Abstract
Human T-lymphotropic virus type 1 (HTLV-1) is a causative agent of adult T cell leukemia/lymphoma and a variety of inflammatory disorders. HTLV-1 encodes a nuclear localizing protein, p30, that selectively alters viral and cellular gene expression, activates G2-M cell cycle checkpoints, and is essential for viral spread. Here, we used immunoprecipitation and affinity pulldown of ectopically expressed p30 coupled with mass spectrometry to identify cellular binding partners of p30. Our data indicate that p30 specifically binds to cellular ATM (ataxia telangiectasia mutated) and REGγ (a nuclear 20 S proteasome activator). Under conditions of genotoxic stress, p30 expression was associated with reduced levels of ATM and increased cell survival. Knockdown or overexpression of REGγ paralleled p30 expression, suggesting an unexpected enhancement of p30 expression in the presence of REGγ. Finally, size exclusion chromatography revealed the presence of p30 in a high molecular mass complex along with ATM and REGγ. On the basis of our findings, we propose that HTLV-1 p30 interacts with ATM and REGγ to increase viral spread by facilitating cell survival.
Keywords: Nucleus, Proteasome, Retrovirus, Viral Protein, Viral Replication, HTLV-1
Introduction
Human T-lymphotropic virus type 1 (HTLV-1)2 is a complex deltaretrovirus and the first reported human retrovirus linked to malignancy (1, 2). It is estimated that ∼20 million people worldwide are infected with HTLV-1 (3). Approximately five percent of these infected individuals will develop one of a variety of immunopathologic or neoplastic conditions (reviewed in Ref. 4). Two well characterized pathological conditions associated with HTLV-1 infection are adult T cell leukemia/lymphoma, a highly aggressive T cell malignancy, and HTLV-1-associated myelopathy/tropical spastic paraparesis, an immune-mediated neurodegenerative condition (5, 6). The prolonged latency between acquisition of infection and disease expression indicates a highly regulated host-virus relationship that promotes survival of HTLV-1-infected cell reservoirs. HTLV-1 contains genes that encode typical retroviral structural and enzymatic proteins such as Gag, Pol, and Env, as well as regulatory proteins Tax and Rex (reviewed in Ref. 4). The pX region of HTLV-1 contains open reading frames that also encode for various nonstructural proteins (p12, p13, HBZ, and p30) that are essential to viral transmission and spread (7–12).
HTLV-1 p30 is a nuclear and nucleolar localizing protein encoded by a doubly spliced mRNA from ORF II of the pX region of the viral genome (13, 14) that modulates viral and cellular gene expression by transcriptional and post-translational mechanisms (14, 15). HTLV-1-infected individuals produce cytotoxic T cells and antibodies to p30 peptides (16, 17). We reported that p30 differentially modulates the activity of HTLV-1 promoter-driven reporter genes (18) through its interaction with the transcriptional coactivator p300 (19). In Jurkat T cells, p30 alters the expression of a variety of cellular genes and also enhances signal pathways important for T cell growth and survival (20). Ectopically expressed p30 leads to the retention of tax/rex mRNA in the nucleus (15, 21). The switch between viral latency and replication has been postulated to occur by the interaction of p30 and Rex, with Rex rescuing p30-mediated nuclear retention of tax/rex mRNA to promote replication and p30 suppressing Rex expression to maintain latency (23). In addition, interaction of p30 with the Myc-Tip60 complex modifies Tip60-mediated transcription and may promote cellular transformation (24). Importantly, p30 is necessary for establishment and maintenance of HTLV-1 infection in rabbit and a non-human primate model (9, 7, 25) and causes the accumulation of cells in the G2-M phase of the cell cycle (27). Collectively, these studies support the role of p30 as a multifunctional protein with transcriptional and post-transcriptional activities that balance the influence of Tax to regulate viral gene expression and modulate host cell factors to promote survival of infected cells. Identification of host protein-p30 interactions and their influence on the host cell-virus relationship is essential to understand how this unique viral protein influences cell survival to promote HTLV-1 transmission and spread.
In this study, we have used immunoprecipitation, affinity pulldown, and mass spectrometry to demonstrate specific interaction of p30 with ATM (ataxia telangiectasia mutated) and REGγ. The functional significance of p30 and ATM interaction is reflected in modulation of the levels of ATM and increased cell survival after irradiation-induced genotoxic stress. Surprisingly, knockdown and overexpression experiments indicated that REGγ, which is typically known to target nuclear proteins to proteasomal degradation, is associated with enhanced p30 protein expression. Our data are the first to demonstrate p30 in high molecular mass fractions together with cellular ATM and REGγ, suggesting a functional significance through multiprotein complex formation. We propose that interaction of p30 with ATM and REGγ modulates cellular pathways to promote survival of infected cells, thereby enhancing viral transmission and spread.
EXPERIMENTAL PROCEDURES
Cell Culture, Transfection, and Transduction
HEK 293T cells were cultured in DMEM containing 10% FBS, 2 mm l-glutamine, 100 μg/ml streptomycin, and 100 units/ml penicillin. Jurkat T cells (ATCC clone E6-1) were cultured in RPMI 1640 medium supplemented with 15% FBS, 2 mm l-glutamine, 100 μg/ml streptomycin, 100 units/ml penicillin, 1× minimum Eagle's medium nonessential amino acids (Sigma), and 1 mm sodium pyruvate. Plasmids were transfected using SuperFect (Qiagen, Valencia, CA) according to the manufacturer's instructions. Transduction of 293T and Jurkat T cells with pWPT-IRES-GFP and pWPT-p30-HA-IRES-GFP was performed as described (20). For ectopic expression of p30, 293T cells were transfected with 10 μg of pTriEx4-Neo (mock), pTriEx-Neo-S-GFP, or pTriEx4-Neo-S-p30-HA (Novagen, Madison, WI). REGγ was expressed in 293T cells by transfecting 5 μg of pCMV-AC-REGγ plasmid (OriGene, Rockville, MD). For knockdown experiments, 293T cells (1 × 106) expressing p30-HA or GFP were transfected twice at 24-h intervals with 85 nm SMARTpool siRNA against REGγ and control negative siRNA (Dharmacon, Lafayette, CO) using a commercial transfection reagent (OligofectamineTM, Invitrogen). The cells were lysed 72 h after the first knockdown transfection, and immunoblotting was performed to detect protein amounts.
