Abstract
ATF5 loss of function has been shown previously to cause apoptotic cell death in glioblastoma and breast cancer cells but not in non-transformed astrocytes and human breast epithelial cells. The mechanism for the cell type-dependent survival function of ATF5 is unknown. We report here that the anti-apoptotic factor BCL-2 is a downstream target of ATF5 that mediates the prosurvival function of ATF5 in C6 glioma cells and MCF-7 breast cancer cells. ATF5 binds to an ATF5-specific regulatory element that is downstream of and adjacent to the negative regulatory element in the BCL-2 P2 promoter, stimulating BCL-2 expression. Highlighting the critical role of BCL-2 in ATF5-dependent cancer cell survival, expression of BCL-2 blocks death of C6 and MCF-7 cells induced by dominant-negative ATF5, and depletion of BCL-2 impairs ATF5-promoted cell survival. Moreover, we found that BCL-2 expression is not regulated by ATF5 in non-transformed rat astrocytes, mouse embryonic fibroblasts, and human breast epithelial cells, where expression of BCL-2 but not ATF5 is required for cell survival. These findings identify BCL-2 as an essential mediator for the cancer-specific cell survival function of ATF5 in glioblastoma and breast cancer cells and provide direct evidence that the cell type-specific function of ATF5 derives from differential regulation of downstream targets by ATF5 in different types of cells.
Keywords: Apoptosis, Cell Death, Tissue-specific Transcription Factors, Transcription Factors, Transcription Regulation, ATF5, C6, MCF-7, Cell Type-specific Cell Survival, Transcription Regulation
Introduction
ATF5 (activating transcription factor 5; also known as ATFx) is a member of the ATF/cAMP response element-binding protein (CREB)2 family of bZIP (basic zipper) proteins (1). It can bind to several transcription regulatory DNA elements that include cAMP response elements (CRE), an amino acid response element, and an ATF5-specific response element (ARE) and regulate gene expression in different types of cells (1–4). ATF5 has been identified as a factor whose down-regulation is essential for differentiation of rat neural progenitor cells and PC12 pheochromocytoma cells (5–7). ATF5 is expressed in a number of cancer cells and down-regulated in those cells following growth factor deprivation; exogenous expression of ATF5 suppresses apoptosis in HeLa cells induced by serum withdrawal and in FL5.12 cells, an IL-3-dependent cell line, by IL-3 deprivation (8). Conversely, dominant-negative ATF5 induces apoptosis of HeLa and FL5.12 cells and a number of glioma and breast cancer cell lines cultured in the presence of growth factors (8–10). Similar interference of ATF5 function in non-neoplastic breast cells or in non-tumor brain cells, such as mature neurons and glial cells, does not affect their survival (9, 10). The mechanism of such selective survival requirement for ATF5 in different cell types is not understood.
The BCL-2 family of proteins includes both anti-apoptotic (BCL-2, BCL-XL, and MCL-1) and apoptotic (BAK, BAX, BID, and BIM) proteins. The regulation and balance of this BCL-2 family of proteins in a particular cell result in inhibition or induction of the apoptotic signaling pathways (11, 12). Notably, the expression pattern of BCL-2 during murine embryogenesis shows that this protein is restricted to zones of survival in various tissues, including B- and T-lymphoid tissues, brain, intestine, skin, and other tissues (13, 14). Increased expression of BCL-2 is also responsible for decreased cell death in follicular lymphoma (15, 16). Although BCL-2 is subject to both tissue-specific and developmental regulation, and transcription regulation of BCL-2 is known to play a major role in BCL-2 expression regulation (17), how various transcription factors affect cell type-dependent BCL-2 regulation remains poorly defined. The BCL-2 gene consists of two distinct promoters, P1 and P2, which can respond to a wide range of transcription factors in different cell types. The P1 promoter resembles structures of some constitutively expressed genes and seems to be the predominant promoter in the natural promoter settings (18). The P2 promoter is located ∼1.3 kb downstream of P1 and, intriguingly, contains a negative regulatory element (NRE) that can block the activity of both P1 and P2 promoters (18, 19). Significantly, the NRE overlaps with regulatory elements that respond to p53 and Rb in a cell type-dependent manner (20, 21).
