue at position 103, which plays a key role in the NS1·CPSF4 complex stability (PDB code 2RHK). The aromatic ring of Phe103 interacts with hydrophobic Leu72, Tyr88, Phe112, and, possibly, with Pro111 of CPSF4 (top). When Phe103 is substituted with Tyr, its hydroxyl group may destroy the accurate network of hydrophobic interactions destabilizing the NS1-CPSF4 assembly (bottom). Side chains of these residues as well as conserved residues at positions 27, 68, 116, 141, and 202 are shown. D, NS1 residues implicated in protein stability, stimulation of translation and inhibition of polyadenylation analyzed in this study. aa, amino acids.
NS1 of H5N1 but Not H5N2 and H1N1/2009 Is an Efficient Inhibitor of Polyadenylation of Cellular Pre-mRNA
NS1 proteins of different influenza A virus isolates have been shown to interact with cellular CPSF4 protein and inhibit polyadenylation of cellular pre-mRNA, thus blocking host RNA transcription (14–16). However, NS1 proteins of pandemic H1N1/2009 strain and several other strains are unable to block general host gene expression, despite interacting with CPSF4 (Fig. 3A and Refs. 14, 15, 31). We hypothesized that the stability of the NS1·CPSF4 complex may play a particular role. Indeed, crystallographic studies identified a critical role for amino acids 103, 106, 108, 125, and 189 of NS1 for NS1-CPSF4 interaction (16). NS1 proteins of avian LP H5N2 have a unique Tyr in position 103. H1N1/2009 and H5N1 NS1 have a Phe at this position (Fig. 1A). A model of CPSF4-NS1 H5N2 based on the complex structure of NS1 ED and zinc finger domains F2 and F3 of CPSF4 (amino acids 63–114, CPSF4F2F3; Protein Data Bank (PDB) code 2RHK) suggested that the hydroxyl group of Tyr103 may not fit correctly into the hydrophobic pocket formed by side chains of Pro111, Phe112, Leu72, and Tyr88 of CPSF4 (Fig. 4C) and, therefore, could destabilize CPSF4-NS1 H5N2 interaction.
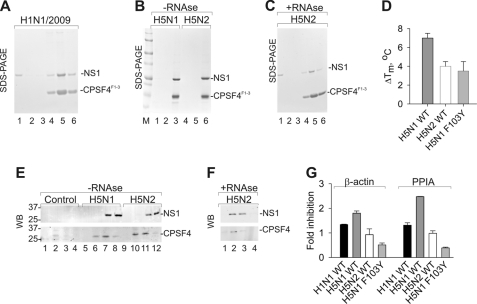
FIGURE 3.
Differential effects of NS1 proteins in pre-mRNA processing. A, NS1 of H1N1/2009 interacts with CPSF4F1–3. His-tagged CPSF4F1–3 and untagged NS1 were incubated with Ni-NTA-agarose beads. Unbound polypeptides were removed with buffer containing 20 mm imidazole/1 M NaCl (lane 1) and 50 mm imidazole/50 mm NaCl (lane 2). Remaining proteins were eluted with TN buffers containing 100 (lane 3), 150 (lane 4), 200 (lane 5), and 250 mm imidazole (lane 6) and analyzed on SDS-PAGE. B, purified His-tagged CPSF4F1–3 and untagged NS1 of H5N1 and H5N2 co-elute from Ni-NTA-agarose beads. Unbound polypeptides were removed with buffer containing 20 mm imidazole/1 m NaCl (lanes 1 and 4) and 50 mm imidazole/50 mm NaCl (lanes 2 and 5). Remaining proteins were eluted with TN buffers containing 250 mm imidazole (lanes 3 and 6). C, RNase treatment of purified His-tagged CPSF4F1–3 and H5N2 NS1 does not affect their subsequent co-purification on Ni-NTA resin. Lanes 1 and 2, flow-through from the beads; lane 3, wash; lane 4, elution with 50 mm imidazole; lane 5, elution with 100 mm imidazole; lane 6, elution with 250 mm imidazole. D, difference in thermal stability (ΔTm) between NS1·CPSF4F1–3 complexes and NS1 alone. Complexes of CPSF4F1–3 with wild-type or mutant F103Y NS1 of H5N1 or wild-type H5N2 NS1 were eluted form Ni-NTA-agarose resin, and their Tm values were measured. Tm of NS1 proteins alone were measured and subtracted from Tm of their