Abstract
To investigate the biochemical mechanism underlying the effect of sterol deprivation on longevity in Caenorhabditis elegans, we treated parent worms (P0) with 25-azacoprostane (Aza), which inhibits sitosterol-to-cholesterol conversion, and measured mean lifespan (MLS) in F2 worms. At 25 μm (∼EC50), Aza reduced total body sterol by 82.5%, confirming sterol depletion. Aza (25 μm) treatment of wild-type (N2) C. elegans grown in sitosterol (5 μg/ml) reduced MLS by 35%. Similar results were obtained for the stress-related mutants daf-16(mu86) and gas-1(fc21). Unexpectedly, Aza had essentially no effect on MLS in the stress-resistant daf-2(e1370) or mitochondrial complex II mutant mev-1(kn1) strains, indicating that Aza may target both insulin/IGF-1 signaling (IIS) and mitochondrial complex II. Aza increased reactive oxygen species (ROS) levels 2.7-fold in N2 worms, but did not affect ROS production by mev-1(kn1), suggesting a direct link between Aza treatment and mitochondrial ROS production. Moreover, expression of the stress-response transcription factor SKN-1 was decreased in amphid neurons by Aza and that of DAF-28 was increased when DAF-6 was involved, contributing to lifespan reduction.
Keywords: Aging, Cholesterol, Insulin, Reactive Oxygen Species (ROS), Sterol, Azacoprostane
Introduction
Sterols are important molecules involved in membrane organization, hormone production, and signal processing. Because Caenorhabditis elegans lacks a de novo sterol biosynthesis pathway, it requires dietary cholesterol or plant sterols (e.g. sitosterol) that can be converted to cholesterol or its most abundant sterol, 7-dehydrocholesterol (1, 2) (Fig. 1A). Sterol depletion experiments have revealed that a restriction in the sterol supply caused by inadequate nutrition produces serious defects in development and reduces the longevity of C. elegans (3–7). For example, sterol starvation leads to an increase in embryonic lethality (7) and a decrease in lifespan (∼40%) in wild-type N2 worms (6). Most of the sterol depletion phenotype also occurs when worms are grown in the presence of sitosterol as a sterol nutrient and 25-azacoprostane (Aza),2 an inhibitor of sterol C24-reductase and dealkylation of desmosterol during its conversion to cholesterol (8). Using proteomic approaches, our laboratory has previously shown that defects in development and growth in C. elegans caused by Aza treatment are associated with changes in the abundance of many proteins (3). The major proteins influenced by Aza treatment were the lipoproteins VIT-2 and VIT-6 and their putative receptors RME-2 and LRP-1, which were previously known to respond to sterols (3). Recently, the endogenous ligands of DAF-12 have been discovered to be 3-keto bile acid-like steroids, called Δ4- and Δ7-dafachronic acids (9). Dafachronic acids and related metabolites regulate longevity and stress resistance (9–11). DAF-36, a Rieske-like oxygenase, and DAF-9, a cytochrome P450 enzyme, produce dafachronic acid ligands that activate the DAF-12 nuclear receptor (9). It was also reported that methyltransferase STRM-1 modifies sterol substrates for the synthesis of dafachronic acid (12).
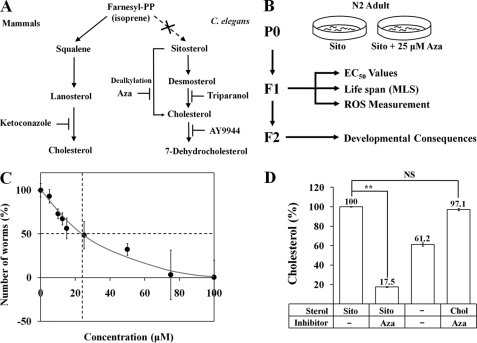
FIGURE 1.
The development of C. elegans is affected by the sterol biosynthesis inhibitor Aza. A, the sterol biosynthesis pathway and sites of action of four sterol biosynthesis inhibitors in the nematode C. elegans and mammals. C. elegans has no de novo sterol biosynthesis pathway, but can utilize various plant sterols (e.g. sitosterol, stigmasterol) by converting them via sterol dealkylation into 7-dehydrocholesterol and then by 7-dehydrocholesterol reductase into cholesterol for normal growth and development. B, experimental flow chart (Sito, Sitosterol). C, total number of offspring plotted against the Aza concentration administered to wild-type N2 worms. The solid black vertical lines represent a 50% reduction in the total number of offspring (EC50 = 23.81 μm). D, total cholesterol levels were measured in the adult stage using a cholesterol oxidase-based procedure and normalized to total protein. Each value represents the average of three independent experiments with three replicates of worms in each condition. Error bars represent the S.D., and p values were determined using Student's t test (**, p ≤ 0.01 versus sitosterol without Aza; NS, not significant).
Oxidative stress, or the chronic generation of reactive oxygen species (ROS), is thought to contribute to the progression of various human diseases including type 2 diabetes. Mitochondrial dysfunction impairs insulin signaling both directly and indirectly through generation of excess ROS. Mitochondrial ROS generation is known to activate protein kinase signaling pathways that suppress insulin signaling downstream of the insulin receptor at the level of insulin receptor substrate-1 and phosphatidylinositol 3-kinase to promote “insulin resistance” (13, 14). ROS have a potential role for enhancement of insulin signaling in vivo (15) but are generated in mitochondria during normal respiration (16). Although the relationship between ROS effects and respiratory rate in C. elegans is very complicated (17, 18) and remains controversial (19), ROS are believed to decrease the lifespan in C. elegans by damaging cellular components (20, 21). For example, the mev-1 and gas-1 mitochondrial complex mutants exhibit increased ROS levels and have a shorter mean lifespan (MLS) (22, 23). Conversely, a reduced respiratory rate can decrease ROS and increase the lifespan of C. elegans (24). Increased ROS also leads to increased translocation of DAF-16 to the nucleus (25, 26).
Sterols have recently been suggested to help protect against O2 and/or ROS. For example, depletion of cholesterol from red blood cells increased their vulnerability to peroxidation (27). In Schizosaccharomyces pombe, where the biosynthesis of sterols is dependent on O2, sterols can serve as O2 sensors (28, 29). Therefore, sterols serve not only as essential molecules for development and aging but also protect cells from ROS attack. These results obtained from other organisms led us to hypothesize that depletion of environmental sterol may increase susceptibility to cellular ROS attacks in C. elegans.