A number of genes, including HSP27, cyclin D3, CYP2B6 (a member of the P450 family), and ID1, are transcriptionally regulated by ATF5 (22–25), although none of these are known for mediating the cell type-dependent prosurvival function of ATF5. A recent report showed that the myeloid leukemia cell differentiation protein (MCL-1), a member of the BCL-2 family of prosurvival proteins, is regulated by ATF5 and contributes to ATF5-promoted cell survival function in mouse glioma GL261 cells. It is anticipated, however, that additional ATF5 targets are yet to be identified (26). We performed a series of survival (rescue) assays using C6 cells transfected with dominant-negative ATF5 (dnATF5), which induces cell death, with reagents that are known to promote cell survival. This screen identified BCL-2 as a gene that can effectively block dnATF5-induced apoptosis in C6 cells. We demonstrate that ATF5 binds directly to the ARE of the BCL-2 P2 promoter, which is located downstream of and adjacent to the NRE region, and transactivates BCL-2 in C6 glioma and MCF-7 breast cancer cells. Moreover, we show that regulation of the ARE by ATF5 is cell type-dependent, indicating that binding to the ARE by ATF5 relieves the inhibitory effect of the NRE in the BCL-2 promoter in selective types of cells. Our results identify BCL-2 as playing an essential role in mediating the cell type-dependent prosurvival function of ATF5.
EXPERIMENTAL PROCEDURES
Plasmids
DNA constructs pCMS-EGFP-FLAG-ATF5, pLeGFP-C1-FLAG-ATF5, pLeGFP-C1-FLAG-dnATF5, pCIN4, pCIN4-FLAG-HA-ATF5, and pQsiren-shRNA-ATF5 were described previously (2, 5). To create a BCL-2 promoter-luciferase reporter and derivatives, we obtained, from Addgene, the plasmid construct LB322, which contains the human BCL-2 gene fragment from ATG to −3934 with both P1 and P2 promoters (15). The BCL-2(P1P2) promoter fragment (3.9 kb) was released from LB322 by BamHI and HindIII double digestion and inserted at the polylinker region between the BglII and HindIII sites in the pGL3-Basic vector, creating the pGL3-BCL-2(P1P2) construct. pGL3-BCL-2(P1) was created by inserting a 2.7-kb BCL-2 P1 promoter fragment, obtained from LB322 by KpnI and BamHI double digestion, at the polylinker region between the KpnI and BglII sites in the pGL3-Basic vector. pGL3-BCL-2(P2) was created by inserting a 1.3-kb BCL-2 P2 promoter fragment, released from LB322 by KpnI and BamHI double digestion, between the KpnI and BamHI sites in the pGL3-Basic vector. pGL3-BCL-2(P1ΔARE), in which the ARE site TTTCTTTCTT (at −81) was changed to TTAGATTTGA, was created by site-directed mutagenesis using pGL3-BCL-2(P1) as a template and primer pairs 5′-GAGGAGGGCTCTTAGATTTGAATGAACCGTG-3′ and 5′-CACGGTTCATTCAAATCTAAGAGCCCTCCTC-3′ following the manufacturer's instructions (QuikChange site-directed mutagenesis kit, Stratagene). pGL3-BCL-2(P2ΔARE), in which the ARE site CCTCTTCTTT (at −1581) was changed to CCAGTTCTGT, was created using pGL3-BCL-2(P2) as a template and primer pairs 5′-GCTGGATTATAACTCCAGTTCTGTGGGGGCCGTGG-3′ and 5′-CCACGGCCCCCACAGAACTGGAGTTATAATCCAGC-3′. pGL3-BCL-2(P1ΔCRE), in which GTGACGTTA (at −1552) was changed to GGGCCTTTA, was created by inserting a 2.7-kb BCL-2(P1ΔCRE) promoter fragment, obtained from LB595 by KpnI and BamHI double digestion, at the polylinker region between the KpnI and BglII sites in the pGL3-Basic vector. To construct BCL-2 shRNA against