Abstract
Prion diseases are infectious neurodegenerative disorders that affect humans and animals and that result from the conversion of normal prion protein (PrPC) into the misfolded prion protein (PrPSc). Chronic wasting disease (CWD) is a prion disorder of increasing prevalence within the United States that affects a large population of wild and captive deer and elk. Determining the risk of transmission of CWD to humans is of utmost importance, considering that people can be infected by animal prions, resulting in new fatal diseases. To study the possibility that human PrPC can be converted into the misfolded form by CWD PrPSc, we performed experiments using the protein misfolding cyclic amplification technique, which mimics in vitro the process of prion replication. Our results show that cervid PrPSc can induce the conversion of human PrPC but only after the CWD prion strain has been stabilized by successive passages in vitro or in vivo. Interestingly, the newly generated human PrPSc exhibits a distinct biochemical pattern that differs from that of any of the currently known forms of human PrPSc. Our results also have profound implications for understanding the mechanisms of the prion species barrier and indicate that the transmission barrier is a dynamic process that depends on the strain and moreover the degree of adaptation of the strain. If our findings are corroborated by infectivity assays, they will imply that CWD prions have the potential to infect humans and that this ability progressively increases with CWD spreading.
Keywords: Amyloid, Neurodegeneration, Neurological Diseases, Prions, Protein Conformation, Prion Diseases
Introduction
Chronic wasting disease (CWD)2 is a disorder associated with infectious prions that affects deer, elk, and moose (1, 2). CWD is so far the only prion disease of wild animals; it is highly contagious, and the exact prevalence is currently unknown. Its origin and mechanism of transmission are also unclear. CWD-affected animals show loss of body condition, changes in behavior, ataxia, head tremors, salivation, and somnolence (1). CWD can reduce the growth and size of wild deer and elk populations in areas where the prevalence is high and is of increasing concern in North America. The disease was thought to be limited in the wild to a relatively small endemic area in northeastern Colorado and southeastern Wyoming. However, in the last decade, it has spread east and west of those areas, even across natural borders, and has been reported in 14 states, two Canadian provinces, and South Korea (1, 2). The risk of transmission of CWD to other animal species and especially to humans is unknown. Defining that risk is of utmost importance, considering that transmission of bovine prions to humans resulted in a new disease (variant Creutzfeldt-Jakob disease (CJD)) and that the number of cervids affected by CWD in the United States is increasing (3).
Transmission of prions between different species of mammals is often limited by the species barrier phenomenon (4). Currently, the strength of the barrier between different species is unpredictable and requires bioassay studies to be assessed. These experiments are costly and time-consuming and, in the case of humans, cannot be measured directly but rely on the use of animal models, such as transgenic mice and primates. It is presumed that a large number of hunters in the United States have been in contact with or consumed CWD-infected meat (5). Fortunately, no clinical evidence linking CWD-exposed humans and CJD patients has yet been found (5, 6). Experimental inoculation of CWD prions into squirrel monkeys produced the disease (7, 8); however, transgenic mice expressing human PrP did not (9–11), suggesting that the species barrier between humans and cervids is greater than that between cattle and humans.
To test the possibility that human PrPC can be converted into the infectious form by CWD PrPSc, we performed experiments using the protein misfolding cyclic amplification (PMCA) technique. PMCA mimics prion replication in vitro at an accelerated speed (12); PrPSc generated in vitro by PMCA has been shown to be infectious to wild-type animals and to maintain the strain properties (13–16). Interestingly, new prion strains can be generated, adapted, and stabilized upon crossing species barriers in vitro by PMCA (16, 17). These findings demonstrate that PMCA is a valuable tool to investigate the strength of the barrier between diverse species, its molecular determinants, and the expected features of the new infectious material produced.
EXPERIMENTAL PROCEDURES
Preparation of Brain Homogenates
Brains from healthy transgenic mice overexpressing either human PrP (129M genotype) (18) or cervid PrP (Tg1536) (19) were extracted after animals were perfused with PBS plus 5 mm EDTA. As inoculum for the PMCA reaction, we used brains from different naturally affected CWD mule deer, cattle with bovine spongiform encephalopathy (BSE), sheep with scrapie, humans affected with diverse forms of CJD, or Tg1536 mice infected with CWD prions. 10% (w/v) brain homogenates were prepared in conversion buffer (PBS containing 150 mm NaCl, 1.0% Triton X-100, and CompleteTM protease inhibitor mixture (Roche Applied Science)).