Reporter Gene p30 Functional Assay
The p30 functional assay was performed as described with slight modifications (28). Briefly, 0.1 μg of HTLV-1 LTR-luciferase reporter was cotransfected with or without 0.1 μg of a Tax expression plasmid in 293T cells, keeping the total DNA concentration constant. Increasing amounts (1.2 and 2.4 μg) of S-p30-HA were cotransfected to analyze the repression activity. After 24 h, the luciferase activity was determined. The transfection efficiency was normalized using Renilla luciferase activity.
S-Tag Affinity Purification and Immunoprecipitation
Cell lysates were prepared with passive lysis buffer (Promega, Madison, WI) in the presence of protease inhibitor mixture (Roche Applied Science). S-Tag purification was performed by rocking cell lysates with S-beads (Novagen) overnight at 4 °C. Immunoprecipitation was performed by rocking cell lysates overnight at 4 °C with protein A-agarose beads (Pierce) that were equilibrated with specific antibodies for 45 min at room temperature. The S-beads or protein A-agarose beads were washed once with high salt (1 m NaCl)-containing radioimmunoprecipitation assay buffer (150 mm NaCl, 0.01 m sodium pyrophosphate, 10 mm EDTA, 10 mm sodium fluoride, 50 mm Tris, 0.1% SDS, 12.8 mm deoxycholic acid, 10% glycerol, and 1% Nonidet P-40 (pH 8.0)), three times with radioimmunoprecipitation assay buffer, and once with PBS. Samples evaluated for ATM binding were washed three times with 50 mm Tris (pH 7.4) and 150 mm NaCl prior to protein elution by boiling the beads in SDS loading buffer and immunoblotting.
Irradiation Experiments and Cell Survival Studies
293T cells transduced with lentivirus (pWPT-p30-HA-IRES-GFP) to stably express p30 or mock pWPT-IRES-GFP were exposed to 10-gray (Gy) γ-irradiation. Cell lysates were prepared in the presence of phosphatase (Sigma) and protease (Roche Applied Science) inhibitor mixtures after 30, 60, and 120 min of recovery time prior to immunoblotting. To test cell survival in the presence of p30, 293T cells and Jurkat T cells transduced with lentivirus (pWPT-p30-HA-IRES-GFP) to stably express p30 or mock pWPT-IRES-GFP were exposed to 10-Gy irradiation. Cell survival was monitored using CellTiter 96® AQueous One solution reagent (Promega) according to the manufacturer's instructions.
Immunoblotting and Antibodies
Cell lysate-derived proteins and proteins from S-Tag purification or immunoprecipitation assays were resolved on a 4–20% gradient SDS-PAGE and transferred to nitrocellulose membranes prior to immunoblotting using the following primary and secondary antibodies: rabbit anti-HA polyclonal and mouse anti-HA monoclonal antibodies (Covance Research Products, Princeton, NJ); mouse anti-ATM monoclonal, mouse anti-phospho-ATM (pATM) (Ser-1981) monoclonal, rabbit anti-γH2AX polyclonal, rabbit anti-REGγ, anti-REGα, and anti-REGβ polyclonal, horse anti-mouse, and goat anti-rabbit antibodies (Cell Signaling, Beverly, MA); and mouse anti-β-actin monoclonal antibody (Sigma). GFP was detected using HRP-conjugated S-protein (Novagen).
Mass Spectrometry and Proteomics
For MALDI-TOF analysis, the protein band of interest was excised from SDS-PAGE, destained, dehydrated, and digested with 1 μg of trypsin in 50 mm NH4HCO3 at 25 °C overnight. Small molecular mass peptides were analyzed by a MALDI-TOF AXIMA-CFR instrument (Shimadzu Scientific Instruments) using an α-cyano-4-hydroxycinnamic acid as a matrix. Protein Prospector v5.6.1 was used to identify detected peptide peaks.
Size Exclusion Chromatography
Experiments were performed with a HiLoad 16/60 Superdex 200 pg column (GE Healthcare) at 1 ml/min in buffer containing 50 mm HEPES (pH 7.4), 150 mm NaCl, 5% glycerol, and 2 mm β-mercaptoethanol. The column was calibrated with the following proteins: thyroglobulin (670,000 Da), γ-globulin (158,000 Da), ovalbumin (44,000 Da), myoglobin (17,000 Da), and vitamin B12 (1350 Da). Proteins were detected by absorbance at 280 nm.