It is well established that insulin/IGF-1-like signaling (IIS) (30–32) is one of the key regulatory systems controlling C. elegans longevity. Specifically, a reduction in IIS has been shown to enhance stress resistance and increase MLS through DAF-16 activation (33). Accordingly, daf-2 and age-1 mutants deficient in IIS have extended lifespans (30, 32). IIS not only inhibits DAF-16 but also directly suppresses SKN-1, which defends against oxidative stress by regulating the conserved phase II detoxification response (34, 35). The stress-resistance function of SKN-1 is mediated by its expression in the intestine (34, 36), although it is also present in nuclei of ASI neurons, where it is required for dietary restriction-induced lifespan extension (36). Among the 40 predicted insulin ligands in C. elegans (37–39), DAF-28, an agonist for the insulin receptor DAF-2, is influenced by sensory neurons (38), and is down-regulated under dauer-inducing conditions, and the lifespan of daf-28(sa191) mutant worms is increased (38, 40). Moreover, DAF-28 expression is increased in daf-6(lf) mutants (38).
daf-6 encodes a patched-related protein containing a sterol-sensing domain (SSD) (41) consisting of ∼180 amino acids organized into a cluster of five consecutive membrane-spanning domains. In C. elegans, the Niemann-Pick disease type C1 homologs ncr-1 and ncr-2 are involved in intracellular cholesterol processing (42, 43), further indicating the importance of C. elegans SSDs in cholesterol homeostasis and cholesterol transport.
Although numerous studies have reported that sterol depletion mediates physiological changes and that IIS profoundly influences the longevity of C. elegans, the biochemical and cellular mechanism by which sterol deprivation exerts its effects and interacts with IIS remains elusive. To determine the key biochemical mechanism underlying sterol-deprivation stress effects on C. elegans longevity, we assessed MLS and ROS levels in wild-type N2 and mutant C. elegans strains treated with Aza, an inhibitor of the conversion of sitosterol to cholesterol. Our data suggest that the stress caused by Aza-mediated sterol depletion leads to activation of IIS and mitochondrial dysfunction, resulting in increased ROS production and reduced MLS in C. elegans.
EXPERIMENTAL PROCEDURES
C. elegans Strains and Culture
The following mutant strains were obtained from the Caenorhabditis Genetics Center: N2, TK22 [mev-1(kn1)], CB1377 [daf-6(e1377)], FK129 [tax-4(ks11)], CF1407 [daf-16(mu86) I; muls71]. The transgenic worm IS007 was kindly provided by Dr. T. K. Blackwell, Harvard Medical School. Worms were grown on nematode growth medium plates. Heat-killed Escherichia coli OP50 was added as a food source.
Measurement of Aza Toxicity
The effects of four sterol biosynthesis inhibitors (AY9944, 25-azacoprostane hydrochloride, triparanol, and ketoconazole) on embryogenesis were tested by assessing embryonic lethality. N2 adults (P0) were grown on nematode growth medium plates in the presence of 5 μg/ml of sitosterol and different concentrations of Aza. L4 F2 progeny were transferred to a new plate and then embryonic lethality was calculated. Lethality was measured by allowing adult worms to lay eggs overnight at 20 °C, after which the adults were removed and the percentage of eggs that hatched after 1 day were determined. Each experiment was repeated three times and at least 100 eggs were counted in each case. The results represent the average of more than three experiments, and the bars represent standard deviations. We also determined Aza toxicity (EC50) in an index-of-reproduction assay as previously described (44), with slight modifications.
Cholesterol Quantification Assay
Total cholesterol levels were determined using an Amplex Red Cholesterol Assay Kit (Invitrogen, Calsbad, CA). Synchronized populations were obtained by allowing adults to lay eggs at 20 °C for 5–6 h on plates with or without Aza. After 4 days, samples were collected and homogenized in 100 μl of 1× buffer included in the kit. The homogenate was centrifuged at 5000 × g for 5 min, and the supernatant was transferred to a new tube. A 10-μl aliquot was assayed according to the kit instructions and measured in an Infinite F500 microplate reader (Tecan Group Ltd., Mannedorf, Switzerland) with a 535-nm excitation filter and a 580-nm emission filter. Cholesterol levels were normalized to protein in each homogenate using a Bradford assay (Bio-Rad, Hercules, CA). Samples were prepared in triplicate; each experiment was repeated at least two times; and the resulting data were pooled. Data were analyzed using unpaired two-tailed Student's t tests with unequal variance (45).
Measurement of Intracellular ROS in C. elegans
Intracellular ROS in C. elegans were measured using 2′,7′-dichlorodihydrofluorescein diacetate (H2DCF-DA) as a molecular probe (Molecular Probes Inc., Eugene, OR). For ROS detection under normal culture conditions, worms that had just reached adulthood were treated with or without 25 μm Aza for 4 days. At the end of the treatment period, worms were collected into 100 μl of phosphate-buffered saline (PBS) with 1% Tween 20 in microcentrifuge tubes. The worms were then sonicated (UP200S, Hielscher Ultrasonics GmbH, Teltow, Germany) and pipetted into microcentrifuge tubes containing H2DCF-DA (final concentration, 50 μm in PBS). Samples were read every 30 min for 1 h at 37 °C in a fluorescence spectrophotometer (SFM-25, Kontron Instruments AG, Zurich, Switzerland) (46) with excitation at 485 nm and emission at 530 nm.
Oxidative Stress Resistance
The young adult worms were transferred to an M9 solution containing 100 mm paraquat (1,1′-dimethyl-4,4′-bipyridinium dichloride) (Sigma, St. Louis, MO) and incubated at 20 °C. Dead worms were counted every 5 h up to the 20-h time point. Thirty worms from each strain were treated with paraquat, and the experiment was performed three times.
GFP Expression Assay
daf-16(mu86) muls71[DAF-16a::GFP], Is007[SKN-1::GFP], and dpy-20 Ex[DAF-28::GFP] worms were transferred to plates with or without Aza. After 3–4 days, the expression of green fluorescent protein (GFP) was visualized using a Zeiss Axioscope microscope. Three independent experiments were performed and used for data processing.
Lifespan Measurement
Synchronized populations were obtained by allowing adults to lay eggs at 20 °C for 5–6 h on plates with or without Aza. After reaching the L4 stage, worms were transferred onto nematode growth medium plates (with heat-killed E. coli OP50) containing floxuridine (80 μm) and Aza (25 μm). The number of live animals was scored every 1–2 days until death, which was defined as the failure to respond to gentle prodding on the head and tail with a platinum wire.