PMCA Procedure
Aliquots (100 μl) of 10% healthy brain homogenate were loaded onto 0.2-ml PCR tubes. Tubes were positioned on an adaptor placed on the plate holder of a microsonicator (Misonix Model 4000), programmed to perform 144 cycles of 30 min of incubation at 37 °C, followed by a 20-s pulse of sonication set at an amplitude of 70. For serial PMCA, after each round of cycles, a 10-μl aliquot of the amplified material was diluted into 90 μl of normal brain homogenate, and a new round of PMCA cycles was performed. In some experiments, 0.05% digitonin and 4 mm EDTA were added to the conversion buffer. The detailed protocol for PMCA, including reagents, solutions and troubleshooting, has been published previously (20, 21).
PrPSc Detection by Western Blotting after Proteinase K Digestion
The standard procedure to digest PrPSc consists of subjecting samples to incubation in the presence of proteinase K (PK; 50 μg/ml) for 60 min with shaking at 37 °C. The digestion was stopped by the addition of electrophoresis sample buffer, and protease-resistant PrP was revealed by Western blotting. Proteins were fractionated by SDS-PAGE, electroblotted onto nitrocellulose membrane, and probed with antibody 6D11 (1:5000 dilution) to recognize cervid, cattle, or sheep PrP proteins or with antibody 3F4 (1:10,000 dilution) to detect exclusively human PrP. The immunoreactive bands were visualized by enhanced chemiluminescence assay (Amersham Biosciences) using a UVP image analysis system.
RESULTS
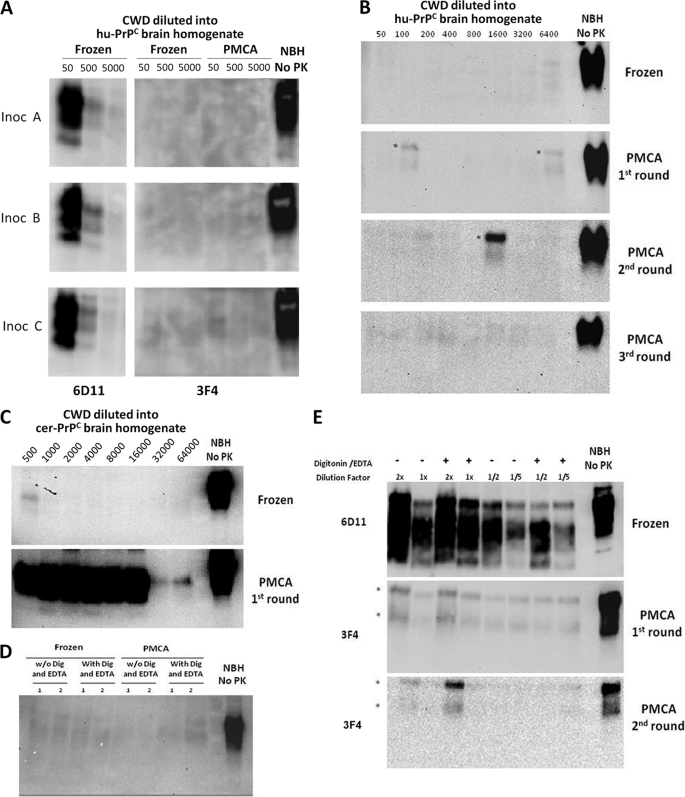
Having validated the PMCA technology to assess the species barrier in vitro (16, 17), we investigated the possibility that CWD PrPSc can convert human PrPC to PrPSc. Standard PMCA using samples from three different CWD brains failed to produce a signal for human PrPSc (Fig. 1A). For these studies, we took advantage of the fact that human (but not deer) PrP is detectable with antibody 3F4 (22), so any PK-resistant signal detectable with this antibody corresponds to human PrPSc material. To further attempt the generation of human PrPSc from deer, we performed several rounds of PMCA, a process that has been shown to dramatically increase the amplification efficiency (23, 24). As shown in Fig. 1B, no human PrPSc signal was detectable even after three rounds of PMCA. Importantly, the same CWD inoculum efficiently induced the conversion of deer PrPC into PrPSc (Fig. 1C). We used brains of transgenic mice expressing human PrPC as the substrate under conditions that were previously shown to efficiently propagate human PrPSc from various forms of sporadic and variant CJD (see also supplemental Fig. 1) (14). To further attempt conversion of human PrPC by deer PrPSc, we added 0.05% digitonin and 4 mm EDTA to the conversion buffer, which we have found to increase the efficiency of PMCA amplification in human samples (supplemental Fig. 1). However, these conditions were unsuccessful in generating PK-resistant human PrPSc (Fig. 1D). In our previous experiments to cross the mouse-hamster species barrier by PMCA, we found that adding a more concentrated solution of the PrPSc inoculum was successful in producing interspecies conversion (17). To evaluate this possibility, we used more concentrated CWD material (from 2- to 0.2-fold) as inoculum. Again, no human PrPSc product was observed under these conditions (Fig. 1E). The conclusion from these experiments is that either the deer-human barrier is absolute or the PMCA conditions were not optimal to cross it. In a previous study, Hoover and co-workers (25) were also unable to replicate CWD PrPSc at the expense of human PrPC in serial PMCA experiments.