human BCL-2 mRNA, oligonucleotides 5′-GATCCGCCGGGAGATAGTGATGAAGTATTCAAGAGATACTTCATCACTATCTCCCGGTTTTTTACGCGTG-3′ and 5′-AATTCACGCGTAAAAAACCGGGAGATAGTGATGAAGTATCTCTTGAATACTTCATCACTATCTCCCGGCG-3′ were annealed to make a double-stranded DNA fragment, which was then cloned into RNAi-Ready pSIREN-RetroQ-ZsGreen between the BamHI and EcoRI sites. Similarly, oligonucleotides 5′-GATCCGCGAGTGGGATACTGGAGATTTCAAGAGAATCTCCAGTATCCCACTCGTTTTTTACGCGTG-3′ and 5′-AATTCACGAGTAAAAAACGAGTGGGATACTGGAGATTCTCTTGAAATCTCCAGTATCCCACTCGCG-3′ were annealed to make a double-stranded DNA fragment. The DNA fragment was subsequently cloned into RNAi-Ready pSIREN-RetroQ-ZsGreen between the BamHI and EcoRI sites to made BCL-2 shRNA against rat BCL-2 mRNA.
Cell Cultures, Preparation of Primary Rat Astrocytes and Human Breast Epithelial Cells, Transfection, and Retrovirus Infection
Rat C6 glioma and human MCF-7 and T47D breast cancer cells were grown in DMEM (Invitrogen) with 10% FBS (Atlanta Biologicals), 100 μg/ml streptomycin, and 100 IU/ml penicillin. Stable cell lines C6-pCIN6 and C6-FLAG-HA-ATF5 were selected and maintained in DMEM with 10% FBS containing 800 μg/ml G418 (Clontech). Primary rat astrocytes were prepared using newborn rats according to procedures described by Cole and Vellis (33) and cultured in DMEM/F-12 (Invitrogen) supplemented with 15 mm HEPES, 1.2 g/liter NaHCO3, and 10% FBS. Human breast epithelial cells (HBECs) were prepared from fresh reduction mammoplasty tissue following a modified protocol described previously (27). Briefly, surgical mammary tissue (200–250 g) with fat and blood clots removed was cut into small pieces under sterile conditions and washed five times with ice-cold Hanks' balanced salt solution (Lonza) containing 0.2% kanamycin. Tissue fragments were treated with type II collagenase (0.15% in Ca2+/Mg2+-free Hanks' balanced salt solution containing 2% BSA; Roche Applied Science) in vials at 37 °C in an agitating water bath for 3–4 h. Digested tissue suspension was sequentially sieved through a 1-mm nylon screen and then a 300-μm nylon screen. The pass-through was centrifuged at 100 × g for 5 min, and cell pellets were resuspended in Hanks' balanced salt solution at a concentration of 2 × 107 cells/ml. The cell suspension was laid onto a preformed discontinuous gradient of 12-ml Percoll (Sigma) in sterile precapped Corning tubes and centrifuged at room temperature in a fixed-angle rotor at 800 × g for 15 min. Epithelial cells formed a cell band at a density of 1.053–1.064 and were collected and seeded at a concentration of 5 × 105 cells/T25 in Ham's F-10 medium (Invitrogen) containing 0.2% kanamycin, 0.24% NaHCO3 (0.12 units/ml), 5% human serum, 10 ng/ml EGF, 6.5 ng/ml triiodothyronine, 5 μg/ml transferrin, and 10 ng/ml cholera toxin. The cell culture was maintained for 5 days at 37 °C in CO2 (5%) incubator medium, which was changed every other day. Cell transfection and retrovirus infection were performed as described previously (2, 5). For serum withdrawal, cells (24 h after transfection if transfected cells were used) were washed with PBS (140 mm NaCl, 2.7 mm KCl, 10 mm Na2HPO4, and 1.8 mm KH2PO4 (pH 7.4)) and maintained in serum-free DMEM. Cell transfection was carried out using FuGENE 6 reagent (Roche Applied Science) according to the manufacturer's instructions. Retrovirus production and infection were performed as described previously (5).