RNA Isolation and Real Time Quantitative Reverse Transcription-Polymerase Chain Reaction (qRT-PCR)
Total RNA was isolated with an RNAspin Mini isolation kit (GE Healthcare, Piscataway, NJ). Total RNA was reverse transcribed using the Transcriptor First Strand cDNA Synthesis Kit (Roche Diagnostics Corp., Indianapolis, IN). The cDNA was quantified using a Nanodrop spectrophotometer (ND-1000) and then used in qRT-PCR. A total of 500 ng of cDNA per sample was used in a total volume of 50 μl, and reactions were performed in an MJ Research Chromo4 Detector using the QuantiTect SYBR Green PCR kit as described by the manufacturer (Qiagen). Targets (INS-1, INS-7, INS-18, and DAF-28) were amplified from treated samples using specific primers (supplemental Table S1). Amplification and analysis of mRNA were performed in triplicate, and mRNA levels in the tested strains were normalized to those in the N2 strain and compared with each other. Serially diluted N2 cDNA (500 to 0.05 ng) was used to construct a standard curve for qRT-PCR using β-actin as an internal control.
Statistics
Unless otherwise specified, the data presented throughout are the mean ± S.D. from triplicate determinations obtained in three independent experiments.
RESULTS
Acute Toxicity of Aza on C. elegans Development
The disturbance of sterol metabolism in C. elegans by Aza toxicity has been well documented (1, 3, 47, 48). Our investigation addressed the biochemical and cellular mechanism underlying Aza toxicity, focusing on Aza toxicity in the second generation (F2) after initial Aza treatment of parental worms (P0). To this end, we modified our previous experimental procedures as outlined in Fig. 1B such that worms were grown in the presence of sitosterol and 25 μm Aza on a plate instead of in liquid culture. Sitosterol, a commonly used sterol source for C. elegans culture, was included in the medium as a supplement throughout these toxicity experiments (1). On the basis of preliminary experiments in which parental worms (P0) were treated with several doses of Aza (0–100 μm), we selected a concentration of 25 μm (10 μg/ml) for routine assessment of Aza toxicity on C. elegans because this is approximately the EC50 of Aza with respect to the reduction in the number of offspring (23.81 μm; Fig. 1C). This dose of Aza (25 μm) was also found to profoundly reduce total body cholesterol (by 82.5%), producing sterol-deprivation conditions (Fig. 1D). There was essentially no toxic effect of Aza on C. elegans when cholesterol was used as the main sterol source (Fig. 1D).
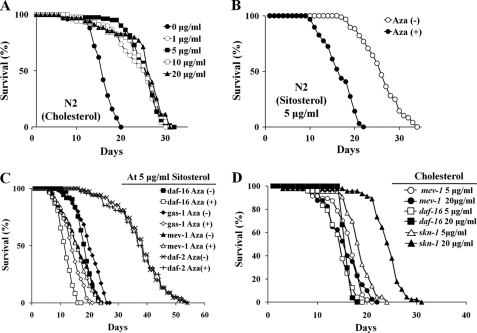
As a prelude to determining whether other biological consequences were associated with Aza toxicity at 25 μm, we first measured the MLS of C. elegans (n = 50/group) that had been grown in different concentrations (0, 1, 5, 10, and 20 μg/ml) of cholesterol without Aza. N2 strain worms grown in the absence of cholesterol showed about a 40% reduction in MLS, whereas all other groups showed essentially the same normal MLS (∼25.09 days), suggesting that as long as worms receive >1 μg/ml of cholesterol, their MLS remained unchanged. Treatment of N2 worms (n ≥ 90) with 25 μm Aza in the presence of 5 μg/ml of sitosterol caused a 35% reduction in MLS (16.5 days) compared with those grown in the presence of sitosterol alone (25.2 days; Fig. 2B and Table 1). Interestingly, similar reductions in MLS (∼35–38%) were observed for stress-sensitive daf-16(mu86) and gas-1(fc21) mutants grown in the presence of 25 μm Aza. In contrast, no significant changes in MLS were observed in the stress-resistant daf-2(e1370) strain (−3.1%) or the mev-1(kn1) mitochondrial complex II mutant strain (−2.8%; Fig. 2C and Table 1). Furthermore, MLS of both daf-16(mu86) and skn-1(zu67) mutants was increased as the cholesterol concentration in the medium was elevated (17% increase in daf-16(mu86) and 26% increase in skn-1(zu67), Fig. 2D). This result indicates that cholesterol profoundly influences downstream genes of IIS, DAF-16, and SKN-1. Thus, cholesterol depletion by Aza may target both IIS and mitochondrial complex II.
FIGURE 2.
Effect of Aza on C. elegans longevity. A, MLS of C. elegans grown in different concentrations of cholesterol: 0 μg/ml, 15.96 days; 1 μg/ml, 23.47 days; 5 μg/ml, 25.09 days; 10 μg/ml, 24.3 days; and 20 μg/ml, 25.08 days (n = 50). B, effect of Aza on the longevity of C. elegans grown in the presence of sitosterol. With Aza (●); without Aza (○) (n ≥ 90 each). C, effect of Aza treatment on the longevity of C. elegans mutants grown in the presence of sitosterol. Mean, standard errors, and statistical analyses of independent experiments performed in triplicate are presented in Table 1. D, MLS of C. elegans mutants grown in different concentrations of cholesterol. In mev-1(kn1) mutant, 16.3 (at 5 μg/ml) and 15.5 days (at 20 μg/ml); in daf-16(mu86) mutant, 13.7 (at 5 μg/ml) and 16 days (at 20 μg/ml); in skn-1(zu67), 19 (at 5 μg/ml) and 24 days (at 20 μg/ml) (n = 40).
TABLE 1.
