Abstract
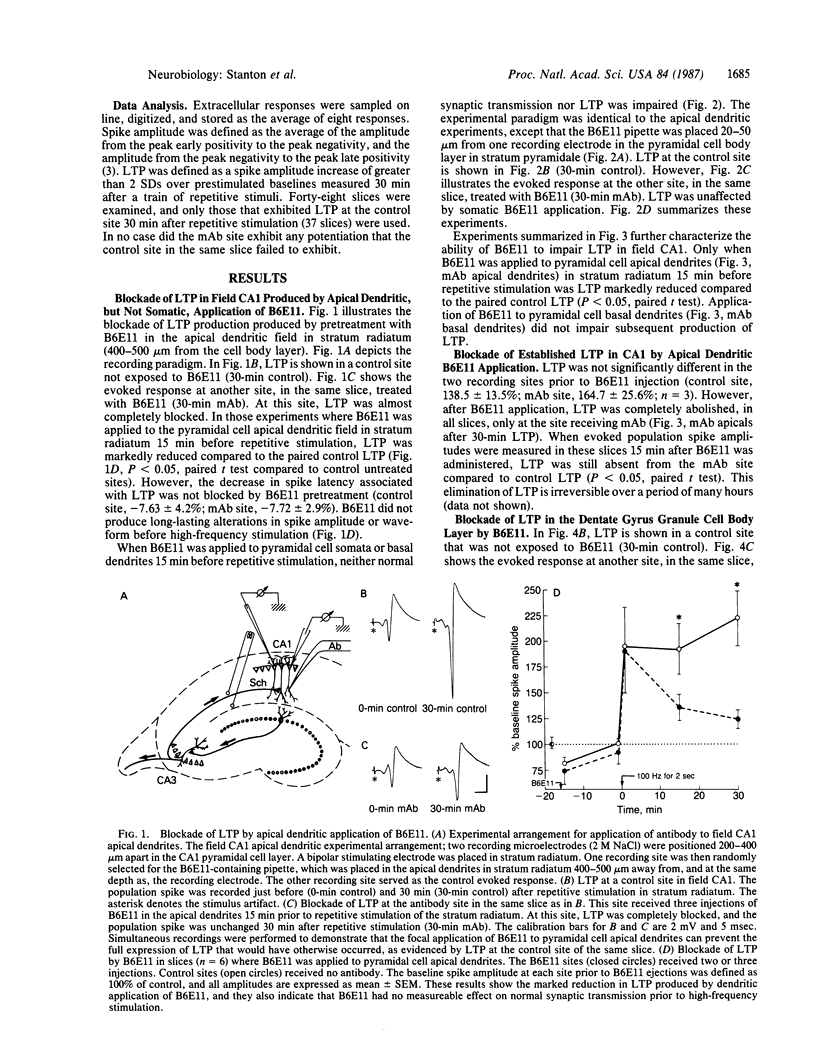
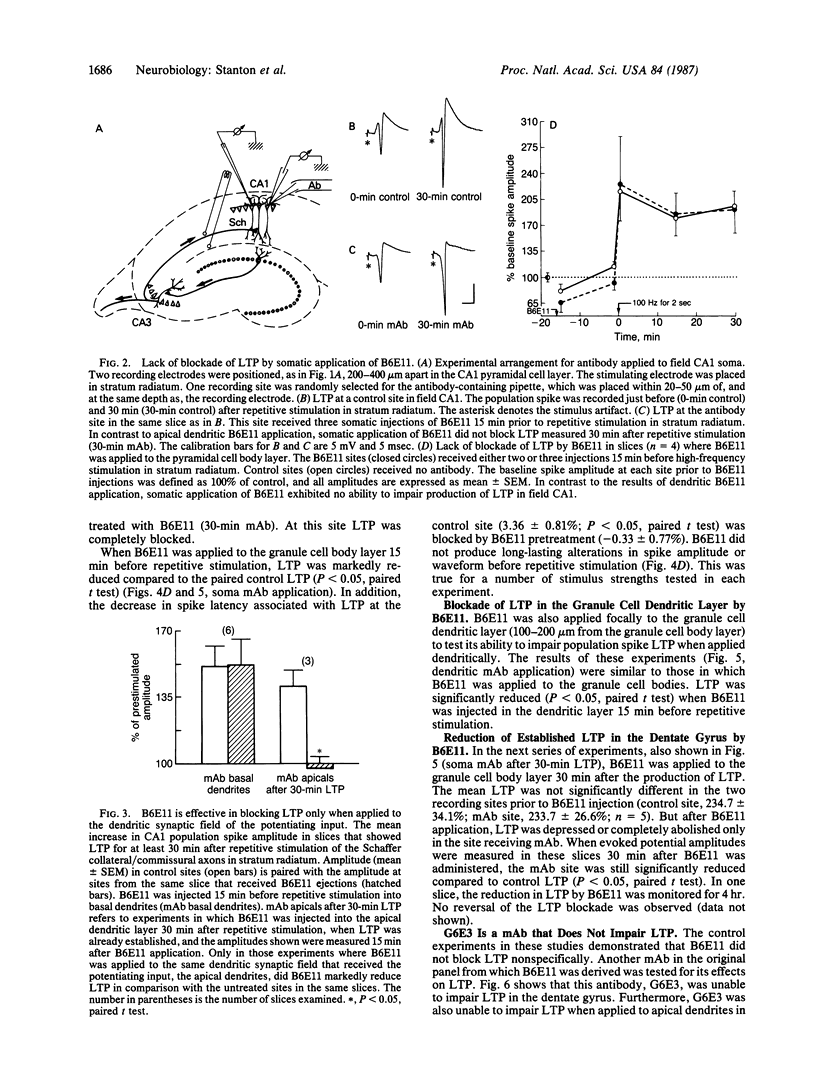
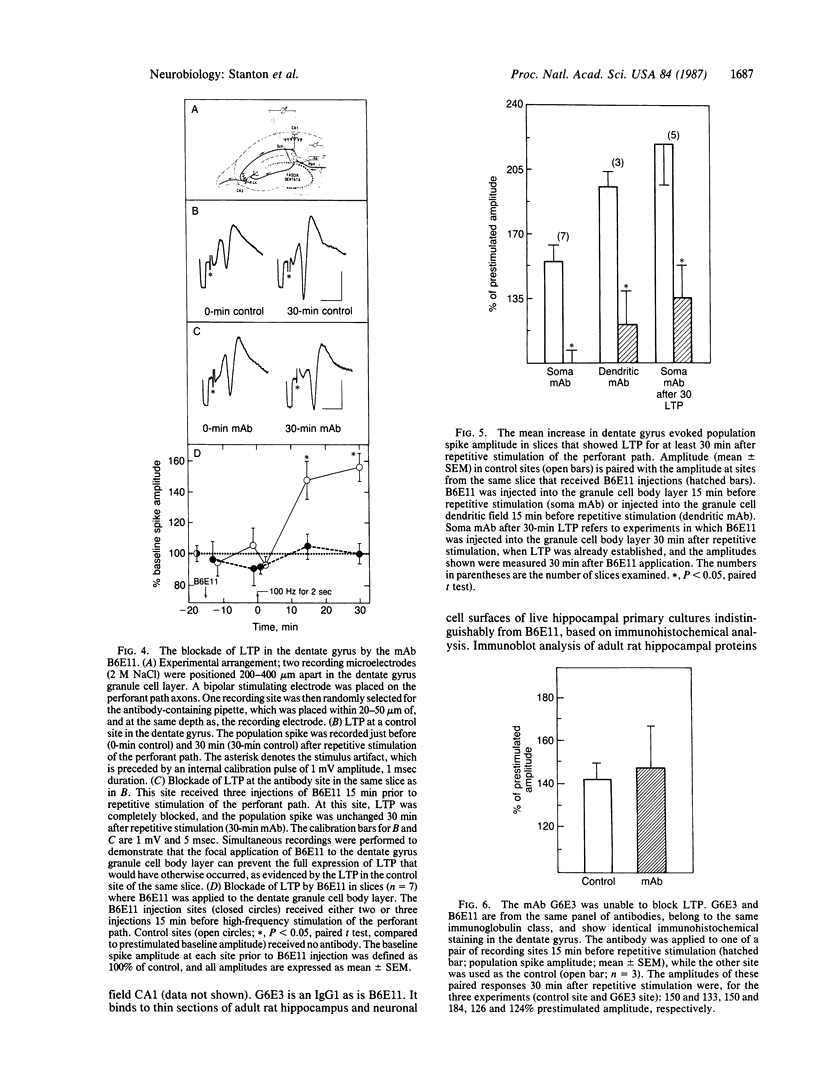
Monoclonal antibodies generated to 5-day-old postnatal rat dentate gyri were tested for their effects on long-term potentiation (LTP) in rat hippocampal slices. One antibody, B6E11, was found to block the production of LTP and to suppress established LTP in both area CA1 of the hippocampus and the dentate gyrus. In CA1, B6E11 was effective only when applied to the apical dendrites synapsing with the potentiating input. The production of LTP could not be blocked if B6E11 was applied to the cell bodies or to the basal dendrites of CA1. In field CA1, B6E11 had no effect on the production of short-term potentiation. Another monoclonal antibody from the same panel as B6E11, of the same immunoglobulin class, which also binds to hippocampal neurons similarly to B6E11, had no effect on LTP production. These results supply evidence that B6E11 modulates LTP by an interaction with a specific cell-surface protein associated with the dendrites of neurons in both dentate gyrus and CA1 regions.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham W. C., Bliss T. V., Goddard G. V. Heterosynaptic changes accompany long-term but not short-term potentiation of the perforant path in the anaesthetized rat. J Physiol. 1985 Jun;363:335–349. doi: 10.1113/jphysiol.1985.sp015714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alger B. E., Teyler T. J. Long-term and short-term plasticity in the CA1, CA3, and dentate regions of the rat hippocampal slice. Brain Res. 1976 Jul 16;110(3):463–480. doi: 10.1016/0006-8993(76)90858-1. [DOI] [PubMed] [Google Scholar]