Immunoblotting
Cells grown on culture plates were rinsed with ice-cold PBS and then lysed in cell lysis buffer (50 mm Tris-HCl (pH 7.4), 150 mm NaCl, 2 mm EDTA, 1% Nonidet P-40, and 0.5% Triton X-100) containing a protease inhibitor mixture (Roche Applied Science). The lysate was kept on ice for 30 min and centrifuged at 12,000 × g for 10 min at 4 °C. The supernatant was boiled with Laemmli sample buffer (63 mm Tris-HCl, 2% SDS, 5% 2-mercaptoethanol, 10% glycerol, and 0.0025% bromphenol blue (pH 6.8)) and loaded onto an SDS-polyacrylamide gel. The antibodies used were rabbit anti-ATF5 (1:250) and mouse anti-β-actin (1:10,000) from Abcam, mouse anti-FLAG (1:1000) from Stratagene, rat anti-HA (1:1000) from Roche Applied Science, mouse anti-BCL-2 (1:250) from Pharmingen, rabbit anti-BCL-X (1:100) from Calbiochem, and mouse anti-MCL-1 (1:200) from Santa Cruz Biotechnology.
RNA Extraction, Reverse Transcription-PCR, and Quantitative Real-time PCR
RNA was extracted using TRIzol reagent (Invitrogen) according to the manufacturer's directions. To make cDNAs, RNA (2 μg) was primed with oligo(dT) and reverse-transcribed with SuperScript reverse transcriptase (Invitrogen) according to the manufacturer's directions. PCR and quantitative real-time (QRT) PCR analyses were carried out using the GoTaq DNA polymerase (Promega) and GoTaq Real-Time PCR (Promega) systems, respectively. Primers used were 5′-CCTTCACCCTCCCGACC-3′ and 5′-CAGAGTCAGTGGAACGGGA-3′ for ATF5, 5′-CGACTTTGCAGAGATGTCCA-3′ and 5′-ATGCCGGTTCAGGTACTCAG-3′ for BCL-2, and 5′-CATCGTGGGCCGCCCTAGGC-3′ and 5′-GGGCCTCGGTGAGCAGCACA-3′ for β-actin. Cycling parameters are available upon request.