Effect of Aza treatment on the lifespan of C. elegans mutants
These results represent the average of three independent experiments; p values were determined using Student's t test.
| Strain(treatment) | MLS (±S.D.) at 20 °C | Change | p value versus control | Total worms (n in three independent experiments) |
|---|---|---|---|---|
| days | % | |||
| N2(−) | 25.24 ± 0.68 | 96 (31, 32, 33) | ||
| N2(AZA) | 16.47 ± 0.49 | −34.8 | <0.001 | 98 (30, 33, 35) |
| daf-16(mu86)(−) | 18.83 ± 1.07 | 74 (24, 25, 25) | ||
| daf-16(mu86)(AZA) | 11.69 ± 0.59 | −38.0 | <0.001 | 84 (26, 29, 29) |
| mev-1(kn1)(−) | 15.96 ± 0.87 | 116 (39, 37, 40) | ||
| mev-1(kn1)(AZA) | 15.52 ± 0.66 | −2.8 | 0.56 | 133 (41, 46, 46) |
| gas-1(fc21)(−) | 20.12 ± 0.47 | 70 (20, 23, 27) | ||
| gas-1(fc21)(AZA) | 12.98 ± 0.88 | −35.5 | <0.001 | 72 (20, 22, 30) |
| daf-2(e1370) (−) | 37.23 ± 0.68 | 90 (25, 30, 34) | ||
| daf-2(e1370) (AZA) | 36.09 ± 0.69 | −3.1 | 0.65 | 68 (20, 22, 24) |
Aza Toxicity Is Coupled to ROS Production via Mitochondrial Dysfunction
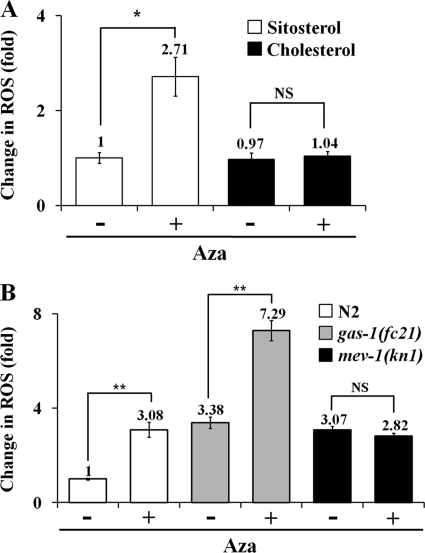
The sterol biosynthesis inhibitor miconazole, which targets eukaryotic lanosterol 14α-demethylase (49), has been previously reported to increase ROS levels (50). To determine whether increased ROS might have contributed to the reduction of the lifespan in the Aza-treated N2 group, we measured ROS production in Aza-treated worms and controls using H2DCF-DA staining (46). We found a 2.71 (± 0.4)-fold increase in ROS levels in Aza-treated worms grown in sitosterol (Fig. 3A), confirming a role for sterol biosynthesis inhibition in ROS production (50). However, worms treated with Aza in the presence of cholesterol exhibited no increase in ROS (Fig. 3A), indicating that Aza treatment not only interrupts the reversible conversion of sitosterol to cholesterol (resulting in cholesterol depletion) (3, 48), but also causes an increase in ROS production through mitochondrial dysfunction (see below). Again, the MLS of Aza-treated worms grown in the presence of cholesterol were not changed (data not shown), confirming the importance of the presence of cholesterol for normal development and aging.
FIGURE 3.
Effects of Aza treatment on ROS production in C. elegans. ROS production was measured using a H2DCF-DA-based fluorometric assay. Worms were grown in the presence of 5 μg/ml of sitosterol or cholesterol plus 25 μm Aza for 4 days, and ROS were measured. A, data are the means of ROS levels in N2 in the presence or absence of Aza. Aza treatment does not directly induce ROS production but instead increases ROS through cholesterol depletion. B, differential production of ROS by Aza treatment in N2 and mev-1(kn1) mutants. Each value represents the average of three independent experiments. Error bars represent the S.D., and p values were determined using Student's t test (**, p ≤ 0.01; *, p ≤ 0.05; NS, not significant).
Because ROS is also generated during normal metabolism in mitochondria (17), we determined the effects of Aza treatment on ROS generation and MLS in the presence of sitosterol in the mev-1(kn1) strain, a mitochondrial complex II mutant with a defective cytochrome b subunit, which is involved in oxidative phosphorylation. This mutant was previously shown to exhibit a reduced MLS, probably due to increased ROS production (51). As shown in Fig. 3B, there was no significant difference in ROS production between Aza-treated and control mev-1(kn1) strain worms (0.92 ± 0.12-fold). In contrast, there was a substantial increase in ROS production in Aza-treated N2 worms (3.08 ± 0.31-fold), and Aza-treated worms of the gas-1(fc21) strain (7.3 ± 0.43-fold), a mitochondrial complex I mutant. In another set of experiments where worms were grown in the medium with different concentrations of cholesterol (0, 5, and 20 μg/ml), it was seen that cholesterol depletion causes reduction in oxidative stress resistance (supplemental Fig. S1). These results clearly indicate that cholesterol depletion (either by Aza treatment or no supplement) increases ROS production in the mitochondria of N2 worms, whereas mev-1(kn1) mutants are insensitive to Aza treatment-mediated cholesterol depletion. Thus, Aza treatment of worms might target mitochondrial complex II, which causes a further increase in ROS (∼3–7-fold) that may subsequently lead to lifespan reduction.
Aza Exerts Its Toxicity by Modulating IIS in ASI Neurons
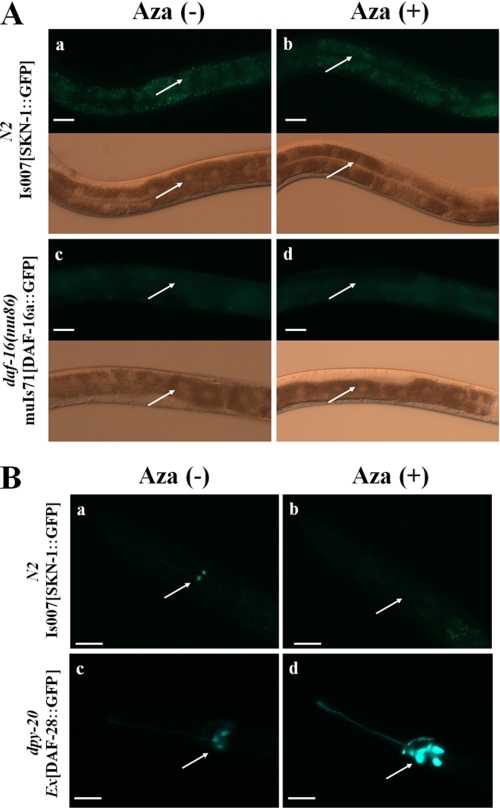
It is well known that a reduction in IIS induces stress resistance and lifespan extension in C. elegans (33), and that cholesterol depletion causes an increase in insulin secretion in mammals (52). Because Aza treatment decreased MLS (35–38%) in N2 worms, and stress-sensitive daf-16(mu86) and gas-1(fc21) mutants (Table 1, Fig. 2, B and C), we tested whether sterol depletion by Aza treatment also caused changes in IIS by measuring changes in the expression and localization of DAF-16 and SKN-1, nuclear-localized transcription factors that are regulated by DAF-2 and oxidative stress (32–34, 53). As shown in Fig. 4A, there was essentially no change in the localization of SKN-1 or DAF-16 in the intestine following treatment of SKN-1::GFP and DAF-16::GFP transgenic worms with 25 μm Aza. However, the expression level of SKN-1, but not DAF-16, was reduced in ASI neurons (Fig. 4B), indicating that the detrimental Aza-induced effect may be independent of DAF-16.
