Abstract
Pregnancy-specific β1 glycoproteins (PSGs) are the most abundant fetal proteins in the maternal bloodstream in late pregnancy. They are secreted by the syncytiotrophoblast and are detected around day 14 postfertilization. There are 11 human PSG genes, which encode a family of proteins exhibiting significant conservation at the amino acid level. We and others have proposed that PSGs have an immune modulatory function. In addition, we recently postulated that they are proangiogenic due to their ability to induce the secretion of VEGF-A and the formation of tubes by endothelial cells. The cellular receptor(s) for human PSGs remain unknown. Therefore, we conducted these studies to identify the receptor for PSG1, the highest expressed member of the family. We show that removal of cell surface glycosaminoglycans (GAGs) by enzymatic or chemical treatment of cells or competition with heparin completely inhibited binding of PSG1. In addition, PSG1 did not bind to cells lacking heparan or chondroitin sulfate on their surface, and binding was restored upon transfection with all four syndecans and glypican-1. Importantly, the presence of GAGs on the surface of endothelial cells was required for the ability of PSG1 to induce tube formation. This finding indicates that the PSG1-GAG interaction mediates at least some of the PSG1 proposed functions.
Keywords: Cell Surface Receptor, Chondroitin Sulfate, Endothelium, Heparan Sulfate, Reproduction, Angiogenesis, Pregnancy-specific Glycoprotein 1, Syndecans
Introduction
Pregnancy success requires that the maternal immune system does not attack the fetal trophoblast cells, which are in direct contact with maternal blood and express genes derived from both the mother and the father. In addition, during pregnancy major vascular adaptations are required to guarantee fetal growth and survival. These include uterine vessel dilation, remodeling of the maternal decidual arteries, and angiogenesis within the placenta villi, as reviewed in Ref. 1. Primates and rodents synthesize a group of placental proteins known as pregnancy-specific glycoproteins (PSGs)2 or Schwangerschafts protein 1 (2–4). PSGs are an early biochemical marker of syncytiotrophoblast formation and are detected at the time of implantation, becoming the most abundant placental protein (200–400 μg/ml) in the maternal bloodstream in late pregnancy (5, 6). Certain pregnancy conditions such as small-for-gestational age fetus are associated with low PSG levels (7, 8). The PSG family belongs to the carcinoembryonic antigen family, which is part of the immunoglobulin gene superfamily. There are 11 members of the PSG family in humans (PSG1–11) and 17 mouse genes (PSG16–32) located on chromosome 19 and chromosome 7, respectively (4, 9). We and others have suggested that human PSGs contribute to the establishment of an immune environment required for the tolerance to the fetal semiallograft (10–13). In addition, we have recently reported that some PSGs may play a role in the development of the placental vasculature (14). Specifically, PSG1 and some murine PSGs induced endothelial tube formation and the secretion of TGF-β1 and of VEGF-A in different cell types. Interestingly, many different cell types respond to PSG treatment, including monocyte/macrophages, endothelial cells, extravillous trophoblast cells lines, and dendritic cells, and human PSGs have activity in mouse cells (11, 13, 14). These observations suggest that cells from different origins express the receptor for these proteins, and there is cross-species binding.
We determined that murine PSG17 and 19 bind to the tetraspanin CD9 (15, 16). In contrast, human PSG1 and murine PSG22 and 23 did not bind to human or murine CD9. Therefore, we undertook the studies reported here to identify the receptor for PSG1. We show that PSG1 binds to heparan and chondroitin sulfate proteoglycans. Specifically, we found that PSG1 binds syndecans 1–4 and glypican-1 on the surface of cells. Importantly, binding of PSG1 to glycosaminoglycans (GAGs) on the surface of endothelial cells mediates tube formation.
EXPERIMENTAL PROCEDURES
Recombinant Protein Production and Purification
Generation of PSG1-Fc has been reported previously (17). Briefly, the PSG1 cDNA encoding the leader peptide, N, A2, and B2 domains was subcloned into the pFuse-IgG1 e3-Fc1 vector (InvivoGen, San Diego, CA), resulting in the addition of the hinge region, CH2 and CH3 domains of the IgG heavy chain which is mutated to reduce binding to Fcγ receptors or complement factors. The control protein, FLAGFc, was harvested and purified from the supernatant of stably transfected Chinese hamster ovary (CHO) cells as described previously (17).
The plasmids encoding the wild-type and mutated PSG1-Fc were transfected into CHO-K1 cells with Lipofectamine 2000 (Invitrogen). Five hours after transfection, the medium was aspirated and replaced with OPTI-MEM I (Invitrogen) for 72 h prior to harvesting of the supernatant. After a short centrifugation and filtration, the recombinant proteins in the collected medium were purified with a 5-ml HiTrap protein A column (GE Healthcare) under the manufacturer's recommendations. The proteins were eluted with 0.1 m glycine (pH 2.9) in 1-ml fractions, which were neutralized by adding 100 μl of 1 m Tris-HCl (pH 8.0). Individual fractions were analyzed by Western blotting with HRP-conjugated anti-human Fc (Thermo Scientific), and positive fractions were pooled, concentrated, and buffer-exchanged with PBS using Amicon Ultra-15 10-KDa MWCO centrifugal units (Millipore Corp.) To quantitate the proteins and assess their purity, recombinant proteins were separated on a 4–12% NuPAGE BisTris gel (Invitrogen) at different dilutions, stained with GelCode Blue (Thermo Scientific), and quantitated against BSA standards (Thermo Scientific).