RESULTS AND DISCUSSION
HTLV-1 p30 Promotes Cell Survival in the Presence of Genotoxic Stress
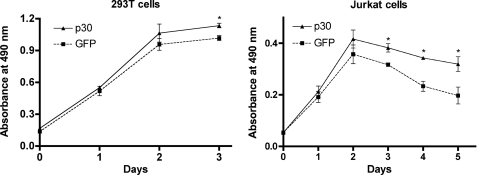
HTLV-1 infection is characterized by lymphocyte-mediated diseases, and the virus has well documented properties that promote the proliferation of infected cells through cell cycle alterations or cell signaling pathways (29, 30). HTLV-1 p30 has been reported by our laboratory and others to modulate cell cycle parameters to favor cell survival or potentially contribute to cellular transformation (24, 27). In this study, we tested the ability of p30 to promote cell survival following genotoxic stress. 293T cells and Jurkat T cells were transduced with lentivirus vectors to stably express p30 (pWPT-p30-HA-IRES-GFP) or mock pWPT-IRES-GFP vectors and then exposed to 10-Gy γ-irradiation. Cell survival was monitored using a tetrazolium dye-based method. Cells expressing p30 exhibited significantly higher cell survival compared with mock-transduced cells (Fig. 1). This effect was more pronounced in Jurkat T cells (62% increase) compared with 293T cells (11% increase), reflecting the natural tropism of HTLV-1 for T cells. These data suggested a role for p30 in protecting HTLV-1-infected cells in circumstances associated with DNA damage, such as Tax expression (31).
FIGURE 1.
p30 expression enhances cell survival under conditions of genotoxic stress. Cell survival was tested in 293T and Jurkat T cells transduced with lentivirus vectors to stably express p30 or mock (GFP vector) prior to exposure to 10-Gy irradiation. Cell survival was monitored using CellTiter 96 AQueous One solution reagent according to the manufacturer's instructions. Data points represent the mean absorbance values of six independent trials plotted over time. Statistical differences were compared using analysis of variance. In 293T cells, statistically significant (asterisk) higher cell survival was noted in the presence of p30 compared with the control (GFP) at day 3 (p ≤ 0.0004), at the time the cells reached confluence. In Jurkat T cells, statistically significant (asterisk) higher cell survival was observed in the presence of p30 compared with the control (GFP) at days 3, 4, and 5 (p ≤ 0.0044, 0.0003, and 0.0185, respectively).
ATM Levels Are Modulated in the Presence of p30 upon DNA Damage
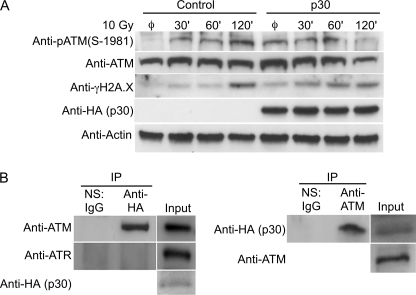
Depending on the extent of DNA damage, cells exhibit a variety of physiological responses, including cell cycle checkpoint activation, DNA repair, and apoptosis. In these circumstances, evolutionarily conserved checkpoint kinases are rapidly induced to prevent replication or segregation of damaged DNA before repair is completed. Upon DNA damage, ATM is activated by autophosphorylation on Ser-1981 (pATM) to recruit and phosphorylate downstream DNA repair proteins (32). ATM also activates cell cycle checkpoints or apoptosis by phosphorylating p53 at Ser-15 (33). To test the potential interaction of p30 with ATM, 293T cells transduced with lentivirus vectors to stably express p30 (pWPT-p30-HA-IRES-GFP) or mock pWPT-IRES-GFP vectors were subjected to DNA damage (10-Gy irradiation), and the levels of ATM and pATM were monitored (Fig. 2A). Elevated levels of γH2AX, a marker of double-stranded DNA breaks, confirmed initiation of the DNA repair pathway. In the absence of p30, the levels of ATM remained constant, and pATM appeared by 30 min and peaked by 120 min. In the presence of p30, the phosphorylation of ATM peaked by 60 min to levels comparable with that at 120 min in the control. The maximum phosphorylation of ATM in cells expressing p30 was achieved at least 60 min earlier than in control cells. The levels of both ATM and pATM were reduced by 120 min after DNA damage, whereas no such decrease was observed in control cells. Coupled with the observation that p30 enhanced cell survival following genotoxic stress (Fig. 1), we hypothesized that p30 expression may modulate levels of pATM to prevent triggering apoptosis, promoting cell survival of viral infected cells (34, 35). Our data suggest a role for p30 in modifying cellular response to DNA damage, influencing the amount of ATM available to induce apoptosis.
FIGURE 2.
p30 binds to ATM and modulates the levels of ATM after genotoxic stress. A, 293T cells were transduced with lentivirus vectors to stably express p30 or mock (GFP vector) prior to exposure to 10-Gy irradiation and were allowed to recover for different times as indicated. Cell lysates were prepared at defined time points and tested by immunoblotting for pATM, ATM, p30-HA, and γH2AX. Equal protein loading was confirmed by testing cell lysates for amounts of β-actin. Base amounts of each evaluated protein were visualized from cells not subjected to DNA damage (denoted by ø). Data represent a minimum of three independent trials. B, cell lysates from Jurkat T cells transduced with lentivirus vectors to stably express p30 or mock (GFP vector) prior to exposure to 10-Gy irradiation were immunoprecipitated (IP) using normal IgG (normal serum (NS)) or rabbit anti-HA antibodies and probed with mouse anti-ATM and anti-ATR monoclonal antibodies. The reverse immunoprecipitation was performed by immunoprecipitation of cell lysates of Jurkat T cells stably expressing p30-HA with normal IgG or rabbit anti-ATM antibody and probed with mouse anti-HA monoclonal antibody. Data represent a minimum of three independent trials for each immunoprecipitation assay. IP, immunoprecipitation.