FIGURE 4.
SKN-1 and DAF-28 expression in Aza-treated C. elegans. A, expression of SKN-1 (a, Aza-untreated; b, Aza-treated) and DAF-16 (c, Aza-untreated; d, Aza-treated) in the intestines of adults in the absence or presence of Aza. Arrows indicate intestinal cell nuclei. B, expression of SKN-1 (a, Aza-untreated; b, Aza-treated) and DAF-28 (c, Aza-untreated; d, Aza-treated) in ASI neurons in the absence or presence of Aza. The arrow indicates the ASI neuron. A representative picture from one of three independent experiments (n = 20) is shown. Scale bar = 20 μm.
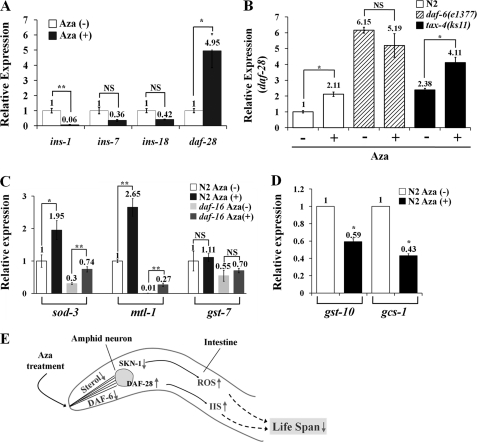
In C. elegans, most insulin ligand candidates are expressed in either ASI neurons or the intestine (39, 54). We selected DAF-28, INS-7, INS-1, and INS-18 from the known insulin ligands and measured changes in their relative expression upon treatment with 25 μm Aza using real time qRT-PCR. As shown in Fig. 5A, mRNA levels of the insulin receptor agonist daf-28 were increased about 5-fold in N2 worms, whereas mRNA levels of the insulin receptor antagonist ins-1 were decreased. Collectively, these results suggest that Aza treatment does not affect DAF-16 expression or localization but causes an increase in DAF-2 signaling in ASI neurons. This conclusion is further supported by the fact that Aza treatment reduced MLS (Fig. 2C and Table 1), and suggests that Aza-mediated sterol depletion causes a decrease in MLS by activating the upstream DAF-2 pathway (Table 1), not by changing DAF-16 expression. Because daf-28 expression is also known to be increased in daf-6(e1377) mutants compared with the N2 strain (38), we examined whether daf-6, which encodes an SSD-containing patched-related protein (41), regulates DAF-28 production under Aza treatment conditions. As shown in Fig. 5B, treatment of worms with Aza caused an increase in daf-28 expression in N2 worms. Although the basal level of daf-28 expression in the daf-6(e1377) mutant was higher than that in N2 in the absence of Aza, daf-28 expression was essentially unchanged by Aza treatment, consistent with previous results (38). However, Aza treatment induced a 1.75 (± 0.33)-fold increase in daf-28 expression in the tax-4(ks11) chemotaxis-defective mutant. Furthermore, Aza treatment of daf-16(mu86) mutants caused an increase in expression of two DAF-16 target genes, sod-3 and mtl-1 (Fig. 5C), indicating that Aza-induced expression of these genes was independent of DAF-16. Expression of gst-7, which encodes a predicted glutathione S-transferase, remained unchanged under the same conditions. However, expression of gcs-1 or gst-10 that are regulated by SKN-1 (34, 35) was decreased by Aza treatment, which is quite contrary to that of sod-3 or mtl-1 (Fig. 5D). This indicates that cholesterol may control SKN-1 expression.
FIGURE 5.
Aza-induced changes in the expression of insulin-related genes in C. elegans. A, expression levels of major insulin-like genes in the C. elegans N2 strain: daf-28 and ins-7 (agonist) versus ins-1 and ins-18 (antagonists). B, expression level of daf-28 in N2, daf-6(e1377), and tax-4(ks11) strains. C, expression levels of the major stress response genes, sod-3, mtl-1, and gst-7, well known DAF-16 target genes in C. elegans. Each value represents the average of three independent experiments. Error bars represent the S.D., and p values were determined using Student's t test (*, p ≤ 0.05; **, p ≤ 0.01; NS, not significant). D, expression levels of SKN-1 target genes, gst-10 and gcs-1, upon Aza treatment. Each value represents the average of three independent experiments. Error bars represent the S.D., and p values were determined using Student's t test (*, p ≤ 0.05). E, working model of Aza effects on aging in C. elegans. Sterols can be sensed by DAF-6 in amphid neurons; subsequent control of SKN-1 and DAF-28 by DAF-6 in amphid neurons regulates respiration and ROS response through changes in endocrine signaling.
DISCUSSION
Here, we explored the potential biochemical mechanism underlying Aza-mediated cholesterol deprivation in the context of oxidative stress and longevity reduction. One impetus for this study was to highlight the importance of sterol metabolism in C. elegans, a metabolic pathway that facilitates the utilization of plant sterols (e.g. sitosterol, stigmasterol) and is also a target of a novel nematicide (55). A few points are worth noting with respect to the persistence of sterol depletion with Aza treatment. First, although the effects of Aza-induced cholesterol depletion in the nematode body are visually striking (Fig. 1D), it is not known exactly when depletion first occurs because we have not yet performed a time course study measuring sterols in worms treated with Aza. Second, in an earlier report, several different sterols in C. elegans were depleted by Aza treatment; cholesterol was depleted to ∼1% of the level typically found in C. elegans after culturing with a concentration of Aza approximately one-half that used in the present study (47). From the results from our laboratory, it was observed that a transgenic worm, called cholegans, which can produce endogenous cholesterol by converting 7-dehydrocholesterol showed a 30% increase in MLS when grown in the cholesterol depletion condition (56). This transgenic worm can live longer (24%) when they were grown in a 7-dehydrocholesterol plate (5 μg/ml), suggesting the importance of cholesterol in C. elegans longevity. Third, earlier work also indicated that, unless cholesterol is restored to the culture medium, sterol depletion lasts until worm death. Finally, Aza inhibits a very specific target (sterol 24-reductase); thus, off-target effects may be minimal. Previously, we observed that both the levels of vit-2 and vit-6 gene expression (3) and ROS production (Fig. 3A) were recovered when cholesterol was added onto the medium. Apparently, the reduction of lifespan by Aza appears to resemble the situation where N2 and mev-1(kn1) are grown in the absence of sterol (Fig. 2A).