Cell Lines
All cells, primary and established cell lines, were cultured in 5% CO2/95% air in a 37 °C humidified incubator. The human invasive trophoblast HTR-8/SVneo cell line was provided to us by Dr. Charles Graham (Queen's University, Kingston, ON, Canada) and was cultured in RPMI 1640 medium (Invitrogen) with 5% FBS, 1 × normocin (InvivoGen), and 100 units/ml penicillin/streptomycin (Invitrogen) (18). The human endometrial endothelial (HEEC) cell line was a gift from Dr. Gil Mor (Yale University School of Medicine, New Haven, CT). HEE cells were cultured as described by Krikun et al. (19). Human monocytes were isolated from the peripheral blood of healthy adult donors and purified by centrifugal elutriation as described previously (11). Monocytes were maintained in DMEM (Invitrogen) and 50 μg/ml gentamicin. Samples were obtained in accordance with NIH IRB-approved protocols. The CHO-K1, CHO-pgsD-677, and CHO-pgsA-745, BHK-21, COS-1, A431, HeLa, HEK 293, NIH 3T3, Jurkat E6-1, Daudi, and FDCP-1 cells were obtained from American Type Culture Collection (Manassas, VA) and cultured in the medium and conditions recommended. Mouse L929, gro2C, and sog9 cells were a kind gift of Dr. Frank Tufaro and Dr. Gary Cohen (20) and were sent to us by Dr. Katherine Spindler (University of Michigan, Ann Arbor). These cells were cultured in DMEM supplemented with 5% fetal bovine serum, 100 units/ml penicillin and streptomycin. The Namalwa human B lymphoblastoid cell line and the Namalwa transfectants expressing the individual syndecans or glypican-1 have been described previously (21) and were cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum, 100 units/ml penicillin and streptomycin, 2 mm glutamine, and 0.5 mg/ml G418. Human CD4+ T cells were purchased from Lonza (Walkersville, MD) and cultured in X-vivo 15 medium (Lonza). For T cell activation, cells were seeded for 48 h at 5 × 105 cells/well on human T cell activation plates (BD Biosciences). Resting cells were kept for the same amount of time in wells, which had no antibody added. Porcine aortic endothelial cells expressing the yellow fluorescent protein (PAE-YFP) were cultured in DMEM/F12 medium (Invitrogen) supplemented with 10% FBS and 100 units/ml penicillin and streptomycin (22).
Digestion of GAGs by Heparinase I and Chondroitinase ABC and of GPI-linked Proteins with Phosphatidylinositol-specific Phospholipase C (PI-PLC)
To remove cell surface GAGs, 106 cells were detached from their culture vessel using Accutase (Innovative Cell Technologies, San Diego, CA) and then incubated with 12 units/ml and 1 unit/ml heparinase I and chondroitinase ABC, respectively, in 100 μl of PBS containing 2% BSA for 1:30 h at 37 °C. Control cultures were incubated for the same period in the same reaction buffer, but without enzyme addition. For removal of GPI-linked proteins in PAE cells, the cells were dislodged from the flask using Accutase and washed with PBS, and 6.2 × 105 cells were treated for 20 min with 5 μl of PI-PLC (100 units/ml) or no enzyme in a 50-μl volume of DMEM with 0.2% ITS (Lonza).
Cell-based Ligand Binding Assays and Flow Cytometry
For FACS analysis, adherent cells were detached with Accutase, and 1 × 106 cells in 100 μl of FACS buffer (PBS with 2% BSA and 0.05% NaN3) were incubated with the indicated proteins or antibodies for 1 h on ice. To detect protein binding to cells, we employed 2.5 μg/ml PE-labeled anti-human Fcγ (eBiosciences, San Diego, CA) for 1 h on ice. In some experiments, protein was detected with 5 μg/ml biotin-conjugated anti-human Fcγ fragment (eBiosciences) followed by streptavidin APC (Invitrogen). Excess protein and antibody were removed by washing with FACS buffer between steps. To determine whether heparin could compete with PSG binding, 2 μg/ml heparin together with the PSG or control protein was added to the cells. Prior to the addition of protein or antibodies to the Daudi, FDCP-1, and Namalwa cells lines and to human monocytes, cells were blocked for 15–30 min on ice with Fc block (Miltenyi Biotec, Auburn, CA). To confirm expression of syndecans in the Namalwa transfectants, cells were incubated with 1 μg FITC-labeled anti-heparan sulfate 10E4 (US Biological, Swampscott, MA) or FITC-labeled IgM, κ isotype control (BioLegend, San Diego, CA). Syndecan and glypican-1 expression in PAE cells was analyzed by FACS with mAbs F58-10H4 (anti-syndecan-2) and F58-1C7 (anti-syndecan-3), F94-8G3 (anti-syndecan-4), S1 (anti-glypican-1) followed by biotin-conjugated anti-mouse IgG and streptavidin APC and with Alexa Fluor 647-conjugated anti-syndecan-1 clone B-A38 (AbD Serotec). Cells were fixed with BD Cytofix and analyzed using the BD LSR II (BD Biosciences). Fifty thousand total events were collected using BD FACS Diva software (BD Biosciences), and the FlowJo software (Tree Star, Ashland, OR) was used for postacquisition analysis.
Solid Phase Binding Assay
96-well ELISA plates were coated overnight at 4 °C with 200 μg of heparin, heparan, or chondroitin sulfate or BSA in quadruplicate. The coated plates were washed and blocked with PBS and 0.5% BSA. Purified recombinant proteins (200 ng/well in 100 μl of PBS) were applied to the coated plates and incubated overnight at 4 °C. After three washes with PBS and 0.05% Tween 20, the proteins were detected with 1 ng/ml HRP-conjugated anti-Fcγ Ab (Thermo Scientific) followed by the addition of TMB substrate reagent (BD Biosciences). The reaction was stopped by the addition of sulfuric acid, and the plate was read at 450 nm in an ELISA plate reader.
Heparin Binding Column
One hundred micrograms of purified protein was diluted 2-fold with loading buffer (10 mm sodium phosphate (pH 7.0)) and passed through a 1-ml heparin column (GE Healthcare) in the AKTA-Plus system (GE Healthcare) at 1 ml/min. The column was washed with 10 column volumes of loading buffer, and bound protein was eluted using a linear gradient of NaCl over 20 column volumes. Eluted samples were separated on a 4–12% NuPAGE BisTris gel followed by immunoblot analysis.