HTLV-1 p30 Interacts with ATM
To test if p30 modulation of ATM was due to interaction between the two proteins, immunoprecipitation experiments were performed. Cell lysates from p30- or mock vector-expressing Jurkat T cells were immunoprecipitated and probed for ATM. ATM and not ATR (ATM- and Rad3-related), another key kinase in the DNA repair pathway, was immunoprecipitated using antibodies against HA (Fig. 2B, left panel), indicating that p30 interacts directly with ATM. The interaction between ATM and p30 was also demonstrated using a reverse immunoprecipitation assay in which p30 was immunoprecipitated with antibodies against ATM (Fig. 2B, right panel). Our data are the first to demonstrate a retroviral protein that specifically interacts with and modulates ATM.
REGγ Co-purifies with HTLV-1 p30
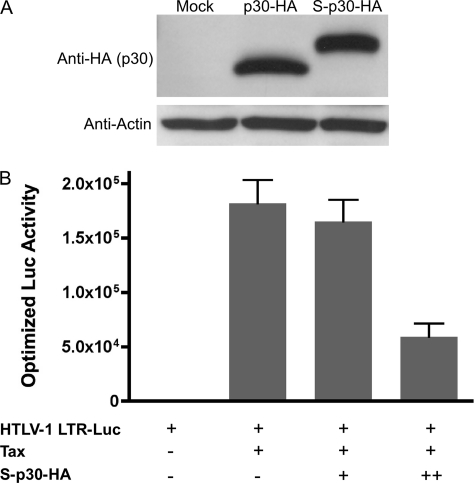
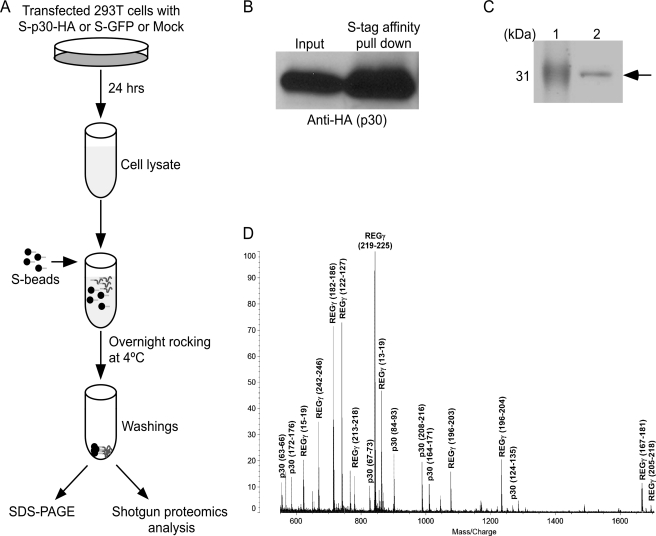
To identify other cellular proteins that interact with p30 and could explain the ability of p30 to modulate ATM, we employed affinity pulldown coupled with mass spectrometry. For these experiments, p30 was expressed with an N-terminal S-Tag and a C-terminal HA tag (S-p30-HA) under the control of the CMV promoter from the pTriEx4-Neo expression plasmid. The expression of S-p30-HA was comparable with that of p30-HA expressed from the pME-p30-HA plasmid in 293T cells reported previously (Fig. 3A) (28). We reported that p30 represses Tax-driven HTLV-1 LTR transcription (28). The ability of S-p30-HA to repress Tax-driven HTLV-1 LTR transcription indicated that S-p30-HA functioned similarly to other forms of the protein (Fig. 3B). The S-Tag was utilized for affinity purification and the HA tag for immunoblot detection. Affinity pulldown of p30 from whole cell lysates was performed according to the protocol shown in Fig. 4A. Immunoblot analysis validated that p30 was enriched in the S-Tag pulldown fraction (Fig. 4B). A protein band corresponding to the molecular mass of p30 was readily detected by Coomassie Blue staining of the S-Tag pulldown fraction in an SDS gel (Fig. 4C). MALDI-TOF analysis of the protein band indicated that it contained two proteins: HTLV-1 p30 and cellular REGγ (Fig. 4D).
FIGURE 3.
Expression, S-Tag affinity purification, and function of S-p30-HA. A, 293T cells were transfected with pME-p30-HA or pTriEx4-Neo-S-p30-HA expression plasmid. p30 was detected using mouse anti-HA monoclonal antibody after resolving cell lysates by 4–20% gradient SDS-PAGE. B, the functional activity of p30 was determined by an HTLV-1 LTR-reporter gene assay as described (28). 293T cells were transfected with a luciferase reporter gene driven by an HTLV-1 LTR-Tax expression plasmid and increasing concentrations of S-p30-HA (1.2 and 2.4 μg). The luciferase (Luc) activity was optimized for transfection efficiency using Renilla luciferase as an internal control. Luciferase activity data represent the means of three independent trials.
FIGURE 4.
Affinity pulldown of p30 and mass spectrometric analysis of interacting proteins. A, schematic of p30 purification using the S-Tag pulldown approach coupled with mass spectrometric analysis. 293T cell lysates were prepared 24 h after transfection with expression plasmids (S-p30-HA, mock, or S-GFP). Each cell lysate was incubated overnight with S-beads at 4 °C. To minimize nonspecific protein binding, beads were washed prior to shotgun proteomics or SDS-PAGE separation. B, S-Tag affinity purification of p30 using S-beads resulted in enrichment of p30 as detected by immunoblotting. C, the proteins from the beads were denatured by boiling in SDS loading dye in the presence of β-mercaptoethanol. The proteins were separated by 14% SDS-PAGE and Coomassie Blue-stained. Lane 1 shows the molecular mass marker, and lane 2 shows the S-Tag affinity-purified p30. The S-p30-HA band indicated by the arrow was excised and subjected to mass spectrometric analysis. D, MALDI-TOF spectrum. The protein band migrating near the 31-kDa marker on SDS-PAGE (indicated by the arrow in C) was cut out and subjected to in-gel proteolysis with trypsin. The resulting peptides were analyzed by a MALDI-TOF AXIMA-CFR instrument. Multiple peptide peaks corresponding to p30 and REGγ proteins have been identified and are indicated. The start and end amino acid positions of peptides are depicted in parentheses. The sequence coverage for p30 and REGγ was 30 and 44%, respectively, enabling unequivocal identification of these proteins.