complexes with CPSF4F1–3. E, H5N1 and H5N2 NS1 proteins co-elute with CPSF4 from an A549 cell lysate. Western blot of affinity purified proteins from A549 cells expressing His-tagged NS1 of HP H5N1, LP H5N2, or GFP. Cells were disrupted 24 h post transfection. Lysates were incubated with Ni-NTA-agarose beads. Unbound proteins were eluted from beads with TN buffers containing 20 mm imidazole (lanes 1, 5, and 9). Remaining proteins were eluted with TN buffers containing 50 (lanes 2, 6, and 10), 100 (lanes 3, 7, and 11), and 250 mm imidazole (lanes 4, 8, and 12). The fractions were resolved by SDS-PAGE, followed by Western blotting using antibodies against His tag and CPSF4. F, Western blot (WB) showing co-purification of NS1 of H5N2 and CPSF4 from the lysate of A549 cells treated with RNase A. G, recombinant NS1 proteins differentially affect polyadenylation of β-actin and peptidylprolyl isomerase A (PPIA) pre-mRNA in A549 cells. A549 cells were transfected with plasmids encoding wild-type H5N2 NS1, H1N1/2009, H5N1, or F103Y mutant of H5N1 NS1. Twenty four hours post transfection, RNA was purified from the cells. Four pools of cDNA obtained by RT with the dT20 primer were used to estimate the amount of the polyadenylated RNA, and another four pools of cDNA were synthesized with random primers and served as a reference for total RNA. Levels of the β-actin and peptidylprolyl isomerase A cDNAs were calculated by quantitative PCR. The ratios between polyadenylated and total RNA were calculated in cells transfected with NS1-expressing constructs and then normalized against the ratio obtained with cells transfected with the control vector. A value >1 indicates that NS1 inhibited the polyadenylation of pre-mRNA. The results show means of three independent experiments.
To test this hypothesis, we reconstituted NS1·CPSF4 complexes from purified recombinant CPSF4F1–3 and NS1 of H5N1 or H5N2 (Fig. 3B). These complexes were resistant to RNase treatment, indicating that NS1 proteins interact directly with CPSF4 in vitro (Fig. 3C). Thermal denaturation analysis showed that although the Tm of NS1 proteins were identical, the Tm of their complexes with CPSF4F1–3 differed by 3 degrees (Fig. 3D). The NS1·CPSF4F1–3 complex containing H5N2 NS1 had lower thermal stability than the complex containing H5N1 NS1. This was in line with our observation that the substitution F103Y destabilized the CPSF4F1–3·NS1 H5N1 complex. These results suggest that Tyr103 of H5N2 NS1 destabilizes its complex with CPSF4 by preventing hydrophobic bonding between NS1 ED and CPSF4F2F3.
To test whether H5N2 NS1 interacts with CPSF4 in human cells, A549 cells expressing His-tagged H5N2 NS1 were disrupted, and proteins were purified on Ni-NTA-agarose resin. In control experiments, proteins were purified from the cells transfected with the control vector or plasmid expressing H5N1 NS1. Western blot analysis showed that CPSF4 together with H5N2 and H5N1 NS1 could be eluted from the resin with high imidazole (Fig. 3E), indicating that both NS1 proteins interact with CPSF4 in vivo. Moreover, these complexes were resistant to RNase treatment, indicating that NS1 proteins interact directly with CPSF4 in A549 cells (Fig. 3F). Altogether, these experiments showed that the NS1 proteins analyzed here bind CPSF4 in vitro and in cell culture, but the resulting NS1·CPSF4 complexes differ in stability.