Chromatin Immunoprecipitation Assay
Cells were fixed with ice-cold 1% formaldehyde in PBS for 15 min at 22 °C. Glycine (2.5 m) was added directly to a final concentration of 0.125 m for 5 min to stop fixation, and the cells were then rinsed twice with ice-cold PBS. The cells were scraped into 1 ml of ChIP radioimmune precipitation assay buffer (50 mm Tris-HCl (pH 8.0), 150 mm NaCl, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS, and 5 mm EDTA) and then sonicated with the tubes in an ice bath with an Ultrasonics sonicator at setting 1 for 1.5 min. The samples were diluted to a protein concentration of 1 mg/ml with ChIP radioimmune precipitation assay buffer and were cleared by centrifugation at 13,000 rpm for 15 min at 4 °C. 100 μl of the samples were saved for input. 1 ml of the chromatin solution was incubated with 1 μg of anti-FLAG antibody (Stratagene) or an irrelevant mouse anti-AKT antibody (Invitrogen) as a control (IgG) and rotated overnight at 4 °C. Immune complexes were immunoprecipitated with 20 μl of 50% protein A-agarose (Roche Applied Science) at 4 °C for 1 h. The immunoprecipitated complexes were washed twice with ChIP radioimmune precipitation assay buffer, three times with cell lysis buffer, twice again with radioimmune precipitation assay buffer, and once with 10 mm Tris-HCl (pH 8.0) and 1 mm EDTA and then eluted in elution buffer (46 mm Tris-HCl (pH 8.0), 1% SDS, and 0.6 mm EDTA) by incubation for 10 min at 65 °C. The cross-linking of the samples was reversed by the addition of NaCl to a final concentration of 0.2 m and incubation at 65 °C overnight. A QIAquick PCR purification kit (Qiagen) was used to purify DNA from the samples, and the purified DNA was eluted into 50 μl of H2O. DNA samples were used for either regular PCRs or QRT-PCRs as indicated. For detection of BCL-2(P1), BCL-2(P1ΔARE), and BCL-2(P1ΔCRE) promoter elements, primers 5′-GCTCAGAGGAGGGCTCTT-3′ and 5′-CTGAGGTGCCTGTCCTCTT-3′ were used. For detection of BCL-2(P2) and BCL-2(P2ΔARE) promoter elements, primers 5′-GTCCAAGAATGCAAAGCACA-3′ and 5′-CCTTCCCAGAGGAAAAGCAA-3′ were used.
Luciferase Assay
This was done as described previously (2), with Renilla as an internal control. Cells were harvested 48 h after transfection or after serum deprivation or staurosporine treatment and were lysed with the lysis buffer provided in the Promega luciferase system. Luciferase and Renilla activities were determined using a TD20/20 luminometer (Turner Designs). Relative luciferase activities were obtained by normalizing the luciferase activity against Renilla activity. Data are presented as means ± S.E. (n = 3).
Survival and Apoptotic Assays
Percent cell survival was determined by trypan blue (0.1% in PBS) staining (2). The percentage of unstained cells among the total cell population (GFP-positive cells only if transfected cells were considered) was calculated and taken as percent surviving cells. Cell apoptosis was determined by Hoechst 33342 staining as described previously (28). Briefly, cells transfected for 48 or 24 h if followed with serum deprivation (SD) or staurosporine (STS) for 48 h were fixed in 4% formaldehyde for 10 min. After washing with PBS three times, the fixed cells were exposed to Hoechst 33342 (Sigma) at 1 μg/ml in PBS and 0.1% Triton X-100 for 15 min. Intact cells (GFP-positive cells if transfected cells were considered) with intact nuclei were scored as viable, whereas those with condensed nuclei and fragmented chromatin were counted as dying or dead. Apoptotic rates are presented as the percentage of apoptotic cells of the total number of cells assessed. All experiments were performed in triplicate and are reported as means ± S.E. (n = 3).
RESULTS
ATF5 Expression Is Down-regulated in C6 and MCF-7 Cells Subject to Serum Deprivation and Staurosporine Treatment
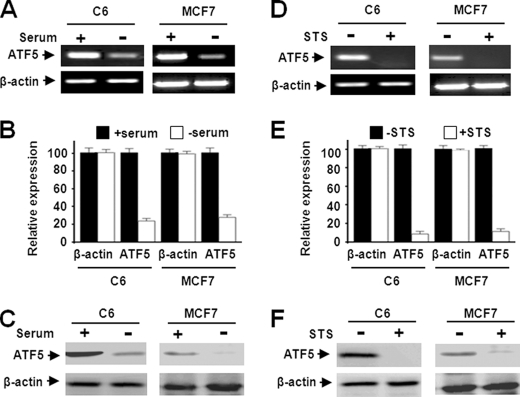
The rat C6 glioma cell and human MCF-7 breast cancer cell were selected for this study because ATF5 function was shown to be required for survival of various glioma and breast cancer cells (9, 10). Given the prosurvival function of ATF5, we anticipated down-regulation of ATF5 in the two cell lines under apoptotic stimulation. Indeed, the ATF5 mRNA transcript was reduced significantly in C6 and MCF-7 cells subjected to SD for 36 h (Fig. 1, A and B). Down-regulation of ATF5 protein was observed 48 h after SD (Fig. 1C). To ascertain that ATF5 down-regulation is not restricted to cell death provoked by SD but instead is a more general response in apoptotic cells, we analyzed ATF5 expression in C6 and MCF-7 cells subjected to additional apoptotic stimuli. Loss of the ATF5 mRNA transcript and ATF5 protein was observed in both C6 and MCF-7 cells treated with the apoptosis-inducing drugs STS (Fig. 1, D and F) (29, 30) and camptothecin (28) (data not shown). These data demonstrate that ATF5 is down-regulated in C6 and MCF-7 cells subjected to a variety of apoptotic stimuli, which is consistent with an earlier report showing that ATF5 is down-regulated in growth factor-deprived FL5.12 and HT-2 cells (8).