To perform a more comprehensive analysis of cellular proteins interacting with p30, 293T cells transfected with S-p30-HA, pTriEx4-Neo (mock), and S-GFP (GFP with an S-Tag on the C terminus expressed from pTriEx4-Neo-S-GFP) were subjected to S-Tag affinity purification (Fig. 4A), followed by shotgun proteomics (LC/MS/MS) analysis of the pulled-down proteins. Quadruplicate experiments with S-p30-HA with two mock and two GFP-negative controls were performed. REGγ consistently co-purified with p30 in four S-p30-HA experiments and was not detected in any of the negative controls. The proteins identified in both controls and p30-expressing samples were considered to be nonspecific bead-interacting proteins. Thus, both MALDI-TOF and LC/MS/MS data indicated that REGγ selectively co-purified with p30. We could not detect ATM in these experiments, likely because ATM is less abundant than REGγ in p30 pulled-down fractions or is contained in lower affinity protein complexes, with p30 requiring the more sensitive immunoblot analyses.
HTLV-1 p30 Specifically Interacts with REGγ
The REG (regulators) family of 20 S proteasome activators includes REGα, REGβ, and REGγ. Despite the fact that REGγ has ∼25% amino acid similarity to REGα and REGβ, the latter proteins were not identified in our MALDI-TOF or LC/MS/MS analysis, indicating that p30 selectively interacts with REGγ. The 20 S proteasome is an inherently latent protease, and the binding of REG proteins activates and modifies protease activity by forming the REG-20 S proteasome complex (36, 37). The proteasome complex is critical in controlling a variety of cellular processes such as cell cycle and transcriptional regulation through regulated proteolysis. REGα and REGβ are cytoplasmic and are involved in antigen processing, whereas REGγ is localized in the nucleus (38).
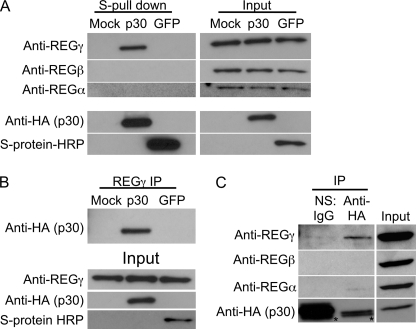
To further confirm the specificity of p30 and REGγ interaction, S-Tag affinity-purified samples were immunoblotted for REGγ. 293T cells were transfected with mock, S-p30-HA, or S-GFP plasmids, and cell lysates were subjected to S-Tag affinity purification and immunoblotted with anti-REGγ antibodies. REGγ co-purified only with p30 and not with GFP or from mock-transfected cell lysates (Fig. 5A), indicating that p30 specifically interacts with REGγ. Both REGα and REGβ did not co-purify with p30, indicating the specificity of the p30 interaction with REGγ (Fig. 5A). To further confirm the specificity of p30 and REGγ interaction, REGγ was immunoprecipitated from 293T cells transfected with mock, S-p30-HA, or S-GFP. p30 was co-immunoprecipitated with REGγ, whereas GFP was not (Fig. 5B), indicating a specific interaction between p30 and REGγ. To demonstrate the interaction in physiologically relevant T cells, Jurkat T cells were transduced with a p30-HA-expressing lentivirus vector and tested for REGγ binding. Antibodies against HA were able to specifically immunoprecipitate REGγ but not REGα or REGβ, whereas nonspecific IgG was unable to immunoprecipitate any REG proteins (Fig. 5C). This also eliminated the possibility of the S-Tag influencing the interaction. Collectively, these data provide further evidence for a novel and specific interaction between HTLV-1 p30 and REGγ.
FIGURE 5.
HTLV-1 p30 specifically interacts with REGγ. A, cell lysates of 293T cells transfected with mock, S-p30-HA, and S-GFP expression plasmids were subjected to S-Tag affinity purification and probed with rabbit anti-REGγ antibody. The specificity of binding was confirmed by probing for REGα and REGβ using rabbit antibodies against each protein. The expression of S-p30-HA and S-GFP was visualized using anti-HA antibody and HRP-conjugated S-protein. B, REGγ was immunoprecipitated (IP) using anti-REGγ antibody from cell lysates of 293T cells transfected with mock, S-p30-HA, or S-GFP and probed with anti-HA antibody for p30. The expression of GFP was confirmed using HRP-conjugated S-protein. C, cell lysate from Jurkat T cells transduced with lentivirus vector expressing p30-HA was immunoprecipitated using normal IgG (normal serum (NS)) or anti-HA antibody and probed with anti-REGα, anti-REGβ, and anti-REGγ antibodies. The membrane was probed with anti-HA antibody to demonstrate the expression of p30. The asterisks indicate the light chain band from the antibodies. Data presented in A–C represent a minimum of three independent trials.
REGγ was thought to be involved in the hydrolysis of small peptides (38). However, recent studies have demonstrated that REGγ specifically interacts and directs full-length folded cellular proteins to proteasomal degradation (39). The significant feature of the REGγ-20 S proteasome complex is its ability to degrade proteins in an ATP- and ubiquitin-independent manner (40). The cellular proteins degraded via REGγ interaction independent of ATP and ubiquitination include SRC-3 (steroid receptor coactivator-3) and p21waf/cip1 (41, 42). However, REGγ also functions as a cofactor in ubiquitin-dependent MDM2-mediated degradation of p53 by facilitating the interaction between MDM2 and p53 (43). REGγ is also involved in the pathogenesis of hepatitis C virus by influencing the turnover of the hepatitis C virus core protein (44, 45). Similarly, the interaction of p30 with REGγ could play an important role in turnover of p30 and thus contribute to the function of p30 in HTLV-1 infection.