- Andersen P., Sundberg S. H., Sveen O., Wigström H. Specific long-lasting potentiation of synaptic transmission in hippocampal slices. Nature. 1977 Apr 21;266(5604):736–737. doi: 10.1038/266736a0. [DOI] [PubMed] [Google Scholar]
- Bliss T. V., Gardner-Medwin A. R. Long-lasting potentiation of synaptic transmission in the dentate area of the unanaestetized rabbit following stimulation of the perforant path. J Physiol. 1973 Jul;232(2):357–374. doi: 10.1113/jphysiol.1973.sp010274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss T. V., Lomo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol. 1973 Jul;232(2):331–356. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning M., Dunwiddie T., Bennett W., Gispen W., Lynch G. Synaptic phosphoproteins: specific changes after repetitive stimulation of the hippocampal slice. Science. 1979 Jan 5;203(4375):60–62. doi: 10.1126/science.214855. [DOI] [PubMed] [Google Scholar]
- Bär P. R., Tielen A. M., Lopes Da Silva F. H., Zwiers H., Gispen W. H. Membrane phosphoproteins of rat hippocampus: sensitivity to tetanic stimulation and enkephalin. Brain Res. 1982 Aug 5;245(1):69–79. doi: 10.1016/0006-8993(82)90340-7. [DOI] [PubMed] [Google Scholar]
- Collingridge G. L., Kehl S. J., McLennan H. Excitatory amino acids in synaptic transmission in the Schaffer collateral-commissural pathway of the rat hippocampus. J Physiol. 1983 Jan;334:33–46. doi: 10.1113/jphysiol.1983.sp014478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas R. M., Goddard G. V. Long-term potentiation of the perforant path-granule cell synapse in the rat hippocampus. Brain Res. 1975 Mar 21;86(2):205–215. doi: 10.1016/0006-8993(75)90697-6. [DOI] [PubMed] [Google Scholar]