FIGURE 1.
Unsuccessful conversion of human PrPC by CWD PrPSc. A, three different samples of brain homogenates from mule deer naturally affected with CWD were used to attempt conversion of human PrPC (hu-PrPC). 10% CWD brain homogenates were diluted 50-, 500-, or 5000-fold into 10% brain homogenates from transgenic mice expressing the human (129M) PrP gene-coding segment. Samples were either frozen or subjected to 144 cycles of PMCA. Formation of PrPSc was assessed by PK digestion, followed by Western blotting using antibody 3F4, which recognizes human, but not cervid, PrP (22). The amount of cervid PrPSc in the inoculum (Inoc) was checked by Western blotting using antibody 6D11. B, to further attempt conversion of human PrPC with cervid prions, several serial rounds of PMCA were done using standard conditions. As reported previously, three serial rounds of PMCA should lead to ∼100,000-fold amplification of any PrPSc signal present in the sample (24). C, the CWD PrPSc conversion of deer PrPC by PMCA was studied using samples of 10% CWD brain homogenate (inoculum A) that were diluted into 10% brain homogenate prepared from healthy transgenic mice expressing cervid PrPC (cer-PrPC). The formation of cervid PrPSc was assessed by Western blotting using antibody 6D11. D, crossing the cervid-human species barrier was further attempted by adding detergents and EDTA to the conversion buffer, which has been shown to increase PMCA efficiency (37), particularly for human samples (supplemental Fig. 1). E, the CWD inoculum was concentrated by the Sarkosyl procedure described under “Experimental Procedures” to add larger amounts of PrPSc. 2-, 1-, 0.5, or 0.2-fold PrPSc relative to the 10% CWD brain homogenate was added to the human transgenic mouse brain homogenate and subjected to PMCA in either the presence or absence of digitonin (Dig) and EDTA. In the experiments shown in all panels, samples were treated with PK (50 μg/ml), except for the normal brain homogenate (NBH No PK) used as control. The asterisks in B and E represent incomplete digestion of PrPC, which is clearly appreciated because the electrophoretic mobility is identical to that of full-length PrPC.
On the basis of our previous studies, we noted that PMCA not only enables interspecies conversion but also allows adaptation of the new strain generated (17). Indeed, a recent study showed that serial rounds of PMCA produce the same adaptation of a natural CWD prion strain as do serial passages in vivo (26). Because the CWD prions used in our experiments are derived from naturally affected mule deer, we hypothesized that the CWD strain most likely corresponds to an unstable prion strain. To assess whether strain adaptation by serial PMCA rounds might influence the ability of CWD prions to cross the human species barrier, we performed several passages of CWD PrPSc into deer PrPC by PMCA (Fig. 2A). For this experiment, six replicate experiments were performed in parallel. Interestingly, after one PMCA round, the deer PrPSc produced was able to convert human PrPC with low efficiency (Fig. 2B, upper panel). One of the six replicates showed a clear protease-resistant band with a molecular weight reminiscent of PrP27–30. Two or three other replicates showed faint immunoreactive bands (Fig. 2B, upper panel). Moreover, after a second and third passage of CWD PrPSc into deer PrPC, a much more clear and efficient conversion of human PrPC to PrPSc was observed (Fig. 2B, middle and lower panels). These results suggest that CWD prions might be able to affect human beings but only after the CWD strain has been stabilized by successive passaging. To further test this hypothesis and to determine that the ability to convert human PrP was not an artifact produced by in vitro PMCA amplification, we tested samples of CWD that were serially passaged in transgenic mice expressing cervid PrPC. Samples from the first and second passages in vivo in mice from two different inocula were used to trigger conversion of human PrPC. As shown in Fig. 3, cervid PrPSc obtained from these two inocula, after either one or two passages in transgenic mice, was able to convert human PrPC into PK-resistant human PrPSc.