FIGURE 1.
ATF5 expression is down-regulated in C6 and MCF-7 cells subjected to serum deprivation and staurosporine treatment. A and B, reverse transcription-PCR (A) and QRT-PCR (B) analyses monitoring expression levels of ATF5 mRNA in C6 and MCF-7 cells subjected to SD for 36 h. C, Western blot analysis monitoring ATF5 expression in C6 and MCF-7 cells following SD for 48 h. D and E, reverse transcription-PCR (D) and QRT-PCR (E) analyses were carried out as for A and B, except that cells were treated with STS (0.1 μm) for 36 h. F, Western blot analysis was carried out as described for C using cells treated with STS (0.1 μm) for 48 h. β-Actin was used as a control in all the experiments. All experiments with statistical analyses were performed at least three times, and the error bars depict means ± S.E.
ATF5 Suppresses Apoptosis in C6 and MCF-7 Cells Induced by Serum Deprivation and Staurosporine Treatment
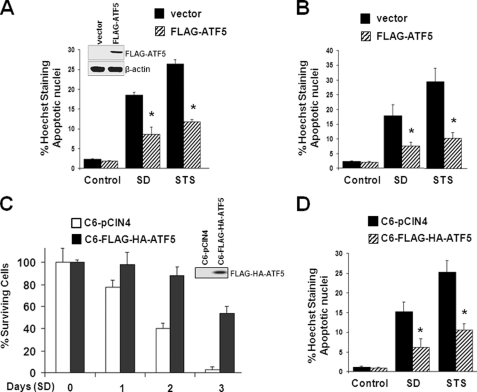
To determine whether ATF5 expression protects C6 and MCF-7 cells from apoptosis, we transiently transfected the two cells with empty vectors or vectors expressing ATF5 and subjected the cells to SD and STS treatment. Both SD and STS treatment evoked apoptosis in vector-transfected C6 (Fig. 2A) and MCF-7 (Fig. 2B) cells, whereas ATF5-expressing C6 and MCF-7 cells displayed significant resistance to cell death (Fig. 2, A and B). Western blot analysis confirmed the presence of FLAG-ATF5 in transfected C6 cells (Fig. 2A, inset) and MCF-7 cells (data not shown). To further determine the anti-apoptotic function of ATF5, we carried out cell survival and apoptotic analyses using a C6 cell line (C6-FLAG-HA-ATF5) in which FLAG-HA-ATF5 is expressed at a level comparable to endogenous ATF5 (2), and therefore, physiological function of ATF5 could be assessed. Fig. 2C shows that C6-pCIN4 cells underwent rapid apoptosis when deprived of serum, whereas C6-FLAG-HA-ATF5 cells showed significant improvement in cell survival under SD. Assessment of apoptotic cells by Hoechst 33342 staining further showed that expression of FLAG-HA-ATF5 at the physiological level in the C6-FLAG-HA-ATF5 cells inhibited apoptosis evoked by SD and STS treatment (Fig. 2D).