- Duffy C., Teyler T. J., Shashoua V. E. Long-term potentiation in the hippocampal slice: evidence for stimulated secretion of newly synthesized proteins. Science. 1981 Jun 5;212(4499):1148–1151. doi: 10.1126/science.7233208. [DOI] [PubMed] [Google Scholar]
- Galfre G., Howe S. C., Milstein C., Butcher G. W., Howard J. C. Antibodies to major histocompatibility antigens produced by hybrid cell lines. Nature. 1977 Apr 7;266(5602):550–552. doi: 10.1038/266550a0. [DOI] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Derivation of specific antibody-producing tissue culture and tumor lines by cell fusion. Eur J Immunol. 1976 Jul;6(7):511–519. doi: 10.1002/eji.1830060713. [DOI] [PubMed] [Google Scholar]
- Lynch G., Baudry M. The biochemistry of memory: a new and specific hypothesis. Science. 1984 Jun 8;224(4653):1057–1063. doi: 10.1126/science.6144182. [DOI] [PubMed] [Google Scholar]
- Milner B. Disorders of learning and memory after temporal lobe lesions in man. Clin Neurosurg. 1972;19:421–446. doi: 10.1093/neurosurgery/19.cn_suppl_1.421. [DOI] [PubMed] [Google Scholar]
- Moskal J. R., Schaffner A. E. Monoclonal antibodies to the dentate gyrus: immunocytochemical characterization and flow cytometric analysis of hippocampal neurons bearing a unique cell-surface antigen. J Neurosci. 1986 Jul;6(7):2045–2053. doi: 10.1523/JNEUROSCI.06-07-02045.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Routtenberg A., Lovinger D. M. Selective increase in phosphorylation of a 47-kDa protein (F1) directly related to long-term potentiation. Behav Neural Biol. 1985 Jan;43(1):3–11. doi: 10.1016/s0163-1047(85)91426-8. [DOI] [PubMed] [Google Scholar]