FIGURE 2.
Conversion of human PrPC induced by CWD PrPSc after PMCA strain adaptation. A, six separate samples of a 1000-fold dilution of CWD brain homogenate (inoculum A) were used to convert cervid PrPC in three successive rounds of 144 PMCA cycles. Protease-resistant deer PrPSc was measured by Western blotting using antibody 6D11. B, samples from the amplified material were used to attempt conversion of human PrPC by PMCA, and the protease-resistant signal was checked using the human-specific antibody 3F4. NBH No PK, normal brain homogenate used as a control.
FIGURE 3.
Conversion of human PrPC induced by CWD PrPSc after in vivo strain adaptation. Transgenic (Tg) mice expressing the cervid PrP gene were used to serially passage CWD prions from two different natural inocula (Inoc). Brain homogenate from sick transgenic mice after one or two in vivo passages was used to convert human PrPC by subjecting the sample to 144 PMCA cycles. The generation of human PrPSc after PMCA was assessed by Western blotting employing antibody 3F4 (right panel). The PrPSc reactivity of the CWD inoculum from cervid transgenic mice was evaluated by Western blotting using antibody 6D11 (left panel). All samples were digested with PK (50 μg/ml), except for the normal brain homogenate (NBH No PK), which was used as a marker of full-length PrPC electrophoretic migration. The asterisk represents incomplete digestion of PrPC. As shown before, amplification of the original CWD inoculum at the expense of human PrPC failed to show any PK-resistant PrPSc band.
Interestingly, when the Western blot profile of this newly generated form of human PrPSc (termed CWD-huPrPSc) was compared with those of known strains of human prions, it was clear that CWD-huPrPSc exhibited a different pattern (Fig. 4A). The electrophoretic migration of this protein after PK digestion is similar to that of the type 1 strain of sporadic CJD, but its glycosylation profile is clearly different, showing a highly predominant diglycosylated form (Fig. 4, A and B). This result suggests that CWD-huPrPSc corresponds to a new human prion strain. Interestingly, a detailed previous study by Chen and co-workers (6) comparing the biochemical characteristics of PrPSc from cervids and humans showed that CWD PrPSc is similar to sporadic CJD MM1 in terms of electrophoretic mobility. However, the misfolded protein associated with CWD is predominantly diglycosylated, whereas PrPSc from type 1 sporadic CJD is mostly monoglycosylated (6). On the basis of the fact that transmission of BSE prions to humans resulted in a new form of PrPSc very similar to the one in cattle (6, 27), these authors predicted that if humans were infected by CWD, it is likely that PrPSc would be type 1, with a predominance of the diglycosylated isoform (6). Our results agree with that prediction and suggest that the newly generated CWD-huPrPSc acquires the biochemical properties of the cervid infectious material (Fig. 4, A and B). We and others have shown that PMCA replication of PrPSc obtained from experimental rodents, sheep, cervid, and human samples faithfully maintains the prion strain characteristics (14, 16, 26, 28–30). To further support the relevance of our results, we performed experiments in which we attempted to convert human PrPC by either cattle BSE PrPSc or sheep scrapie PrPSc. Whereas the typical variant CJD type of PrPSc was generated when human PrPC was converted by BSE PrPSc, no human PrPSc was generated under any condition when sheep scrapie PrPSc was used as inoculum (Fig. 4B). These results further validate our PMCA assay.
FIGURE 4.