REGγ Enhances Detection of HTLV-1 p30
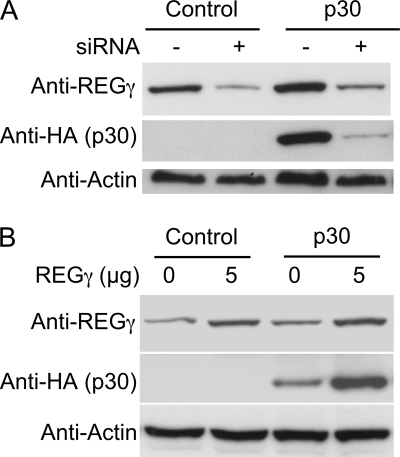
To investigate the influence of p30 and REGγ interaction on the ability to detect p30, REGγ was knocked down using siRNA in 293T cells stably expressing p30-HA. The levels of REGγ and not REGα or REGβ were decreased, indicating that the siRNA knockdown was specific (data not shown). Considering the role of REGγ in 20 S proteasome activation, one would expect the levels of p30 to increase. For example, the amounts of hepatitis C virus core protein, SRC-3, and p21waf/cip1 were shown to increase upon reduction of the levels of REGγ. In contrast, the levels of p30 decreased upon knocking down REGγ (Fig. 6A), suggesting that the interaction with REGγ stabilizes p30. To further confirm these results, REGγ was overexpressed in 293T cells stably expressing p30-HA. Overexpression of REGγ resulted in increased amounts of p30 (Fig. 6B), again suggesting that REGγ stabilizes p30. Bioinformatic analysis suggests that p30 is a labile and unstable protein with a very high pI value of 11.7; thus, the interaction with REGγ may be important for the stabilization and function of p30.
FIGURE 6.
HTLV-1 p30 appears to be stabilized by interaction with REGγ. A, SMARTpool siRNA-mediated knockdown of REGγ in stably transduced 293T cells expressing p30-HA resulted in reduced levels of p30. siRNA against REGγ and nonspecific control siRNA are indicated as + and −, respectively. Immunoblotting for REGα and REGβ indicates specific REGγ knockdown. B, overexpression of REGγ using the REGγ expression plasmid in 293T cells stably expressing p30-HA resulted in increased amounts of p30 detected. In A and B (minimum of two independent trials), β-actin immunoblotting served as a loading control, and 293T cells stably expressing only GFP were used as a control.
It is noteworthy that REGγ is involved in the degradation of both p21waf/cip1 and p53, which play critical roles in cell cycle regulation (46, 47). In addition, REGγ−/− mouse embryonic fibroblasts show slow growth and accumulation in the S phase and are more susceptible to apoptosis (48, 49). The biological role of REGγ in cell cycle regulation and cell survival is underlined by these observations. Our laboratory has reported previously that p30 causes an activation of the G2-M checkpoint (27). On the basis of the current data, we hypothesize that interaction of p30 with REGγ could alter cell cycle regulation to benefit early viral spread or replication. Our data demonstrate for the first time that REGγ stabilizes a key retroviral protein, p30, known to be important in early viral spread (7, 9, 25).
HTLV-1 p30 Is Associated with ATM and REGγ in High Molecular Mass Multiprotein Complexes
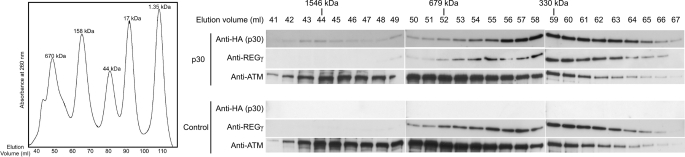
REGγ and ATM function as a heptamer (∼225 kDa) (50) and a dimer (∼600 kDa) (22), respectively. To investigate whether p30 associates with these high molecular mass species, we subjected lysates of 293T cells stably expressing p30-HA to size exclusion chromatography. No p30 band was detected in the fractions corresponding to monomeric p30. Instead, two distinct peaks of p30 were observed in higher molecular mass fractions. The first weaker peak was observed at ∼1546 kDa, and the second strong peak was at ∼330 kDa (Fig. 7). REGγ, which exists as a heptamer in cells, co-eluted with the second peak of p30 (Fig. 7).
FIGURE 7.
p30, REGγ, and ATM co-elute, consistent with a multiprotein complex. Cell lysate of 293T cells with no expression or with stable expression p30-HA was subjected to size exclusion chromatography (representative of two independent trials). Different fractions as indicated were subjected to immunoblotting and probed with anti-HA, anti-REGγ, and anti-ATM antibodies. The molecular mass fractions of the protein peak elution are indicated. The chromatograph of the protein standard with different molecular masses is shown and was used to plot the standard curve to derive molecular masses of relevant fractions.
The molecular mass of ATM is ∼350 kDa, and it exists in a non-activated state as a dimer associated with DNA. Consistent with these properties of ATM, we detected ATM in two separate elution peaks. The first ATM peak was at ∼1546 kDa and exhibited a high absorbance at 260 nm, indicating that this peak likely corresponds to DNA-associated ATM (Fig. 7). The DNA-associated ATM peak coincided with the first weaker peak of p30 (Fig. 7), indicating that a small portion of p30 is associated with ATM that is bound to DNA. The second peak of ATM at ∼679 kDa corresponded to the dimeric form of the protein. This peak exhibited a broad shoulder overlapping with p30 and REGγ peaks (Fig. 7), suggesting that these three proteins assemble into a multiprotein complex. The elution profiles of REGγ and ATM did not change in the absence of p30 (Fig. 7).