To study the effects of Tyr103 of H5N2 NS1 protein on polyadenylation of cellular pre-mRNA, A549 cells were transfected with plasmids encoding wild-type His-tagged H5N2 NS1. In the control experiments, cells were transfected with the control vector or plasmids expressing NS1 of H5N1, its F103Y mutant or NS1 of H1N1/2009. These plasmids also expressed GFP from a bicistronic mRNA. GFP fluorescence was used to reach the transfection efficiency of 70%. Twenty four hours post transfection, RNA was purified from the cells. Four pools of cDNA obtained by RT with the dT20 primer were used to estimate the amount of the polyadenylated RNA, another four pools of cDNA were synthesized with random primers and served as a reference for total RNA. Levels of the β-actin and peptidylprolyl isomerase A cDNAs were calculated by quantitative PCR. The ratios between polyadenylated and total RNA were calculated in cells transfected with NS1-expressing constructs and then normalized against the ratio obtained with cells transfected with the control vector. The effect of the different NS1 proteins on polyadenylation of β-actin and peptidylprolyl isomerase A pre-mRNA is shown in Fig. 3G. A reproducible increase in inhibition of polyadenylation of two cellular pre-mRNAs was observed upon H5N1 NS1 transfection in A549 cells, whereas H5N2 NS1 and F103Y mutant of H5N1 as well as H1N1/2009 NS1 were almost unable to inhibit polyadenylation of the pre-mRNAs (Fig. 3G). Thus, the F103Y mutation present in the H5N2 NS1 attenuates polyadenylation inhibition.
Altogether, these experiments show that recombinant NS1 proteins of the analyzed influenza strains differ in their ability to inhibit pre-mRNA polyadenylation of two cellular pre-mRNAs. This difference may partly correlate with the amino acid at position 103 that contributes to the structural stability of the NS1·CPSF4 complex. We conclude that NS1·CPSF4 complex stability is important for inhibition of cellular pre-mRNA processing.
DISCUSSION
NS1 protein ensures efficient influenza A virus replication for almost 2 days without detection by the host immune system (4), taking advantage of its multiple interactions with host factors (Table 1). It has been proposed that NS1 proteins of different influenza A virus strains differentially affect basic cellular processes (18). Using highly purified NS1 proteins we showed here that the NS1 of human pandemic H1N1/2009, avian HP H5N1 and LP H5N2 strains differentially affect cellular pre-mRNA polyadenylation and mRNA translation. This finding allowed us to identify key amino acids within NS1 that contribute to its structural stability and interactions with host CPSF4 and translation factors during pre-mRNA polyadenylation and mRNA translation, respectively.
TABLE 1.
Sequence identity and similarity between human and chicken NS1-interacting partners
| NS1 cellular partner | Identity/similarity (%) | Ref. |
|---|---|---|
| CPSF4 | 96/98 | 6 |
| PABC | 98/98 | 20 |
| eIF4GI | 45/55 | 21 |
| PABPN1 | 45/54 | 22 |
| Staufen1 | 62/66 | 23 |
| CrkL | 92/96 | 24 |
| ISG15 | 31/50 | 25 |
| NS1-BP | 91/96 | 26 |
| Nucleolin | 63/76 | 27 |
| NXF1 | 12/13 | 28 |
| NXT1/NTF2-related export protein 2 | 80/93 | 28 |
| Rae1 | 55/58 | 28 |
| E1B-AP5 | 38/49 | 28 |
| PI3K (p85ß) | 71/81 | 29 |
| PKR | 37/52 | 30 |
| TRIM25 | 48/65 | 31 |
Different Structural Stability of NS1 Proteins