Western blot profile of human PrPSc generated in vitro from CWD. A, aliquots of CWD-huPrPSc obtained by in vitro conversion of human PrPC with CWD after two rounds of PMCA in deer (see Fig. 2) were loaded together with huPrPSc associated with variant CJD (vCJD) and sporadic CJD types MM1 and MM2. Samples were treated with PK and developed using antibody 3F4. A schematic representation of the mobility and intensity of the different PrP glycoforms in each sample is shown to the right. B, samples of PrPSc from either cattle affected with BSE or sheep with typical scrapie were used to induce conversion of human PrPC (Hu-PrPC) by 144 PMCA cycles. Newly generated human PrPSc was detected by Western blotting using antibody 3F4, which recognizes human, but not cattle or sheep, PrP. As a control of the electrophoretic pattern of human PrPSc associated with variant CJD, a brain sample from a patient affected with this disease was loaded. The asterisk represents a signal coming from incomplete digestion of PrPC. NBH No PK, normal brain homogenate used as a control.
DISCUSSION
CWD is possibly the most worrisome prion zoonosis because it affects free-ranging animals, making it very difficult to control its spread, and because it is highly efficiently transmitted (1, 2). Indeed, in dense free-ranging cervid populations, CWD prevalence can reach as high as 30%, and among captive herds, the prevalence can climb to nearly 100%. The mechanisms and routes of transmission are currently unknown but likely involve horizontal spread through exposure to prion-infected secretions, excretions, or decomposed carcasses (1, 2). Moreover, it is likely that CWD prions are progressively accumulating in the environment because PrPSc binds tightly to soil and can maintain infectivity for a long time (31–33). Currently, it is unknown what proportion of natural CWD cases arises sporadically or comes from horizontal transmission among animals. Based on the available knowledge of the emergence, adaptation, and stabilization of prion strains, it is likely that prions appear either spontaneously, through interspecies transmission, or by genetic mutations. These “first generation” prions are unstable strains that begin a progressive and gradual process of adaptation that may take several passages and years or decades to complete. In addition, natural strain stabilization may take considerably more time than the controlled adaptation done by intracerebral inoculation of brain homogenates in experimental animals. In natural cases, animals usually get infected by peripheral (most likely oral) exposure to small quantities of prions present in peripheral tissues or secretion fluids. Recent data indicate that, in some cases, the strain characteristics of natural prions in peripheral organs are different from those in the brain even in the same individuals.3,4 In cervids, there are at least two different strains that can be differentiated by the incubation time and neuropathological characteristics produced when inoculated into transgenic mice expressing deer PrP (34). The susceptibility of these two strains to human transmission is currently unknown.
Our findings demonstrate that upon strain adaptation by serial passages in vitro or in cervid transgenic mice, cervid PrPSc is capable of converting human PrPC to produce PrPSc with unique biochemical properties, likely representing a new human prion strain. The newly generated CWD-huPrPSc material has been inoculated into transgenic mice expressing human PrP to study infectivity and disease phenotype, and these data will be published elsewhere. It is highly unlikely that the human PrPSc generated in these studies is coming from spontaneous “de novo generation” because, under the conditions used, no spontaneous PK-resistant band was detected in brain homogenates of humans or transgenic mice expressing human PrPC, even after >20 serial rounds of PMCA (35). Furthermore, none of the many controls included in our experiments in which no PrPSc was added to the reaction showed any PK-resistant PrP band.
Analyses of the transmission of CWD to transgenic mice expressing human PrP have consistently given negative results (9–11), indicating a strong species barrier. This conclusion is consistent with our many failed attempts to convert human PrPC with natural CWD, even after pushing the PMCA conditions (see Fig. 1). We found successful conversion only after adaptation of the CWD prion strain by successive passages in vitro or in cervid transgenic mice. We are not aware that the inoculum used in any of the transgenic mice studies was a previously stabilized CWD strain. Although it has been shown that strain stabilization in vitro by PMCA (17, 26) and in vivo using experimental rodents (36) has similarities to the strain adaptation process occurring in natural hosts, we cannot rule out that the type of CWD strain adaptation that is required to produce strains transmissible to humans may take much longer time in cervids or not occur at all. An important experiment will be to study transmissibility to humanized transgenic mice of CWD passaged experimentally in deer several times.
Besides the importance of our results for public health in relation to the putative transmissibility of CWD to humans, our data also illustrate a very important and novel scientific concept related to the mechanism of prion transmission across species barriers. Today, the view is that the species barrier is controlled mostly by the degree of similarity of the sequence of the prion protein between the host and the infectious material (4). In our study, we have shown that the strain and, moreover, the stabilization of the strain play a major role in the interspecies transmission. In our system, there is no change in the protein sequence, yet strain adaptation results in a complete change in prion transmissibility with potentially dramatic consequences. Therefore, our findings lead to a new view of the species barrier that should not be seen as a static process but rather a dynamic biological phenomenon that can change over time when prion strains mature and evolve. It remains to be investigated whether other species barriers also change upon progressive strain adaptation of other prion forms (e.g. the sheep-human barrier).