In Vitro Tube Formation Assay
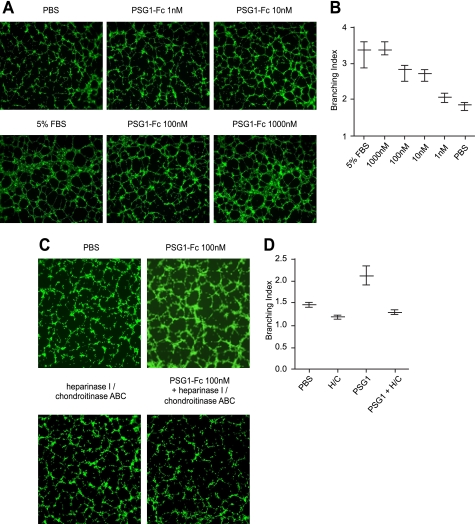
PAE cells were seeded on 96-well plates at 2.7 × 104 cells/well over 50 μl of gelled, reduced growth factor Geltrex (Invitrogen). Cultures were immediately treated with PBS (negative controls), 5% FBS (positive controls), and various concentrations of recombinant PSG1 (1, 10, 100, and 1,000 nm). Tubular structures were visualized with a 5 × objective and photographed after a 5-h incubation with an AxioCam camera connected to a Zeiss Axiovert 200M microscope. In a separate experiment, PAE cells were pretreated with heparinase I and chondroitinase ABC as described above or reaction buffer for 1.5 h at 37 °C, and the potential of PSG1 (100 nm) to form tubes was evaluated. The enzymes were kept during the incubation of PSG1 or the negative control with the endothelial cells. The complexity of capillary-like structures induced by PSG1 was evaluated using ImageJ, the Java-based image processing program developed at the National Institutes of Health (Bethesda, MD). The branching index, which reflects the numbers of tubes connected to cell clusters, was employed to quantitate the images.
Reagents
Heparin, heparan, and chondroitin sulfate, BSA, sodium chlorate, heparinase I and chondroitinase ABC were obtained from Sigma-Aldrich. Fully de-O-sulfated heparin was obtained from Neoparin (Alameda, CA). PI-PLC was obtained from Invitrogen. The hybridoma secreting anti-PSG1 mAb number 4 was generated by Dr. Stipan Jonjic (University of Rijeka, Croatia).
Statistics
SPSS (SPSS, Inc., Chicago, IL) and Microsoft EXCEL were used for data statistical analysis. The two-tailed Student's t test was used to determine statistical significance in experiments comparing protein treated versus untreated cells, with a p value of <0.05 as a cut-off. For tube formation statistical analysis, we employed ANOVA and Bonferroni's multiple comparison tests. All data are representative of at least three independent experiments.
RESULTS
PSG1 Binds to Cell Surface Proteoglycans
Several cell types derived from different species and tissues, besides the PSG target cells previously identified in our laboratory (14, 17), were tested by FACS for their ability to bind recombinant PSG1. These cells included BHK-21, COS-1, A431, HeLa, HEK 293, NIH 3T3, Jurkat, FDC-P1, Daudi, L929, and CHO-K1. We found that PSG1 bound to all cells tested, albeit at different levels, with the exception of the murine lymphoblast FDC-P1 and the human B lymphoblast Daudi cell lines (data not shown). These observations prompted us to investigate whether PSG1 could bind to heparan sulfate proteoglycans which are not expressed by FDC-P1 (23) and have been reported to be absent or expressed at very low levels in several pre-B stage cells (24).
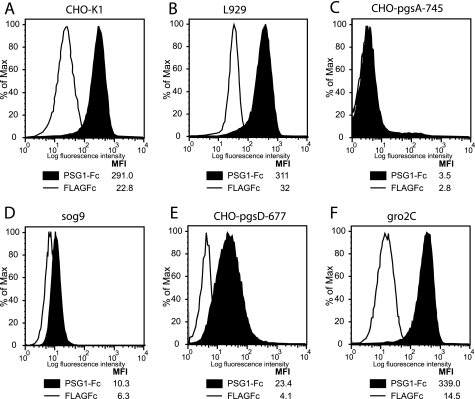
To this end, we obtained CHO-K1 cells and CHO-K1-derived mutants (CHO-pgsD-677 and CHO-pgsA-745) and murine L929 and its derived mutants (gro2C and sog9). The CHO-K1 and L929 mutants are defective in proteoglycan synthesis. Specifically, CHO-pgsD-677 lacks both N-acetylglucosaminyl- and glucuronosyl-transferase activities, and like the gro2C cells, which has a splice site mutation in the EXT1 gene encoding the HS-synthesizing enzyme, it lacks the ability to synthesize heparan sulfate. CHO-pgsA-745 produces neither chondroitin nor heparan-sulfated proteoglycans (HSPGs) (20, 25–27), and sog9 cells have the same mutation as the gro2c cells with an additional mutation in the chondrotin 4-O-sulfotransferase-1 enzyme, resulting in CS of reduced length and degree of 4-O-sulfation (28). In contrast to parental CHO-K1 and L929, PSG1-Fc showed no binding to CHO-pgsA-745 and minimal binding to sog9 cells (Fig. 1, A–D). On the other hand, PSG1 bound significantly over control protein to CHO-pgsD-677, which has three times more CS on their surface than CHO-K1 cells, and to gro2C cells (Fig. 1, E–F). The binding to the HS-null/CS-containing cells was much lower than the binding observed compared with the respective parental cell lines. These results suggested that PSG1 binds to cell surface HSPGs and to a lesser extent to CSPGs on the surface of these cells.
FIGURE 1.
PSG1 binds to plasma membrane proteoglycans. CHO-K1 (A), L929 (B), and their derived mutant cell lines CHO-pgsA-745 (C), sog9 (D), CHO-pgsD-677 (E), and gro2c (F) were treated with 30 μg/ml PSG1-Fc or the control protein FLAGFc followed by PE-conjugated anti-human Fcγ. The median fluorescence intensity (MFI) of each treatment is shown.