The association of p30 together with REGγ and ATM in a high molecular mass complex supports a possible mechanism for the reduced levels of ATM/pATM after DNA damage. These reduced levels ATM/pATM could help the cell to avoid apoptosis under DNA damage conditions and promote cell survival. The kinase activity of ATM is activated by acetylation of ATM by Tip60, a histone acetyltransferase (26), and it has been reported that p30 interacts with Tip60 (24). The other possible mechanism for reduced levels of pATM may be due to the ability of p30 to interfere with Tip60 to acetylate ATM, which is required for its autophosphorylation and activation.
In summary, we have reported the interaction of HTLV-1 p30 with two cellular host proteins, ATM and REGγ, which are involved in DNA damage repair and proteasomal degradation machinery, respectively. Both ATM and REGγ are involved in cell survival in different ways, and the interaction of HTLV-1 p30 with these proteins could lead to enhanced survival of normal and DNA-damaged cells. Our size exclusion chromatography experiments suggest that these proteins assemble into multiprotein complexes. Our data indicate that p30 modulates the survival of infected cells through modulation of ATM and provide a plausible mechanism for how the retroviral protein facilitates early viral spread by enhancing cell survival during the critical phases of early mucosal transmission. Further studies are also needed to investigate the functional significance of these interactions to extend our understanding of DNA damage repair mechanisms in retroviral transmission and the biological role of REGγ in cell survival.
This work was supported, in whole or in part, by National Institutes of Health Program Project Grant P01 CA100730 from NCI (to M. D. L.).
- HTLV-1
- human T-lymphotropic virus type 1
- Gy
- gray
- pATM
- phospho-ATM.
REFERENCES
- 1. Poiesz B. J., Ruscetti F. W., Gazdar A. F., Bunn P. A., Minna J. D., Gallo R. C. (1980) Proc. Natl. Acad. Sci. U.S.A. 77, 7415–7419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yoshida M., Miyoshi I., Hinuma Y. (1982) Proc. Natl. Acad. Sci. U.S.A. 79, 2031–2035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yoshida M. (2005) Oncogene 24, 5931–5937 [DOI] [PubMed] [Google Scholar]
- 4. Lairmore M., Franchini G. (2007) in Fields Virology (Knipe D. M. ed) pp. 2071–2105, Wolters Kluwer/Lippincott Williams and Wilkins, Philadelphia, PA [Google Scholar]
- 5. Gonçalves D. U., Proietti F. A., Ribas J. G., Araújo M. G., Pinheiro S. R., Guedes A. C., Carneiro-Proietti A. B. (2010) Clin. Microbiol. Rev. 23, 577–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Satou Y., Matsuoka M. (2010) J. Clin. Exp. Hematop. 50, 1–8 [DOI] [PubMed] [Google Scholar]
- 7. Valeri V. W., Hryniewicz A., Andresen V., Jones K., Fenizia C., Bialuk I., Chung H. K., Fukumoto R., Washington P. R., Ferrari M. G., Nicot C., Cecchinato V., Ruscetti F., Franchini G. (2010) Blood 116, 3809–3817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Collins N. D., Newbound G. C., Albrecht B., Beard J. L., Ratner L., Lairmore M. D. (1998) Blood 91, 4701–4707 [PubMed] [Google Scholar]
- 9. Silverman L. R., Phipps A. J., Montgomery A., Ratner L., Lairmore M. D. (2004) J. Virol. 78, 3837–3845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hiraragi H., Kim S. J., Phipps A. J., Silic-Benussi M., Ciminale V., Ratner L., Green P. L., Lairmore M. D. (2006) J. Virol. 80, 3469–3476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Arnold J., Yamamoto B., Li M., Phipps A. J., Younis I., Lairmore M. D., Green P. L. (2006) Blood 107, 3976–3982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Taylor J. M., Brown M., Nejmeddine M., Kim K. J., Ratner L., Lairmore M., Nicot C. (2009) J. Virol. 83, 11467–11476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Koralnik I. J., Fullen J., Franchini G. (1993) J. Virol. 67, 2360–2366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ghorbel S., Sinha-Datta U., Dundr M., Brown M., Franchini G., Nicot C. (2006) J. Biol. Chem. 281, 37150–37158 [DOI] [PubMed] [Google Scholar]
- 15. Younis I., Khair L., Dundr M., Lairmore M. D., Franchini G., Green P. L. (2004) J. Virol. 78, 11077–11083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chen Y. M., Chen S. H., Fu C. Y., Chen J. Y., Osame M. (1997) Int. J. Cancer 71, 196–202 [DOI] [PubMed] [Google Scholar]