Purified NS1 proteins of human pandemic H1N1/2009 and avian HP H5N1 and LP H5N2 strains have similar oligomeric status but differ in their thermal stability. The NS1 of the pandemic H1N1/2009 had a significantly higher melting temperature than the H5N1 and H5N2 NS1. H1N1/2009 NS1 in contrast to NS1 of avian strains has hydrophobic residues at positions 67, 84, 112, 119, and 171 (Fig. 1A). According to the available structures of NS1 (PDB codes 3EE8, 3EE9, 3F5T, 3D6R, and 2GX9), amino acids 67, 112, and 171 reside in the hydrophobic core of RBD or ED (Fig. 4A). H1N1/2009 NS1 residues Trp67, Ile112, and Tyr171, but not Arg/Asp67, Thr112, and Thr/Asn171 of H5N1/H5N2 NS1 could be involved in the stabilization of NS1 hydrophobic core via binding to conserved hydrophobic residues 27, 68, 116, 141, and 202 (Fig. 4, A and B). However, the R67W mutation introduced into H5N1 NS1 did not increase NS1 stability (TmR67W = 49.0 °C and TmWT = 50.5 °C).6 Therefore, Ile112 and Tyr171 could increase structural stability of H1N1/2009 NS1. Several residues on the surface of NS1 monomer could also contribute to structural stability of the NS1 dimer and perhaps multimer because it was shown that NS1 is able to form long filaments along double-stranded RNA molecules (8). The enhanced structural stability of H1N1/2009 NS1 oligomers in comparison with H5N1 and H5N2 NS1 may result in the depletion of “active” NS1 molecules from reactions, i.e. cellular pre-mRNA polyadenylation and viral mRNA translation. Altogether, our results showed that NS1 proteins of different influenza strains differ in their structural stability.
Differential Effects of NS1 Proteins in Translation
It has been shown that NS1 enhances the translation of mRNA in infected cells (7, 13). Here, we used an in vitro translation system to compare activities of purified recombinant NS1 proteins and to provide molecular insight into this process. We demonstrated that NS1 of avian HP H5N1 and LP H5N2, but not human pandemic H1N1/2009, efficiently stimulated translation of reporter mRNA. Surprisingly, the RNA-binding residues Arg38 and Lys41 of NS1 were not essential for the stimulation of translation.
It has been shown that the 1–114 amino acid region of NS1 is required for the stimulation of viral mRNA translation in transfected COS-1 cells (19). We demonstrate that an even shorter region of NS1 (amino acids 1–74) is required for the stimulation of mRNA translation in rabbit reticulocyte lysates. Moreover, we found that residues at positions 25/26, 48, and 67 are involved in translational stimulation (Fig. 4A). Because these residues are solvent exposed in NS1 structures, we hypothesized that the stimulation of translation by NS1 is mediated by interaction of NS1 with translation factors, PABC being a likely candidate because it binds the 1–81 amino acid region of NS1 (20). Altogether, these results demonstrate that stimulation of translation by NS1 is strain-specific and that amino acids at positions 25/26, 48, and 67 contribute to stimulation efficiency via interaction with host translation factors.
Differential Effects of NS1 Proteins in Pre-mRNA Processing
It has been shown that NS1 inhibits post-transcriptional 3′-end processing of cellular pre-mRNA by binding evolutionary conserved CPSF4 (Table 1), thereby blocking host gene expression (6, 14). However, NS1 protein of pandemic H1N1/2009 strain was unable to block general host gene expression, despite its binding to CPSF4 (32). We found that NS1 of H5N2 and H5N1 also bind CPSF4 in vitro and in vivo; however, NS1 of H5N2, like H1N1/2009 NS1, was unable to inhibit the polyadenylation of two host pre-mRNAs. We hypothesized that this effect may be associated with Tyr at position 103 in H5N2 NS1, which hydroxyl group may affect the interaction between NS1 ED and the CPSF4F2F3 (Fig. 4C). We showed that Tyr103, in contrast to Phe, affected the NS1·CPSF4 complex stability and weakened the inhibition of polyadenylation of two cellular pre-mRNAs. This observation is in line with previous study, in which Leu at position 103 also destabilized the NS1-CPSF4 interaction and weakened the inhibition of cellular pre-mRNA polyadenylation (15, 17). Thus, amino acid at position 103 in NS1 is essential for NS1·CPSF4 complex stability and inhibition of cellular pre-mRNA polyadenylation.