FIGURE 2.
ATF5 expression suppresses apoptotic cell death of C6 and MCF-7 cells induced by serum deprivation and STS treatment. A, C6 cells were transfected with pCMS-EGFP or pCMS-EGFP-FLAG-ATF5 for 24 h and subjected to SD or STS (0.1 μm) for 2 days. Transfected (GFP-positive) cells were scored for the presence of apoptotic nuclei by Hoechst 33342 staining. *, p < 0.01, statistical significance between cells without or with overexpressed ATF5. Inset, Western blot analysis monitoring expression of ATF5 in transfected cells. B, transfection and apoptotic analysis were carried out as described for A, except that MCF-7 cells were used. *, p < 0.01, statistical significance between cells without or with overexpressed ATF5. C and D, C6 cell lines stably transfected with empty vector (C6-pCIN4) or vector expressing ATF5 (C6-Flag-HA-ATF5) were subjected to SD or STS (0.1 μm) treatment for 2 days, and the percentage of cell survival (C) or the percentage of apoptotic nuclei (D) was assessed. The inset in C shows Western blot analysis monitoring the ectopically expressed ATF5. *, p < 0.01, statistical significance between cells without or with overexpressed ATF5.
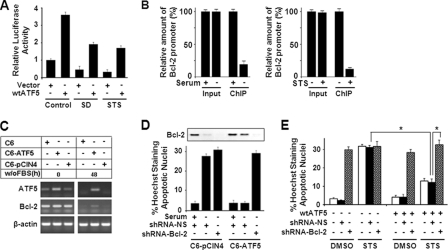
ATF5 Stimulates and dnATF5 Represses BCL-2(P2) Promoter Activity in an ARE-dependent Manner
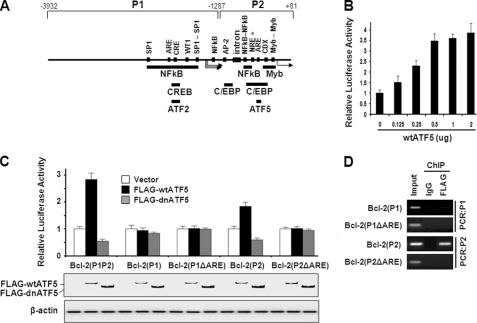
To identify the molecules and pathways that are implicated in the ATF5-dependent prosurvival function, we carried out a series of survival assays using C6 cells transiently transfected with dnATF5, which induces cell death, with reagents that are known to promote cell survival. This screen identified BCL-2 as a gene that can effectively block dnATF5-induced apoptosis in C6 cells. Given that the BCL-2 promoter is known to be regulated via the CRE site in the P1 promoter region by the ATF/CREB family members CREB and ATF2 (31, 32) and contains ARE in both P1 and P2 (Fig. 3A) (2), our data raised the possibility that ATF5 is directly involved in transcription regulation of BCL-2. To test this possibility, we performed BCL-2 promoter-luciferase reporter assays. As shown in Fig. 3 (B and C), coexpression of ATF5 activated BCL-2 promoter (the full promoter contains tandem P1P2) activity by 3–4-fold in an ATF5 expression level-dependent manner, whereas coexpression of dnATF5 repressed the BCL-2 promoter reporter by 50% in C6 cells. Identical BCL-2 promoter reporter results were obtained in MCF-7 cells (data not shown). We next determined how individual BCL-2 P1 and P2 promoters and promoter mutants with deletions of ARE or CRE respond to ATF5 activity. As shown in Fig. 3C, the BCL-2 P2 promoter but not the P1 promoter was activated by ATF5 and repressed by dnATF5 in a pattern similar to that of the full-length BCL-2(P1P2) promoter. In addition, deletion of the ARE in P2 (Fig. 3C) but not the CRE (data not shown) in the BCL-2 P2 promoter abolished its ability to respond to ATF5. Immunoblot analysis using an anti-FLAG antibody confirmed that both FLAG-wtATF5 and FLAG-dnATF5 were expressed when transfected (Fig. 3C, lower panels). A ChIP experiment demonstrated that ATF5 bound to the BCL-2 P2 promoter but not the P1 promoter in an ARE-dependent manner (Fig. 3D). These results indicate that ATF5 stimulates and dnATF5 represses BCL-2 promoter activity and that this regulation is dependent on the ARE site in the P2 promoter.