Our results have far-reaching implications for human health because they indicate that cervid PrPSc can trigger the conversion of human PrPC into PrPSc, suggesting that CWD might be infectious to humans. Interestingly, our findings suggest that unstable strains from CWD-affected animals might not be a problem for humans, but upon strain stabilization by successive passages in the wild, this disease might become progressively more transmissible to man.
Supplementary Material
This work was supported, in whole or in part, by National Institutes of Health Grants R01NS049173, P01AI77774, and P01AG14359. Dr. Soto is the inventor of several patents related to PMCA technology and is currently Founder, Chief Scientific Officer, and Vice President of Amprion Inc., a biotechnological company focusing on the commercial exploitation of PMCA for prion diagnosis.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. 1.
G. C. Telling, unpublished data.
M. A. Barria and C. Soto, unpublished data.
- CWD
- chronic wasting disease
- CJD
- Creutzfeldt-Jakob disease
- PMCA
- protein misfolding cyclic amplification
- BSE
- bovine spongiform encephalopathy
- PK
- proteinase K.
REFERENCES
- 1. Miller M. W., Williams E. S. (2004) Curr. Top. Microbiol. Immunol. 284, 193–214 [DOI] [PubMed] [Google Scholar]
- 2. Sigurdson C. J., Aguzzi A. (2007) Biochim. Biophys. Acta 1772, 610–618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bosque P. J. (2002) Curr. Neurol. Neurosci. Rep. 2, 488–495 [DOI] [PubMed] [Google Scholar]
- 4. Moore R. A., Vorberg I., Priola S. A. (2005) Arch. Virol. Suppl. 19, 187–202 [DOI] [PubMed] [Google Scholar]
- 5. Belay E. D., Gambetti P., Schonberger L. B., Parchi P., Lyon D. R., Capellari S., McQuiston J. H., Bradley K., Dowdle G., Crutcher J. M., Nichols C. R. (2001) Arch. Neurol. 58, 1673–1678 [DOI] [PubMed] [Google Scholar]
- 6. Xie Z., O'Rourke K. I., Dong Z., Jenny A. L., Langenberg J. A., Belay E. D., Schonberger L. B., Petersen R. B., Zou W., Kong Q., Gambetti P., Chen S. G. (2006) J. Biol. Chem. 281, 4199–4206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Marsh R. F., Kincaid A. E., Bessen R. A., Bartz J. C. (2005) J. Virol. 79, 13794–13796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Race B., Meade-White K. D., Miller M. W., Barbian K. D., Rubenstein R., LaFauci G., Cervenakova L., Favara C., Gardner D., Long D., Parnell M., Striebel J., Priola S. A., Ward A., Williams E. S., Race R., Chesebro B. (2009) Emerg. Infect. Dis. 15, 1366–1376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kong Q., Huang S., Zou W., Vanegas D., Wang M., Wu D., Yuan J., Zheng M., Bai H., Deng H., Chen K., Jenny A. L., O'Rourke K., Belay E. D., Schonberger L. B., Petersen R. B., Sy M. S., Chen S. G., Gambetti P. (2005) J. Neurosci. 25, 7944–7949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tamgüney G., Giles K., Bouzamondo-Bernstein E., Bosque P. J., Miller M. W., Safar J., DeArmond S. J., Prusiner S. B. (2006) J. Virol. 80, 9104–9114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sandberg M. K., Al-Doujaily H., Sigurdson C. J., Glatzel M., O'Malley C., Powell C., Asante E. A., Linehan J. M., Brandner S., Wadsworth J. D., Collinge J. (2010) J. Gen. Virol. 91, 2651–2657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Saborio G. P., Permanne B., Soto C. (2001) Nature 411, 810–813 [DOI] [PubMed] [Google Scholar]
- 13. Castilla J., Saá P., Hetz C., Soto C. (2005) Cell 121, 195–206 [DOI] [PubMed] [Google Scholar]