The NS1 protein of influenza A virus influences multiple cellular events that are important for virus replication. NS1 inhibits polyadenylation of host pre-mRNAs in the nucleus of infected cells (6). This ultimately leads to an impaired synthesis of cellular proteins in the cytoplasm and thereby to an attenuation of the host immune response. We showed here that NS1 of avian HP H5N1, in contrast to human pandemic H1N1/2009 and avian LP H5N2, tightly controls cellular pre-mRNA polyadenylation. NS1 of avian HP H5N1 and LP H5N2 but not human pandemic H1N1/2009 strains stimulate the translation of mRNA. Thus, NS1 of HP H5N1, in contrast to NS1 of human pandemic H1N1/2009 and avian LP H5N2 strains, may contribute to increased virus proliferation. In conclusion, our results suggest strain-specific ways in which different NS1 proteins influence cellular pre-mRNA processing and mRNA translation to ensure preferential and efficient virus replication and identify critical amino acids of NS1 proteins that contribute to replication efficiency of influenza A virus strains.
Acknowledgments
Chicken cDNA was a kind gift from Lukasz Kowalik. Yeast tRNAAsp was a kind gift from Anne-Catherine DockBregeon. pNic28-Bsa4 vector was a gift from Opher Gileadi. We thank Aurélie Sausy, Emilie Charpentier, Sébastien De Landtsheer, and Maxim Bespalov for technical support and Andrey Golubtsov for critical reading of the manuscript.
This work was supported by a grant from the Centre de Recherche Public-Santé, the European Cooperation in Science and Technology-B28, Erkko, the National Research Fund (Luxembourg), and Center for International Mobility Ph.D. fellowships.
N. Gerloff, unpublished data.
C. Snoeck, unpublished data.
D. E. Kainov, K. H. Müller, L. L. Theisen, M. Anastasina, M. Kaloinen, and C. P. Muller, unpublished data.
- RBD
- RNA-binding domain
- ED
- effector domain
- HP
- highly pathogenic
- LP
- low pathogenic
- Ni-NTA
- nickel-nitrilotriacetic acid
- RK/AA
- R38A/K41A
- PDB
- Protein Data Bank.
REFERENCES
- 1. Gambotto A., Barratt-Boyes S. M., de Jong M. D., Neumann G., Kawaoka Y. (2008) Lancet 371, 1464–1475 [DOI] [PubMed] [Google Scholar]
- 2. Kuiken T., Holmes E. C., McCauley J., Rimmelzwaan G. F., Williams C. S., Grenfell B. T. (2006) Science 312, 394–397 [DOI] [PubMed] [Google Scholar]
- 3. Scholtissek C. (1994) Eur. J. Epidemiol. 10, 455–458 [DOI] [PubMed] [Google Scholar]
- 4. Moltedo B., López C. B., Pazos M., Becker M. I., Hermesh T., Moran T. M. (2009) J. Immunol. 183, 3569–3573 [DOI] [PubMed] [Google Scholar]
- 5. Ehrhardt C., Seyer R., Hrincius E. R., Eierhoff T., Wolff T., Ludwig S. (2010) Microbes Infect. 12, 81–87 [DOI] [PubMed] [Google Scholar]
- 6. Nemeroff M. E., Barabino S. M., Li Y., Keller W., Krug R. M. (1998) Mol. Cell 1, 991–1000 [DOI] [PubMed] [Google Scholar]
- 7. de la Luna S., Fortes P., Beloso A., Ortín J. (1995) J. Virol. 69, 2427–2433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bornholdt Z. A., Prasad B. V. (2008) Nature 456, 985–988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Owoade A. A., Gerloff N. A., Ducatez M. F., Taiwo J. O., Kremer J. R., Muller C. P. (2008) Emerg. Infect Dis. 14, 1731–1735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kainov D. E., Selth L. A., Svejstrup J. Q., Egly J. M., Poterzsman A. (2010) DNA Repair 9, 33–39 [DOI] [PubMed] [Google Scholar]