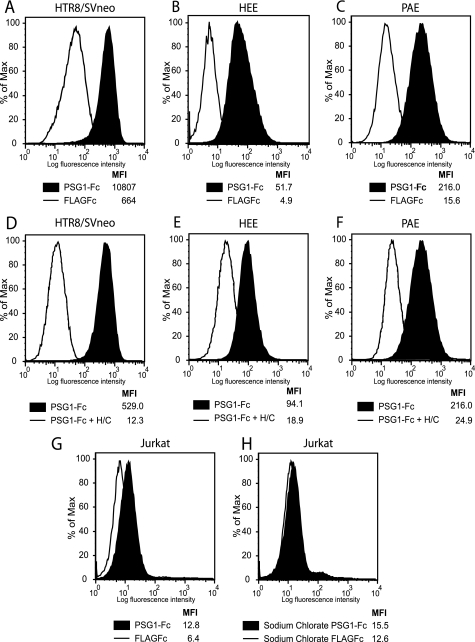
To confirm whether PSG1 binds to proteoglycans, we performed different experiments including: (i) enzymatic removal of GAGs, (ii) chemical treatment of cells to reduce GAG sulfation, and (iii) competition of binding with heparin, a structural homolog of highly sulfated HS (29). Treatment of HTR-8/SVneo, HEE, and PAE cells with enzymes that selectively degrade highly sulfated domains of HS-heparinase I and CS-chondroitinase ABC was performed as indicated under “Experimental Procedures.” As shown in Fig. 2, binding of PSG1 to these cells was significantly reduced after enzymatic treatment. Treatment of cells with heparinase I only was sufficient to observe a significant reduction of PSG1 binding (data not shown).
FIGURE 2.
Treatment of cells with heparinase I and chondroitinase ABC or with sodium chlorate inhibits PSG1 binding to HTR8/SVneo, HEE, PAE, and Jurkat cells. HTR8/SVneo (A and D), HEE (B and E), and PAE (C and F) cells were treated with a combination of heparinase I and chondroitinase ABC (designated as H/C) or left untreated. Jurkat cells were left untreated (G) or were treated with sodium chlorate (25 mm) for 3 days (H). Cells were incubated with 30 μg/ml PSG1-Fc or the control protein FLAGFc followed by PE-conjugated anti-human Fcγ. The median fluorescence intensity (MFI) of each treatment is shown.
Of all the cells tested, human monocytes and Jurkat cells showed low but significant PSG binding over the control protein. Monocytes express HS and CS B on their cell surface, and the T cell line Jurkat expresses low levels of HS (30). Treatment with sodium chlorate, which competitively inhibits the formation of 3′-phosphoadenosine 5′-phosphosulfate, the high energy sulfate donor in cellular sulfation reactions without affecting protein synthesis or other posttranslational modifications, has been previously shown to reduce GAG sulfation efficiently in these cells (31). Binding to human monocytes (data not shown) and Jurkat cells was reduced to the level of the control protein by growing the cells in medium containing 25 or 50 mm sodium chlorate for 3 days (Fig. 2, G and H). As expected, the presence of sodium chlorate did not reduce cell viability as determined by trypan blue exclusion.
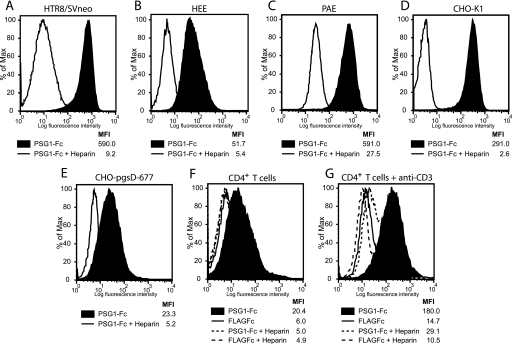
Fig. 3, A–C, shows that PSG1 does not bind to HTR-8/SVneo, HEE, or PAE cells in the presence of heparin. When lower concentrations of heparin were utilized as competitor (0.5–1 μg/ml), binding of PSG1 was partially reduced (data not shown). Heparin also inhibited binding of PSG1 to CHO-K1 and CHO-pgsD-677 cells (Fig. 3, D and E).
FIGURE 3.
Heparin treatment inhibits PSG1 binding to HTR8/SVneo, HEE, PAE, CHO, and human CD4+ T cells. HTR8/SVneo (A), HEE (B), PAE (C) CHO-K1 (D), and CHO-pgsD-677 (E) cells were treated with PSG1-Fc (30 μg/ml) alone or in combination with heparin (2 μg/ml) followed by PE-conjugated anti-human Fcγ. Human CD4+ T cells were left untreated (resting) or were activated with 10 μg/ml plate-bound anti-CD3 for 48 h. Resting (F) and activated (G) CD4+ T cells were treated with 30 μg/ml PSG1-Fc or the control FLAGFc alone or in combination with heparin (2 μg/ml) followed by PE-conjugated anti-human Fcγ. The median fluorescence intensity (MFI) of each treatment is shown.
Recently, it has been shown that human resting CD4+ T cells express low levels of syndecans on their cell surface, but upon activation the levels of syndecan-1 and -4 are significantly increased (32). Therefore, we tested whether PSG1 binding to CD4+ T cells activated with plate-bound anti-CD3 differs from binding to nonactivated cells. As shown in Fig. 3, F and G, PSG1 binds significantly more to activated CD4+ T cells. In addition, binding of PGS1 to resting and activated T cells was inhibited by the addition of heparin (Fig. 3, C and G).
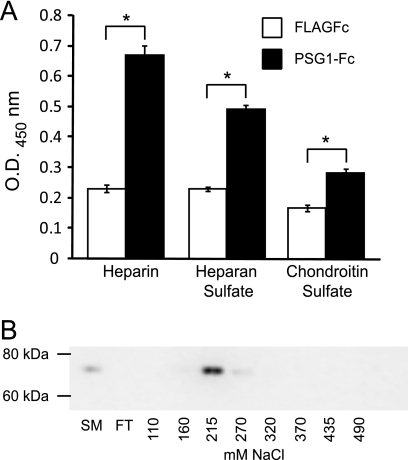
PSG1 Binds to Immobilized HS and Heparin
To investigate whether the GAG-dependent binding to cells correlates with direct binding to heparin/HS, we examined the ability of these proteins to bind to these molecules on a solid matrix. PSG1-Fc bound to heparin, HS, and to a lesser extent to CS-coated wells (Fig. 4A). These results strongly suggested that PSG1 is a heparin-binding protein. To confirm these observations further, we evaluated the ability of PSG1-Fc to bind to heparin-Sepharose. Purified PSG1-Fc was applied to a heparin-Sepharose column, and after several washes the proteins were eluted with a linear salt gradient of NaCl. Aliquots of eluted fractions were assessed by immunoblot analysis with anti-PSG1 mAb number 4. As shown in Fig. 4B, PSG1-Fc eluted from the column between 0.21 and 0.27 m NaCl. When full-length PSG1 with a His6 tag rather than an Fc tag was loaded onto the heparin-Sepharose column, the protein eluted between 0.3 and 0.37 m NaCl (data not shown).