It is conceivable that the failure to convert sitosterol to cholesterol might have an impact on redox balance. However, this conversion involves one simple hydrogenation, which should be quantitatively undetectable against the backdrop of all other hydrogen transfers taking place in the worm (48). Because Aza lacks double bonds and C. elegans does not degrade cholesterol to CO2 (57), we would expect Aza to be quite stable. Furthermore, earlier experiments with radiolabeled cholesterol, desmosterol, and sitosterol in bacteria-free medium showed no evidence for degradation into CO2 (i.e. all radioactivity was recovered in the medium; none was lost), something that would have been evident if the sterols were being degraded.3 Therefore, we would expect a saturated steroid like Aza to remain intact in medium or in C. elegans for months.
It has been known for some time that cholesterol depletion caused by either nutritional modulation (7) or defects in mammalian cholesterol biosynthesis (58, 59) is closely linked to an increase in embryonic lethality. In fact, we also observed about 30% embryonic lethality in F2 worms after growing adult (P0) worms in the presence of 25 μm Aza (∼EC50) at 25 °C (data not shown). This F2 embryonic lethality following Aza treatment of P0 worms may be among the best currently available means to quantitatively assess the toxicity of Aza on C. elegans development. Note that the embryonic-lethal phenotype associated with knockdown of a mitochondrial subunit gene using small interfering RNAs (60) is very similar to that observed under our sterol-depletion conditions. Thus, the embryonic lethality caused by sterol depletion may also be due to mitochondrial dysfunction. The toxic reaction of Aza is reversible and thus can be rescued by adding cholesterol to the medium.
An increase in ROS production caused by Aza-mediated cholesterol depletion would more likely be induced by complex II dysfunction than by complex I dysfunction, resulting in appearance of resistance in the mev-1(kn1) strain. That is, sterol depletion caused by Aza might only increase ROS production in the gas-1(fc21) strain, and not in the mev-1(kn1) strain (Fig. 3B). This is very similar to the previous report that the gas-1(fc21) strain was sensitive to anesthetic conditions but the mev-1(kn1) strain was not (61), suggesting that mitochondrial complex II is involved in cholesterol deprivation-mediated ROS production. Thus, Aza treatment not only caused mitochondrial dysfunction and decreased SKN-1 expression, which consequently led to ROS overproduction, but also stimulated DAF-28 expression in amphid neurons (Fig. 4, B–D). Down-regulation of SKN-1 expression in ASI neurons by Aza treatment might be responsible for the observed mitochondrial dysfunction, given that SKN-1 expression is known to be involved in mitochondrial respiration and is activated by dietary restriction, which leads to lifespan extension in C. elegans (36). Taken together, these results imply that Aza treatment causes an induction of DAF-28 that is coupled to SKN-1 suppression, culminating in lifespan reduction (Fig. 5D).
In daf-2(e1370) mutants that received Aza treatment, there was no apparent decrease in MLS (data not shown). This may be due to the fact that this mutant does not have any sensitivity to change in insulin level but contains a defense system against oxidative stress that would have been caused by Aza treatment. Upon Aza treatment, the elevated level of ROS would also have stimulated DAF-16 and SKN-1. At the same time, IIS would also have been activated, which might offset the activation of DAF-16 and SKN-1 in Aza-treated daf-2(e1370) mutants. This result suggests again that cholesterol is a key sterol controlling IIS. Contrary to sod-3, the expression levels of gcs-1 and gst-10, SKN-1 target genes that are regulated independently of daf-16, were decreased by Aza treatment (Fig. 5D). This result agrees that sterol depletion causes IIS activation (Fig. 5A). In contrast to N2 worms or tax-4 mutants, expression of daf-28 was not increased in Aza-treated daf-6 mutants (Fig. 5B), in which ciliated neurons might not be able to perceive environmental signals due to defects in amphid lumen formation (41). Like many cholesterol regulatory proteins or biosynthesis enzymes, notably 3-hydroxy-3-methylglutaryl-coenzyme A reductase (42), DAF-6 may respond to changes in sterol through its SSD (41, 62) and alter the expression of associated genes (i.e. daf-28). Thus, DAF-6 can sense cholesterol depletion by Aza treatment and then activates IIS through DAF-28, resulting in reduction of MLS.
This is perhaps the most reasonable connection point in amphidial sensory neurons between sterols and IIS, both of which are essential for worms to respond to environmental insults. With respect to the sterols-amphid connection, intense filipin staining in the amphids has been reported, indicating accumulation of cholesterol in this region (63). Earlier data (64) also suggest that the amphid and phasmid socket cells accumulate a sterol metabolite rather than unmodified, exogenously added cholesterol itself. Thus, when sterol is depleted by Aza treatment, C. elegans may interpret this as an environmental insult. This may be responded to by amphid neurons, which subsequently activate IIS signaling with SKN-1 suppression in ASI neurons, resulting in reduction of lifespan (Fig. 5D).
It was thought that cholesterol depletion might have caused dafachronic acid deficiency, which subsequently induces dauer development through daf-9 and daf-12 signaling (65). However, it was observed that cholesterol depletion also caused a lifespan reduction in daf-9(e1406); daf-12(m20) mutant (6). Thus, longevity regulation by cholesterol depletion may be independent of the steroid signaling pathway where DAF-12 and dafachonic acid are involved.
Our data show that cholesterol depletion was achieved by Aza treatment at 25 μm (∼EC50), establishing specific conditions under which sterol depletion-related stress can be observed. We found that the MLS of Aza-treated worms, including N2 and several stress-resistant mutants, were significantly reduced due to increased ROS production. The potential targets of Aza-mediated sterol depletion may be IIS and the mitochondrial oxidative phosphorylation system. Our findings suggest that sterol depletion by Aza causes a disturbance in mitochondrial membranes, thereby triggering an overproduction of ROS that is sensed by amphidial neurons, which subsequently activate IIS but decrease SKN-1, resulting in reduced longevity of adult C. elegans. We also found that DAF-6, through its SSD, may be responsible for sensing the sterol depletion caused by Aza, providing the most reasonable connection point between sterols and IIS in amphid neurons, which are important for the ability of worms to respond to environmental insults.