- 17. Pique C., Ureta-Vidal A., Gessain A., Chancerel B., Gout O., Tamouza R., Agis F., Dokhélar M. C. (2000) J. Exp. Med. 191, 567–572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhang W., Nisbet J. W., Bartoe J. T., Ding W., Lairmore M. D. (2000) J. Virol. 74, 11270–11277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhang W., Nisbet J. W., Albrecht B., Ding W., Kashanchi F., Bartoe J. T., Lairmore M. D. (2001) J. Virol. 75, 9885–9895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Michael B., Nair A. M., Hiraragi H., Shen L., Feuer G., Boris-Lawrie K., Lairmore M. D. (2004) Retrovirology 1, 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nicot C., Dundr M., Johnson J. M., Fullen J. R., Alonzo N., Fukumoto R., Princler G. L., Derse D., Misteli T., Franchini G. (2004) Nat. Med. 10, 197–201 [DOI] [PubMed] [Google Scholar]
- 22. Goodarzi A. A., Lees-Miller S. P. (2004) DNA Repair 3, 753–767 [DOI] [PubMed] [Google Scholar]
- 23. Sinha-Datta U., Datta A., Ghorbel S., Dodon M. D., Nicot C. (2007) J. Biol. Chem. 282, 14608–14615 [DOI] [PubMed] [Google Scholar]
- 24. Awasthi S., Sharma A., Wong K., Zhang J., Matlock E. F., Rogers L., Motloch P., Takemoto S., Taguchi H., Cole M. D., Lüscher B., Dittrich O., Tagami H., Nakatani Y., McGee M., Girard A. M., Gaughan L., Robson C. N., Monnat R. J., Jr., Harrod R. (2005) Mol. Cell. Biol. 25, 6178–6198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bartoe J. T., Albrecht B., Collins N. D., Robek M. D., Ratner L., Green P. L., Lairmore M. D. (2000) J. Virol. 74, 1094–1100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sun Y., Jiang X., Chen S., Fernandes N., Price B. D. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 13182–13187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Datta A., Silverman L., Phipps A. J., Hiraragi H., Ratner L., Lairmore M. D. (2007) Retrovirology 4, 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Michael B., Nair A. M., Datta A., Hiraragi H., Ratner L., Lairmore M. D. (2006) Virology 354, 225–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pise-Masison C. A., Jeong S. J., Brady J. N. (2005) Arch. Immunol. Ther. Exp. 53, 283–296 [PubMed] [Google Scholar]
- 30. Giam C. Z., Jeang K. T. (2007) Front. Biosci. 12, 1496–1507 [DOI] [PubMed] [Google Scholar]
- 31. Chlichlia K., Khazaie K. (2010) Chem. Biol. Interact. 188, 359–365 [DOI] [PubMed] [Google Scholar]
- 32. Bakkenist C. J., Kastan M. B. (2003) Nature 421, 499–506 [DOI] [PubMed] [Google Scholar]
- 33. Banin S., Moyal L., Shieh S., Taya Y., Anderson C. W., Chessa L., Smorodinsky N. I., Prives C., Reiss Y., Shiloh Y., Ziv Y. (1998) Science 281, 1674–1677 [DOI] [PubMed] [Google Scholar]
- 34. Tichý A., Vávrová J., Pejchal J., Rezácová M. (2010) Acta Med. 53, 13–17 [DOI] [PubMed] [Google Scholar]
- 35. Derheimer F. A., Kastan M. B. (2010) FEBS Lett. 584, 3675–3681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ma C. P., Slaughter C. A., DeMartino G. N. (1992) J. Biol. Chem. 267, 10515–10523 [PubMed] [Google Scholar]
- 37. Dubiel W., Pratt G., Ferrell K., Rechsteiner M. (1992) J. Biol. Chem. 267, 22369–22377 [PubMed] [Google Scholar]
- 38. Rechsteiner M., Hill C. P. (2005) Trends Cell Biol. 15, 27–33 [DOI] [PubMed] [Google Scholar]
- 39. Realini C., Jensen C. C., Zhang Z., Johnston S. C., Knowlton J. R., Hill C. P., Rechsteiner M. (1997) J. Biol. Chem. 272, 25483–25492 [DOI] [PubMed] [Google Scholar]
- 40. Zhou P. (2006) Cell 124, 256–257 [DOI] [PubMed] [Google Scholar]
- 41. Li X., Lonard D. M., Jung S. Y., Malovannaya A., Feng Q., Qin J., Tsai S. Y., Tsai M. J., O'Malley B. W. (2006) Cell 124, 381–392 [DOI] [PubMed] [Google Scholar]
- 42. Li X., Amazit L., Long W., Lonard D. M., Monaco J. J., O'Malley B. W. (2007) Mol. Cell 26, 831–842 [DOI] [PubMed] [Google Scholar]
- 43. Zhang Z., Zhang R. (2008) EMBO J. 27, 852–864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Moriishi K., Mochizuki R., Moriya K., Miyamoto H., Mori Y., Abe T., Murata S., Tanaka K., Miyamura T., Suzuki T., Koike K., Matsuura Y. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 1661–1666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Moriishi K., Okabayashi T., Nakai K., Moriya K., Koike K., Murata S., Chiba T., Tanaka K., Suzuki R., Suzuki T., Miyamura T., Matsuura Y. (2003) J. Virol. 77, 10237–10249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Weinberg W. C., Denning M. F. (2002) Crit. Rev. Oral Biol. Med. 13, 453–464 [DOI] [PubMed] [Google Scholar]
- 47. Meek D. W. (2009) Nat. Rev. Cancer 9, 714–723 [DOI] [PubMed] [Google Scholar]
- 48. Murata S., Kawahara H., Tohma S., Yamamoto K., Kasahara M., Nabeshima Y., Tanaka K., Chiba T. (1999) J. Biol. Chem. 274, 38211–38215 [DOI] [PubMed] [Google Scholar]
- 49. Barton L. F., Runnels H. A., Schell T. D., Cho Y., Gibbons R., Tevethia S. S., Deepe G. S., Jr., Monaco J. J. (2004) J. Immunol. 172, 3948–3954 [DOI] [PubMed] [Google Scholar]
- 50. Li J., Gao X., Ortega J., Nazif T., Joss L., Bogyo M., Steven A. C., Rechsteiner M. (2001) EMBO J. 20, 3359–3369 [DOI] [PMC free article] [PubMed] [Google Scholar]