FIGURE 4.
PSG1 binds to immobilized GAGs. A, 96-well ELISA plates were coated with 200 μg/well heparin, heparan sulfate, chondroitin sulfate, or BSA and treated with PSG1-Fc or the control protein FLAGFc (200 ng/well). Bound proteins were detected with HRP-conjugated anti-Fcγ Ab. All treatments were done in quadruplicate, and data are representative of at least three independent experiments. Statistical significance between the protein control (FLAGFc) and PSG1-Fc treated wells was determined by two-tailed Student's t test (*, p < 0.05). Error bars represent the S.E. B, PSG1-Fc was applied to a 1-ml heparin-Sepharose column, and after washing, the protein was eluted with a linear salt gradient from 0 to 1 m NaCl. Aliquots of starting material (SM), flow-through (FT), and eluted fractions were assessed by immunoblot analysis with anti-PSG1 mAb number4 after separation on a 4–12% NuPAGE BisTris gel.
PSG1 Binds to All Four Syndecans
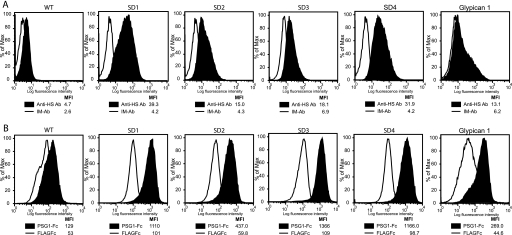
Mammalian cells express two types of membrane-bound HSPG, the glypicans and the syndecans. There are four members of the syndecan family (syndecan 1–4) which are type I transmembrane proteins with up to five GAG attachment sites substituted mainly with HS chains (for review, see Ref. 33). Namalwa cells are a human B lymphoblastoid cell line, which expresses low levels of HSPG, mainly glypican-1 (21). Namalwa cells stably transfected with each of the four syndecans and with glypican-1 were generated as described previously, and as expected, they express much higher levels of HSPG on their surface than wild-type cells (Fig. 5A). The ability of PSG1-Fc to bind to the syndecan-transfected cells was determined and compared with the binding of this protein to the plasmid control Namalwa transfectants. Fig. 5B shows that PSG1 bound significantly more to each of the syndecan-expressing cells than to the wild-type cells. In addition, PSG1 bound more to Namalwa cells transfected with glypican-1 than to control cells (Fig. 5B).
FIGURE 5.
PSG1 binds to syndecans 1–4 and glypican-1. A, Namalwa and Namalwa cells stably transfected with syndecans-1, -2, -3, -4, or glypican-1 were incubated with 1 μg of FITC-labeled anti-heparan sulfate 10E4 antibody (Anti-HS Ab) or FITC-labeled isotype matched (IM-Ab) as the control. B, the cells shown in A were incubated with 30 μg/ml PSG1-Fc or the control protein FLAGFc followed by PE-conjugated anti-human Fcγ. The median fluorescence intensity (MFI) of each treatment is shown.
Binding of PSG1 to GAG Chains Mediates Endothelial Tube Formation
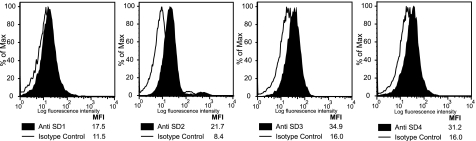
We have shown previously that PSG1 induces endothelial tube formation in primary endothelial cells in the absence of added growth factors (17). Recently, several laboratories demonstrated that syndecans on the surface of endothelial cells play a role in angiogenesis (34). Therefore, we hypothesized that the observed PSG1-elicited endothelial tube formation could be mediated by the interaction between PSG1 and syndecans on the endothelial cell membrane. To investigate this possibility we treated a PAE cell line, which does not express the VEGF kinase insert domain receptor (KDR) (22), with PSG1 at different concentrations. As determined by FACS analysis and shown for other endothelial cells, PAE cells express all four syndecans (Fig. 6). As observed previously with primary endothelial cells, PSG1-Fc induced formation of tubes in a dose-dependent manner with significant differences in branching index compared with the PBS control starting at a concentration of 10 nm (Fig. 7A). To determine whether the presence of GAGs on the cell surface was required for the formation of tubes induced by PSG1, PAE cells were incubated with heparinase I and chondroitinase ABC as indicated under “Experimental Procedures.” As shown in Fig. 7B, the ability of PSG1 to induce tube formation was significantly reduced upon treatment of the cells with heparinase I and chondroitinase ABC, whereas there was a small but not statistically significant effect of enzymatic treatment of the cells in the negative control. Besides syndecans, the other cell surface PG reported to be expressed in endothelial cells is glypican-1 (35). Therefore, we examined whether PAE cells express this GPI-linked protein. PAE cells express very low levels of glypican-1 (supplemental Fig. 1). To determine whether binding of PSG1 to glypicans rather than syndecans could be mediating the observed capillary-like structures, we treated PAE cells with PI-PLC, which removes GPI-linked proteins from the plasma membrane (36). Treatment of cells with PI-PLC did not inhibit PSG1-mediated tubulogenesis. These results indicate that the ability of PSG1 to induce the formation of tubes by endothelial cells is related to its binding to GAGs attached to proteins which have a transmembrane domain rather than GAGs on proteins anchored to the membrane by a GPI linkage.
FIGURE 6.
Expression of syndecans in PAE cells. PAE cells were incubated with 1 μg of anti-syndecan-1, anti-syndecan-2, anti-syndecan-3, or anti-syndecan-4 mAb followed by biotin-conjugated anti-mouse IgG and streptavidin APC. The expression of each syndecan is shown as the median fluorescence intensity (MFI).
FIGURE 7.