Supplementary Material
Acknowledgments
We thank the Caenorhabditis Genetics Center for kindly providing the mutants used in this study. We also thank Prof. T. K. Blackwell for his kind gift of transgenic worm IS007.
This work was supported, in part, by the Korea Health 21 R&D project, Ministry of Health and Welfare of Republic of Korea Grant A030003 (to Y. K.-P.), and the WCU (World Class University) program through the National Research Foundation of Korea, funded by Ministry of Education, Science, and Technology Grant R31-2008-000-10086-0.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Table S1 and Fig. S1.
D. J. Chitwood, unpublished data.
- Aza
- 25-azacoprostane
- MLS
- mean lifespan
- ROS
- reactive oxygen species
- IIS
- insulin/IGF-1-like signaling
- SSD
- sterol-sensing domain
- H2DCF-DA
- 2′,7′-dichlorodihydrofluorescein diacetate.
REFERENCES
- 1. Chitwood D. J., Lusby W. R. (1991) Lipids 26, 619–627 [DOI] [PubMed] [Google Scholar]
- 2. Hieb W. F., Rothstein M. (1968) Science 160, 778–780 [DOI] [PubMed] [Google Scholar]
- 3. Choi B. K., Chitwood D. J., Paik Y. K. (2003) Mol. Cell. Proteomics 2, 1086–1095 [DOI] [PubMed] [Google Scholar]
- 4. Gerisch B., Weitzel C., Kober-Eisermann C., Rottiers V., Antebi A. (2001) Dev. Cell 1, 841–851 [DOI] [PubMed] [Google Scholar]
- 5. Lee S. J., Murphy C. T., Kenyon C. (2009) Cell Metab. 10, 379–391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Merris M., Kraeft J., Tint G. S., Lenard J. (2004) J. Lipid Res. 45, 2044–2051 [DOI] [PubMed] [Google Scholar]
- 7. Shim Y. H., Chun J. H., Lee E. Y., Paik Y. K. (2002) Mol. Reprod. Dev. 61, 358–366 [DOI] [PubMed] [Google Scholar]
- 8. Lozano R., Chitwood D. J., Lusby W. R., Thompson M. J., Svoboda J. A., Patterson G. W. (1984) Comp. Biochem. Physiol. C 79, 21–26 [DOI] [PubMed] [Google Scholar]
- 9. Motola D. L., Cummins C. L., Rottiers V., Sharma K. K., Li T., Li Y., Suino-Powell K., Xu H. E., Auchus R. J., Antebi A., Mangelsdorf D. J. (2006) Cell 124, 1209–1223 [DOI] [PubMed] [Google Scholar]
- 10. Held J. M., White M. P., Fisher A. L., Gibson B. W., Lithgow G. J., Gill M. S. (2006) Aging Cell 5, 283–291 [DOI] [PubMed] [Google Scholar]
- 11. Gerisch G., Weber I. (2007) Cell Adh. Migr. 1, 145–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hannich J. T., Entchev E. V., Mende F., Boytchev H., Martin R., Zagoriy V., Theumer G., Riezman I., Riezman H., Knölker H. J., Kurzchalia T. V. (2009) Dev. Cell 16, 833–843 [DOI] [PubMed] [Google Scholar]
- 13. Veal E. A., Day A. M., Morgan B. A. (2007) Mol. Cell 26, 1–14 [DOI] [PubMed] [Google Scholar]
- 14. Kim J. A., Wei Y., Sowers J. R. (2008) Circ. Res. 102, 401–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Newsholme P., Haber E. P., Hirabara S. M., Rebelato E. L., Procopio J., Morgan D., Oliveira-Emilio H. C., Carpinelli A. R., Curi R. (2007) J. Physiol. 583, 9–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Warner H. R. (1994) Free Radic. Biol. Med. 17, 249–258 [DOI] [PubMed] [Google Scholar]
- 17. Sedensky M. M., Morgan P. G. (2006) Exp. Gerontol. 41, 957–967 [DOI] [PubMed] [Google Scholar]
- 18. Van Raamsdonk J. M., Hekimi S. (2010) Antioxid. Redox Signal. 13, 1911–1953 [DOI] [PubMed] [Google Scholar]
- 19. Gems D. (2009) SEB Exp. Biol. Ser. 62, 31–56 [PubMed] [Google Scholar]
- 20. Lee C. K., Klopp R. G., Weindruch R., Prolla T. A. (1999) Science 285, 1390–1393 [DOI] [PubMed] [Google Scholar]
- 21. Vanfleteren J. R., De Vreese A. (1996) J. Exp. Zool. 274, 93–100 [DOI] [PubMed] [Google Scholar]
- 22. Ishii N. (2000) Free Radic. Res. 33, 857–864 [DOI] [PubMed] [Google Scholar]
- 23. Kayser E. B., Morgan P. G., Hoppel C. L., Sedensky M. M. (2001) J. Biol. Chem. 276, 20551–20558 [DOI] [PubMed] [Google Scholar]
- 24. Dillin A., Hsu A. L., Arantes-Oliveira N., Lehrer-Graiwer J., Hsin H., Fraser A. G., Kamath R. S., Ahringer J., Kenyon C. (2002) Science 298, 2398–2401 [DOI] [PubMed] [Google Scholar]
- 25. Hartwig K., Heidler T., Moch J., Daniel H., Wenzel U. (2009) Genes Nutr. 4, 59–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kondo M., Senoo-Matsuda N., Yanase S., Ishii T., Hartman P. S., Ishii N. (2005) Mech. Ageing Dev. 126, 637–641 [DOI] [PubMed] [Google Scholar]
- 27. López-Revuelta A., Sánchez-Gallego J. I., Hernández-Hernández A., Sánchez-Yagüe J., Llanillo M. (2005) Biochim. Biophys. Acta 1734, 74–85 [DOI] [PubMed] [Google Scholar]
- 28. Garza R. M., Hampton R. Y. (2005) Nature 435, 37–38 [DOI] [PubMed] [Google Scholar]
- 29. Hughes A. L., Todd B. L., Espenshade P. J. (2005) Cell 120, 831–842 [DOI] [PubMed] [Google Scholar]
- 30. Friedman D. B., Johnson T. E. (1988) J. Gerontol. 43, B102–109 [DOI] [PubMed] [Google Scholar]
- 31. Gottlieb S., Ruvkun G. (1994) Genetics 137, 107–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kenyon C., Chang J., Gensch E., Rudner A., Tabtiang R. (1993) Nature 366, 461–464 [DOI] [PubMed] [Google Scholar]