- Scharfman H. E., Sarvey J. M. Postsynaptic firing during repetitive stimulation is required for long-term potentiation in hippocampus. Brain Res. 1985 Apr 8;331(2):267–274. doi: 10.1016/0006-8993(85)91552-5. [DOI] [PubMed] [Google Scholar]
- Scharfman H. E., Sarvey J. M. gamma-Aminobutyrate sensitivity does not change during long-term potentiation in rat hippocampal slices. Neuroscience. 1985 Jul;15(3):695–702. doi: 10.1016/0306-4522(85)90071-5. [DOI] [PubMed] [Google Scholar]
- Schwartzkroin P. A., Wester K. Long-lasting facilitation of a synaptic potential following tetanization in the in vitro hippocampal slice. Brain Res. 1975 May 16;89(1):107–119. doi: 10.1016/0006-8993(75)90138-9. [DOI] [PubMed] [Google Scholar]
- Stanton P. K., Sarvey J. M. Blockade of long-term potentiation in rat hippocampal CA1 region by inhibitors of protein synthesis. J Neurosci. 1984 Dec;4(12):3080–3088. doi: 10.1523/JNEUROSCI.04-12-03080.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton P. K., Sarvey J. M. Blockade of norepinephrine-induced long-lasting potentiation in the hippocampal dentate gyrus by an inhibitor of protein synthesis. Brain Res. 1985 Dec 30;361(1-2):276–283. doi: 10.1016/0006-8993(85)91299-5. [DOI] [PubMed] [Google Scholar]
- Stanton P. K., Sarvey J. M. Depletion of norepinephrine, but not serotonin, reduces long-term potentiation in the dentate gyrus of rat hippocampal slices. J Neurosci. 1985 Aug;5(8):2169–2176. doi: 10.1523/JNEUROSCI.05-08-02169.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]