PSG1-mediated tube formation by PAE cells is dose-dependent and is inhibited by treatment of the cells with heparinase I and chondroitinase ABC. A, PAE-YFP cells were seeded on 96-well plates over gelled-reduced growth factor Geltrex and immediately treated with PBS (negative controls), 5% FBS (positive controls) and various concentrations of PSG1-Fc as shown. B, the branching index, obtained as described under “Experimental Procedures, ” for the different treatments shown in A is represented in the form of a whiskers plot with minimal and maximal values indicated. C, PAE cells were pretreated with heparinase I and chondroitinase ABC as described under “Experimental Procedures ” or left untreated for 1.5 h at 37 °C prior to the addition of PSG1 (100 nm). D, branching index for the treatments shown in C is represented in the form of a whiskers plot with minimal and maximal values indicated. Tubular structures shown in A and C were photographed after 5 h of incubation with each treatment.
DISCUSSION
We have proposed that members of the human and murine PSG family have both immune-regulatory and proangiogenic activity. We undertook the studies reported here to identify the receptor for PSG1 and found that PSG1 binds to cell surface PGs. PGs consist of a protein core and covalently attached GAG chains (37). The syndecans are considered hybrid PGs because they contain mixtures of the two major types of GAG chains found in animal cells, HS and CS. There are four members of the syndecan family, syndecan-1 (CD138), syndecan-2 (fibrogycan), syndecan-3 (N-syndecan), and syndecan-4 (ryudocan or amphiglycan). The other major family of membrane PGs comprises the glypicans (glypicans-1 to -6), which contain GPI anchors instead of a membrane-spanning segment. Cell surface HSPGs have been shown to interact with diverse ligands, including growth factors/morphogens and their receptors, enzymes and enzyme inhibitors, cell adhesion molecules, chemokines, various extracellular matrix proteins, and microbial pathogens (for review, see Ref. 38).
The importance of PG expression in cells for PSG1 binding is supported by studies with hamster and murine cell lines defective in GAG synthesis. No binding to CHO-pgsA-745 cells lacking heparan and CS could be detected. In addition, low levels of binding to sog9 cells, which are HS-deficient but express CS chains of reduced length and degree of 4-O-sulfation, compared with the wild-type L929 cells was observed. These observations strongly suggest that cell surface proteoglycans are the only PSG1-binding moieties on the cells studied. Binding to the CS-containing cells, gro2C and CHO-pgsD-677, was much lower than binding to the parental cell lines, which contain both CS and HS. In addition, binding to CS on a solid phase assay although significant, was consistently lower than to heparan sulfate. These results suggest that PSG1 binds to cell surface HSPGs and to a lesser extent to CSPGs on the surface of cells. This conclusion has to be drawn with caution because PSG1 may bind with different affinity to a form of CS different from the one we have tested in the solid phase assay.
We found that PSG1 binds PGs on the surface of all human cells investigated, which included an extravillous trophoblast cell line, T cells, monocytes, and endothelial cells. When GAGs were removed enzymatically or sulfation was inhibited with sodium chlorate, PSG1 did not bind to any of these cells. In addition, binding was completely inhibited with heparin in a dose-dependent manner, observing small to no inhibition of PSG1 binding by fully de-O-sulfated heparin, a heparin derivative in which all O-sulfate esters are removed. Therefore as established for CHO-K1 and murine fibroblast L929 cells, GAGs contained in cell surface proteoglycans appear to be the binding sites for PSG1 in all cells investigated.
The significance of binding of PSG1 to cell surface PGs was further analyzed. As shown in Fig. 5, PSG1 bound to cells transfected with any of the four members of the syndecan family and to cells transfected with glypican-1, indicating that the interaction is most likely independent of the core protein. The presence of syndecans and glypican-1 on the endothelial cell surface has been very well documented (39), and their importance in angiogenesis was first realized by their observed role as low affinity co-receptors for several growth factors, including the heparin-binding splice isoforms of VEGF-A (40), and FGF-2 (41). Interestingly, a defect in placental development was reported in syndecan-4-null mice involving abnormalities in blood vessels (42).
Besides their ability to modulate growth factor binding, additional roles for syndecans but not for glypicans in angiogenesis have recently been appreciated (34). Syndecan-1 regulates two critical integrins in angiogenesis, αvβ3 and αvβ5 (43), and syndecan-4 regulates Akt activation in a PKCα-dependent manner in endothelial cells (44). In addition, down-regulation of syndecan-2 in microvascular endothelial cells has been shown to impair the formation of capillary-like structures. Therefore, we postulated that binding of PSG1 to syndecans in endothelial cells could mediate the formation of tubes we observed upon PSG1 treatment. Our results show that enzymatic removal of GAGs from the surface of endothelial cells negated the ability of PSG1 to induce tube formation, whereas removal of glypicans with PI-PLC did not. These results suggest that binding of PSG1 to syndecans on endothelial cells in the placenta, together with the ability of PSG1 to induce TGF-β1 and VEGF-A by immune cells and trophoblasts, mediates the proangiogenic activity of these proteins (17). Although as stated above, three of the four syndecans have been reported to play a role in the formation of capillary-like structures, the biological relevance of PSG1 binding to each syndecan requires further investigation in the context of an analysis of the expression of the individual syndecans and other cell surface proteoglycans by endothelial cells in the fetal-placental interface.
The functional consequence of binding of PSG1 to PGs expressed on the different cell types requires further investigation. Recently, binding of DC-HIL to syndecan-4 on activated T cells was shown to inhibit human allogeneic T cell responses (32). In addition, syndecans have been shown to regulate TGF-β activity and to prevent toxic shock by their ability to suppress the production of proinflammatory cytokines by macrophages (45, 46).
In the placenta, the syncytiotrophoblast in the chorionic villi exhibit apical plasma membrane expression of syndecan-1, which is reduced in preeclampsia (47). Therefore, it is possible that at least some PSGs could remain bound to the cells that secrete them. Other cells such as fetal macrophages, villous mesenchyme, and core fetal vessels also express syndecan-1 (48). Syndecan-2 expression is intense in the villous stroma, and syndecans-2 and -4 are expressed mainly in the villous cytotrophoblast and extravillous cytotrophoblastic cells at early pregnancy stages. Syndecan-3 staining has been reported in extravillous trophoblasts (48). A possible role for syndecans in the remodeling of extracellular matrix during the development of the villous tree was suggested after their expression was found to be altered in invasive mole and choriocarcinoma (49), but further studies are required to define their function(s) in the placenta.