- 33. Kenyon C. (2005) Cell 120, 449–460 [DOI] [PubMed] [Google Scholar]
- 34. An J. H., Blackwell T. K. (2003) Genes Dev. 17, 1882–1893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tullet J. M., Hertweck M., An J. H., Baker J., Hwang J. Y., Liu S., Oliveira R. P., Baumeister R., Blackwell T. K. (2008) Cell 132, 1025–1038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bishop N. A., Guarente L. (2007) Nature 447, 545–549 [DOI] [PubMed] [Google Scholar]
- 37. Honjoh S., Yamamoto T., Uno M., Nishida E. (2009) Nature 457, 726–730 [DOI] [PubMed] [Google Scholar]
- 38. Li W., Kennedy S. G., Ruvkun G. (2003) Genes Dev. 17, 844–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pierce S. B., Costa M., Wisotzkey R., Devadhar S., Homburger S. A., Buchman A. R., Ferguson K. C., Heller J., Platt D. M., Pasquinelli A. A., Liu L. X., Doberstein S. K., Ruvkun G. (2001) Genes Dev. 15, 672–686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Malone E. A., Inoue T., Thomas J. H. (1996) Genetics 143, 1193–1205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Perens E. A., Shaham S. (2005) Dev. Cell 8, 893–906 [DOI] [PubMed] [Google Scholar]
- 42. Gil G., Faust J. R., Chin D. J., Goldstein J. L., Brown M. S. (1985) Cell 41, 249–258 [DOI] [PubMed] [Google Scholar]
- 43. Li J., Brown G., Ailion M., Lee S., Thomas J. H. (2004) Development 131, 5741–5752 [DOI] [PubMed] [Google Scholar]
- 44. Boyd W. A., Smith M. V., Kissling G. E., Freedman J. H. (2010) Neurotoxicol. Teratol. 32, 68–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Horner M. A., Pardee K., Liu S., King-Jones K., Lajoie G., Edwards A., Krause H. M., Thummel C. S. (2009) Genes Dev. 23, 2711–2716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gutierrez-Zepeda A., Santell R., Wu Z., Brown M., Wu Y., Khan I., Link C. D., Zhao B., Luo Y. (2005) BMC Neurosci. 6, 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chitwood D. J., Lusby W. R., Lozano R., Thompson M. J., Svoboda J. A. (1984) Lipids 19, 500–506 [DOI] [PubMed] [Google Scholar]
- 48. Chitwood D. J. (1999) Crit. Rev. Biochem. Mol. Biol. 34, 273–284 [DOI] [PubMed] [Google Scholar]
- 49. Marichal P., Koymans L., Willemsens S., Bellens D., Verhasselt P., Luyten W., Borgers M., Ramaekers F. C., Odds F. C., Bossche H. V. (1999) Microbiology 145, 2701–2713 [DOI] [PubMed] [Google Scholar]
- 50. Kobayashi D., Kondo K., Uehara N., Otokozawa S., Tsuji N., Yagihashi A., Watanabe N. (2002) Antimicrob. Agents Chemother. 46, 3113–3117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kayser E. B., Sedensky M. M., Morgan P. G. (2004) Mech. Ageing Dev. 125, 455–464 [DOI] [PubMed] [Google Scholar]
- 52. Hao M., Head W. S., Gunawardana S. C., Hasty A. H., Piston D. W. (2007) Diabetes 56, 2328–2338 [DOI] [PubMed] [Google Scholar]
- 53. Kimura K. D., Tissenbaum H. A., Liu Y., Ruvkun G. (1997) Science 277, 942–946 [DOI] [PubMed] [Google Scholar]
- 54. Patel D. S., Fang L. L., Svy D. K., Ruvkun G., Li W. (2008) Development 135, 2239–2249 [DOI] [PubMed] [Google Scholar]
- 55. Oh W. S., Jeong P. Y., Joo H. J., Lee J. E., Moon Y. S., Cheon H. M., Kim J. H., Lee Y. U., Shim Y. H., Paik Y. K. (2009) PLoS One 4, e7593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lee E. Y., Shim Y. H., Chitwood D. J., Hwang S. B., Lee J., Paik Y. K. (2005) Biochem. Biophys. Res. Commun. 328, 929–936 [DOI] [PubMed] [Google Scholar]
- 57. Rothstein M. (1968) Comp. Biochem. Physiol. 27, 309–317 [DOI] [PubMed] [Google Scholar]
- 58. Roux C., Wolf C., Mulliez N., Gaoua W., Cormier V., Chevy F., Citadelle D. (2000) Am. J. Clin. Nutr. 71, 1270S-1279S [DOI] [PubMed] [Google Scholar]
- 59. Waterham H. R., Koster J., Romeijn G. J., Hennekam R. C., Vreken P., Andersson H. C., FitzPatrick D. R., Kelley R. I., Wanders R. J. (2001) Am. J. Hum. Genet. 69, 685–694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ichimiya H., Huet R. G., Hartman P., Amino H., Kita K., Ishii N. (2002) Mitochondrion 2, 191–198 [DOI] [PubMed] [Google Scholar]
- 61. Hartman P. S., Ishii N., Kayser E. B., Morgan P. G., Sedensky M. M. (2001) Mech. Ageing Dev. 122, 1187–1201 [DOI] [PubMed] [Google Scholar]
- 62. Radhakrishnan A., Sun L. P., Kwon H. J., Brown M. S., Goldstein J. L. (2004) Mol. Cell 15, 259–268 [DOI] [PubMed] [Google Scholar]
- 63. Merris M., Wadsworth W. G., Khamrai U., Bittman R., Chitwood D. J., Lenard J. (2003) J. Lipid Res. 44, 172–181 [DOI] [PubMed] [Google Scholar]
- 64. Matyash V., Geier C., Henske A., Mukherjee S., Hirsh D., Thiele C., Grant B., Maxfield F. R., Kurzchalia T. V. (2001) Mol. Biol. Cell 12, 1725–1736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Matyash V., Entchev E. V., Mende F., Wilsch-Bräuninger M., Thiele C., Schmidt A. W., Knölker H. J., Ward S., Kurzchalia T. V. (2004) PLoS Biol. 2, e280. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.