- 11. Kainov D. E., Pirttimaa M., Tuma R., Butcher S. J., Thomas G. J., Jr., Bamford D. H., Makeyev E. V. (2003) J. Biol. Chem. 278, 48084–48091 [DOI] [PubMed] [Google Scholar]
- 12. Schote A. B., Turner J. D., Schiltz J., Muller C. P. (2007) Mol. Immunol. 44, 1436–1445 [DOI] [PubMed] [Google Scholar]
- 13. Salvatore M., Basler C. F., Parisien J. P., Horvath C. M., Bourmakina S., Zheng H., Muster T., Palese P., García-Sastre A. (2002) J. Virol. 76, 1206–1212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Noah D. L., Twu K. Y., Krug R. M. (2003) Virology 307, 386–395 [DOI] [PubMed] [Google Scholar]
- 15. Twu K. Y., Noah D. L., Rao P., Kuo R. L., Krug R. M. (2006) J. Virol. 80, 3957–3965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Das K., Ma L. C., Xiao R., Radvansky B., Aramini J., Zhao L., Marklund J., Kuo R. L., Twu K. Y., Arnold E., Krug R. M., Montelione G. T. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 13093–13098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kuo R. L., Krug R. M. (2009) J. Virol. 83, 1611–1616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hale B. G., Randall R. E., Ortín J., Jackson D. (2008) J. Gen. Virol. 89, 2359–2376 [DOI] [PubMed] [Google Scholar]
- 19. Marión R. M., Aragón T., Beloso A., Nieto A., Ortín J. (1997) Nucleic. Acids Res. 25, 4271–4277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Burgui I., Aragón T., Ortín J., Nieto A. (2003) J. Gen. Virol. 84, 3263–3274 [DOI] [PubMed] [Google Scholar]
- 21. Aragón T., de la Luna S., Novoa I., Carrasco L., Ortín J., Nieto A. (2000) Mol. Cell. Biol. 20, 6259–6268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chen Z., Li Y., Krug R. M. (1999) EMBO J. 18, 2273–2283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Falcón A. M., Fortes P., Marión R. M., Beloso A., Ortín J. (1999) Nucleic Acids Res. 27, 2241–2247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Heikkinen L. S., Kazlauskas A., Melén K., Wagner R., Ziegler T., Julkunen I., Saksela K. (2008) J. Biol. Chem. 283, 5719–5727 [DOI] [PubMed] [Google Scholar]
- 25. Zhao C., Hsiang T. Y., Kuo R. L., Krug R. M. (2010) Proc. Natl. Acad. Sci. U.S.A. 107, 2253–2258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wolff T., O'Neill R. E., Palese P. (1998) J. Virol. 72, 7170–7180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Murayama R., Harada Y., Shibata T., Kuroda K., Hayakawa S., Shimizu K., Tanaka T. (2007) Biochem. Biophys. Res. Commun. 362, 880–885 [DOI] [PubMed] [Google Scholar]
- 28. Satterly N., Tsai P. L., van Deursen J., Nussenzveig D. R., Wang Y., Faria P. A., Levay A., Levy D. E., Fontoura B. M. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 1853–1858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hale B. G., Kerry P. S., Jackson D., Precious B. L., Gray A., Killip M. J., Randall R. E., Russell R. J. (2010) Proc. Natl. Acad. Sci. U.S.A. 107, 1954–1959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Li S., Min J. Y., Krug R. M., Sen G. C. (2006) Virology 349, 13–21 [DOI] [PubMed] [Google Scholar]
- 31. Gack M. U., Albrecht R. A., Urano T., Inn K. S., Huang I. C., Carnero E., Farzan M., Inoue S., Jung J. U., García-Sastre A. (2009) Cell Host Microbe 5, 439–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hale B. G., Steel J., Medina R. A., Manicassamy B., Ye J., Hickman D., Hai R., Schmolke M., Lowen A. C., Perez D. R., García-Sastre A. (2010) J. Virol. 84, 6909–6922 [DOI] [PMC free article] [PubMed] [Google Scholar]