Whether other human PSGs bind to cell surface proteoglycans has yet to be determined. There is between 54 and 78.5% identity between domains when all human PSGs are compared. The highest identity is found when comparing the A2 domains. Which of the three domains present in our recombinant protein (N, A2, or B2) binds to heparan/chondroitin sulfate is not known, but it is important to note that five different types of domain arrangements have been reported for human PSGs with some lacking the A2 domain. We are in the process of testing whether other members of the human PSG family also bind to syndecans and which domain of the protein mediates this interaction. Interestingly, YKL-40, a heparin-binding secreted glycoprotein of the chitinase gene family, was recently reported to induce the collaboration of syndecan-1 with integrin αvβ3 through HS (50). Based on reported studies, it would be interesting to explore whether PSGs induce the collaboration of syndecans with integrins on endothelial cells and the possible role of the RGD sequence present in the N domain of some PSGs during this process. In addition, whether members of the non-human primate or rodent PSG families use syndecans as their receptors is not known at this time.
Although the results presented here indicate that cell surface PGs appear to be the only receptors for PSG1 in the various cells examined, we cannot completely rule out that PSG1 may bind to another, as yet unidentified receptor. Preliminary results from our laboratory indicate that the ability of PSG1 to induce TGF-β1 does not require the presence of PGs and appears to be the result of a direct interaction between PSG1 and the growth factor. Further studies are needed to dissect the mechanism by which PSGs exert their immune-regulatory and proangiogenic functions during pregnancy and whether there is a requirement for binding to cellular receptors for all its reported activities. In summary, the results reported here show that PSG1 binding to cell surface proteoglycans mediates at least some of the reported proangiogenic properties of this placentally secreted glycoprotein.
Supplementary Material
Acknowledgments
We thank Kateryna Lund and Karen Wolcott for technical help with FACS experiments.
This work was supported, in whole or in part, by National Institutes of Health Grant R01HD035832 (to G. S. D.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. 1.
- PSG
- pregnancy-specific glycoprotein
- BisTris
- bis(2-hydroxyethyl)iminotris(hydroxymethyl)methane
- CS
- chondroitin sulfate
- CSPG
- CS proteoglycan
- GAG
- glycosaminoglycan
- GPI
- glycosylphosphatidylinositol
- HEE
- human endometrial endothelial
- HS
- heparan sulfate
- HSPG
- HS proteoglycan
- PAE
- porcine aortic endothelial
- PE
- phycoerythrin
- PG
- proteoglycan
- PI-PLC
- phosphatidylinositol-specific phospholipase C.
REFERENCES
- 1. Wulff C., Weigand M., Kreienberg R., Fraser H. M. (2003) Reproduction 126, 569–577 [DOI] [PubMed] [Google Scholar]
- 2. Tatarinov Iu S., Masiukevich V. N. (1970) Biull. Eksp. Biol. Med. 69, 66–68 [PubMed] [Google Scholar]
- 3. Zhou G. Q., Hammarström S. (2001) Biol. Reprod. 64, 90–99 [DOI] [PubMed] [Google Scholar]
- 4. McLellan A. S., Fischer B., Dveksler G., Hori T., Wynne F., Ball M., Okumura K., Moore T., Zimmermann W. (2005) BMC Genomics 6, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lin T. M., Halbert S. P., Spellacy W. N. (1974) J. Clin. Invest. 54, 576–582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Camolotto S., Racca A., Rena V., Nores R., Patrito L. C., Genti-Raimondi S., Panzetta-Dutari G. M. (2010) Placenta 31, 312–319 [DOI] [PubMed] [Google Scholar]
- 7. Towler C. M., Horne C. H., Jandial V., Campbell D. M., MacGillivray I. (1977) Br. J. Obstet. Gynaecol. 84, 258–263 [DOI] [PubMed] [Google Scholar]
- 8. Pihl K., Larsen T., Laursen I., Krebs L., Christiansen M. (2009) Prenat. Diagn. 29, 1256–1261 [DOI] [PubMed] [Google Scholar]
- 9. Thompson J., Koumari R., Wagner K., Barnert S., Schleussner C., Schrewe H., Zimmermann W., Müller G., Schempp W., Zaninetta D. (1990) Biochem. Biophys. Res. Commun. 167, 848–859 [DOI] [PubMed] [Google Scholar]
- 10. Wessells J., Wessner D., Parsells R., White K., Finkenzeller D., Zimmermann W., Dveksler G. (2000) Eur. J. Immunol. 30, 1830–1840 [DOI] [PubMed] [Google Scholar]
- 11. Snyder S. K., Wessner D. H., Wessells J. L., Waterhouse R. M., Wahl L. M., Zimmermann W., Dveksler G. S. (2001) Am. J. Reprod. Immunol. 45, 205–216 [DOI] [PubMed] [Google Scholar]
- 12. Motrán C. C., Díaz F. L., Gruppi A., Slavin D., Chatton B., Bocco J. L. (2002) J. Leukocyte Biol. 72, 512–521 [PubMed] [Google Scholar]
- 13. Motrán C. C., Diaz F. L., Montes C. L., Bocco J. L., Gruppi A. (2003) Eur. J. Immunol. 33, 3007–3016 [DOI] [PubMed] [Google Scholar]
- 14. Wu J. A., Johnson B. L., Chen Y., Ha C. T., Dveksler G. S. (2008) Biol. Reprod. 79, 1054–1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ha C. T., Waterhouse R., Warren J., Zimmermann W., Dveksler G. S. (2008) Am. J. Reprod. Immunol. 59, 251–258 [DOI] [PubMed] [Google Scholar]