- 14. Castilla J., Morales R., Saá P., Barria M., Gambetti P., Soto C. (2008) EMBO J. 27, 2557–2566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Deleault N. R., Harris B. T., Rees J. R., Supattapone S. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 9741–9746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Green K. M., Castilla J., Seward T. S., Napier D. L., Jewell J. E., Soto C., Telling G. C. (2008) PLoS Pathog. 4, e1000139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Castilla J., Gonzalez-Romero D., Saá P., Morales R., De Castro J., Soto C. (2008) Cell 134, 757–768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Telling G. C., Scott M., Mastrianni J., Gabizon R., Torchia M., Cohen F. E., DeArmond S. J., Prusiner S. B. (1995) Cell 83, 79–90 [DOI] [PubMed] [Google Scholar]
- 19. Browning S. R., Mason G. L., Seward T., Green M., Eliason G. A., Mathiason C., Miller M. W., Williams E. S., Hoover E., Telling G. C. (2004) J. Virol. 78, 13345–13350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Castilla J., Saá P., Morales R., Abid K., Maundrell K., Soto C. (2006) Methods Enzymol. 412, 3–21 [DOI] [PubMed] [Google Scholar]
- 21. Saá P., Castilla J., Soto C. (2005) Methods Mol. Biol. 299, 53–65 [DOI] [PubMed] [Google Scholar]
- 22. Kascsak R. J., Rubenstein R., Merz P. A., Tonna-DeMasi M., Fersko R., Carp R. I., Wisniewski H. M., Diringer H. (1987) J. Virol. 61, 3688–3693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Castilla J., Saá P., Soto C. (2005) Nat. Med. 11, 982–985 [DOI] [PubMed] [Google Scholar]
- 24. Saá P., Castilla J., Soto C. (2006) J. Biol. Chem. 281, 35245–35252 [DOI] [PubMed] [Google Scholar]
- 25. Kurt T. D., Telling G. C., Zabel M. D., Hoover E. A. (2009) Virology 387, 235–243 [DOI] [PubMed] [Google Scholar]
- 26. Meyerett C., Michel B., Pulford B., Spraker T. R., Nichols T. A., Johnson T., Kurt T., Hoover E. A., Telling G. C., Zabel M. D. (2008) Virology 382, 267–276 [DOI] [PubMed] [Google Scholar]
- 27. Collinge J., Sidle K. C., Meads J., Ironside J., Hill A. F. (1996) Nature 383, 685–690 [DOI] [PubMed] [Google Scholar]
- 28. Soto C., Anderes L., Suardi S., Cardone F., Castilla J., Frossard M. J., Peano S., Saa P., Limido L., Carbonatto M., Ironside J., Torres J. M., Pocchiari M., Tagliavini F. (2005) FEBS Lett. 579, 638–642 [DOI] [PubMed] [Google Scholar]
- 29. Jones M., Peden A. H., Wight D., Prowse C., Macgregor I., Manson J., Turner M., Ironside J. W., Head M. W. (2008) Neuroreport 19, 1783–1786 [DOI] [PubMed] [Google Scholar]
- 30. Thorne L., Terry L. A. (2008) J. Gen. Virol. 89, 3177–3184 [DOI] [PubMed] [Google Scholar]
- 31. Seidel B., Thomzig A., Buschmann A., Groschup M. H., Peters R., Beekes M., Terytze K. (2007) PLoS ONE 2, e435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Brown P., Gajdusek D. C. (1991) Lancet 337, 269–270 [DOI] [PubMed] [Google Scholar]
- 33. Johnson C. J., Pedersen J. A., Chappell R. J., McKenzie D., Aiken J. M. (2007) PLoS Pathog. 3, e93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Angers R. C., Kang H. E., Napier D., Browning S., Seward T., Mathiason C., Balachandran A., McKenzie D., Castilla J., Soto C., Jewell J., Graham C., Hoover E. A., Telling G. C. (2010) Science 328, 1154–1158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Barria M. A., Mukherjee A., Gonzalez-Romero D., Morales R., Soto C. (2009) PLoS Pathog. 5, e1000421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Race R., Meade-White K., Raines A., Raymond G. J., Caughey B., Chesebro B. (2002) J. Infect. Dis. 186, S166–S170 [DOI] [PubMed] [Google Scholar]
- 37. Murayama Y., Yoshioka M., Yokoyama T., Iwamaru Y., Imamura M., Masujin K., Yoshiba S., Mohri S. (2007) Neurosci. Lett. 413, 270–273 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.