- 16. Waterhouse R., Ha C., Dveksler G. S. (2002) J. Exp. Med. 195, 277–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ha C. T., Wu J. A., Irmak S., Lisboa F. A., Dizon A. M., Warren J. W., Ergun S., Dveksler G. S. (2010) Biol. Reprod. 83, 27–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Graham C. H., Hawley T. S., Hawley R. G., MacDougall J. R., Kerbel R. S., Khoo N., Lala P. K. (1993) Exp. Cell Res. 206, 204–211 [DOI] [PubMed] [Google Scholar]
- 19. Krikun G., Mor G., Alvero A., Guller S., Schatz F., Sapi E., Rahman M., Caze R., Qumsiyeh M., Lockwood C. J. (2004) Endocrinology 145, 2291–2296 [DOI] [PubMed] [Google Scholar]
- 20. Gruenheid S., Gatzke L., Meadows H., Tufaro F. (1993) J. Virol. 67, 93–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhang Z., Coomans C., David G. (2001) J. Biol. Chem. 276, 41921–41929 [DOI] [PubMed] [Google Scholar]
- 22. Rönnstrand L., Mori S., Arridsson A. K., Eriksson A., Wernstedt C., Hellman U., Claesson-Welsh L., Heldin C. H. (1992) EMBO J. 11, 3911–3919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Minguell J. J., Hardy C., Tavassoli M. (1992) Exp. Cell Res. 201, 200–207 [DOI] [PubMed] [Google Scholar]
- 24. Kim C. W., Goldberger O. A., Gallo R. L., Bernfield M. (1994) Mol. Biol. Cell 5, 797–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Esko J. D., Stewart T. E., Taylor W. H. (1985) Proc. Natl. Acad. Sci. U.S.A. 82, 3197–3201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Esko J. D., Elgavish A., Prasthofer T., Taylor W. H., Weinke J. L. (1986) J. Biol. Chem. 261, 15725–15733 [PubMed] [Google Scholar]
- 27. Banfield B. W., Leduc Y., Esford L., Schubert K., Tufaro F. (1995) J. Virol. 69, 3290–3298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Uyama T., Ishida M., Izumikawa T., Trybala E., Tufaro F., Bergström T., Sugahara K., Kitagawa H. (2006) J. Biol. Chem. 281, 38668–38674 [DOI] [PubMed] [Google Scholar]
- 29. Nader H. B., Dietrich C. P., Buonassisi V., Colburn P. (1987) Proc. Natl. Acad. Sci. U.S.A. 84, 3565–3569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Vanpouille C., Deligny A., Delehedde M., Denys A., Melchior A., Liénard X., Lyon M., Mazurier J., Fernig D. G., Allain F. (2007) J. Biol. Chem. 282, 24416–24429 [DOI] [PubMed] [Google Scholar]
- 31. den Dekker E., Grefte S., Huijs T., ten Dam G. B., Versteeg E. M., van den Berk L. C., Bladergroen B. A., van Kuppevelt T. H., Figdor C. G., Torensma R. (2008) J. Immunol. 180, 3680–3688 [DOI] [PubMed] [Google Scholar]
- 32. Chung J. S., Bonkobara M., Tomihari M., Cruz P. D., Jr., Ariizumi K. (2009) Eur. J. Immunol. 39, 965–974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Häcker U., Nybakken K., Perrimon N. (2005) Nat. Rev. Mol. Cell Biol. 6, 530–541 [DOI] [PubMed] [Google Scholar]
- 34. Noguer O., Villena J., Lorita J., Vilaró S., Reina M. (2009) Exp. Cell Res. 315, 795–808 [DOI] [PubMed] [Google Scholar]
- 35. Mertens G., Cassiman J. J., Van den Berghe H., Vermylen J., David G. (1992) J. Biol. Chem. 267, 20435–20443 [PubMed] [Google Scholar]
- 36. Bobardt M. D., Salmon P., Wang L., Esko J. D., Gabuzda D., Fiala M., Trono D., Van der Schueren B., David G., Gallay P. A. (2004) J. Virol. 78, 6567–6584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Schaefer L., Schaefer R. M. (2010) Cell Tissue Res. 339, 237–246 [DOI] [PubMed] [Google Scholar]
- 38. Bernfield M., Götte M., Park P. W., Reizes O., Fitzgerald M. L., Lincecum J., Zako M. (1999) Annu. Rev. Biochem. 68, 729–777 [DOI] [PubMed] [Google Scholar]
- 39. Marcum J. A., Atha D. H., Fritze L. M., Nawroth P., Stern D., Rosenberg R. D. (1986) J. Biol. Chem. 261, 7507–7517 [PubMed] [Google Scholar]
- 40. Robinson C. J., Mulloy B., Gallagher J. T., Stringer S. E. (2006) J. Biol. Chem. 281, 1731–1740 [DOI] [PubMed] [Google Scholar]
- 41. Rapraeger A. C., Krufka A., Olwin B. B. (1991) Science 252, 1705–1708 [DOI] [PubMed] [Google Scholar]
- 42. Ishiguro K., Kadomatsu K., Kojima T., Muramatsu H., Nakamura E., Ito M., Nagasaka T., Kobayashi H., Kusugami K., Saito H., Muramatsu T. (2000) Dev. Dyn. 219, 539–544 [DOI] [PubMed] [Google Scholar]
- 43. Beauvais D. M., Ell B. J., McWhorter A. R., Rapraeger A. C. (2009) J. Exp. Med. 206, 691–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Partovian C., Ju R., Zhuang Z. W., Martin K. A., Simons M. (2008) Mol. Cell 32, 140–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chen L., Klass C., Woods A. (2004) J. Biol. Chem. 279, 15715–15718 [DOI] [PubMed] [Google Scholar]
- 46. Ishiguro K., Kadomatsu K., Kojima T., Muramatsu H., Iwase M., Yoshikai Y., Yanada M., Yamamoto K., Matsushita T., Nishimura M., Kusugami K., Saito H., Muramatsu T. (2001) J. Biol. Chem. 276, 47483–47488 [DOI] [PubMed] [Google Scholar]
- 47. Jokimaa V. I., Kujari H. P., Ekholm E. M., Inki P. L., Anttila L. (2000) Am. J. Obstet. Gynecol. 183, 1495–1498 [DOI] [PubMed] [Google Scholar]
- 48. Ball M., Carmody M., Wynne F., Dockery P., Aigner A., Cameron I., Higgins J., Smith S. D., Aplin J. D., Moore T. (2009) Placenta 30, 649–653 [DOI] [PubMed] [Google Scholar]
- 49. Crescimanno C., Marzioni D., Paradinas F. J., Schrurs B., Mühlhauser J., Todros T., Newlands E., David G., Castellucci M. (1999) J. Pathol. 189, 600–608 [DOI] [PubMed] [Google Scholar]
- 50. Shao R., Hamel K., Petersen L., Cao Q. J., Arenas R. B., Bigelow C., Bentley B., Yan W. (2009) Oncogene 28, 4456–4468 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.