FIGURE 3.
ATF5 binds and stimulates the BCL-2 P2 promoter in an ARE-dependent manner. A, schematic illustration of the transcription regulatory elements and regions responsive to various transcription factors in the human BCL-2 promoter. One ARE element, CCTCTTCTTT, is located in P2 from −81 to −72, which is downstream of and adjacent to the NRE from −119 to −84, and another ARE element, TTTCTTTCTT, is in P1 from −1581 to −1572. The NRE is ∼1.3 kb in length, with its most potent region, marked with an asterisk, located close to the ARE. B, BCL-2 promoter reporter analysis determining the effect of increasing levels of transfected ATF5 on the activity of the full-length BCL-2 promoter. C, BCL-2 promoter-luciferase reporter assay determining the response of various BCL-2 promoters and mutant derivatives to expression of wtATF5 and dnATF5. The Western blot panels show the expression of FLAG-wtATF5 and FLAG-dnATF5 detected by an anti-FLAG antibody. β-Actin was used as loading control. D, PCR analysis of ChIP samples monitoring binding of FLAG-HA-ATF5 to transiently transfected BCL-2 promoter reporters in C6-FLAG-HA-ATF5 cells.
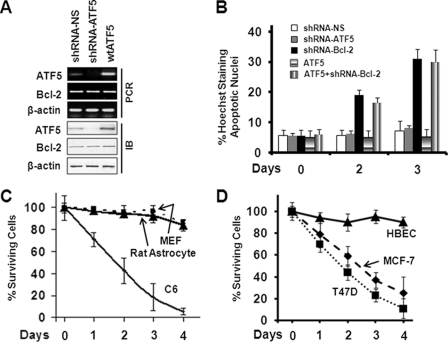
BCL-2 Is Specifically Regulated by ATF5 in C6 and MCF-7 Cells
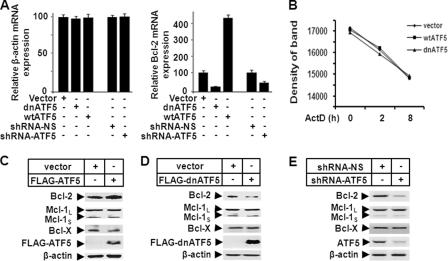
To examine whether expression of the endogenous BCL-2 transcript is regulated by ATF5 activity, we performed QRT-PCR analysis on BCL-2 mRNA in C6 cells infected with empty retroviruses or retroviruses expressing wtATF5 or dnATF5. As shown in Fig. 4A, although the amount of control β-actin mRNA did not change (right panel), BCL-2 mRNA was up-regulated by wtATF5 and down-regulated by dnATF5 48 h after virus infection (left panel). Because the stability of BCL-2 mRNA was not affected in the C6 cells infected with empty retroviruses or those expressing wtATF5 or dnATF5 (Fig. 4B), our data support the conclusion that ATF5 regulates BCL-2 mRNA abundance primarily at the transcription level.
FIGURE 4.
BCL-2 is specifically transactivated by ATF5. A, QRT-PCR analysis monitoring β-actin (left panel) and BCL-2 (right panel) mRNA abundance in C6 cells transiently transfected with empty vector (Vector), vector expressing wtATF5 or dnATF5, or vector expressing non-silencing control shRNA (shRNA-NS) or ATF5 shRNA. The non-regulated β-actin mRNA was used as a control. Expression levels in cells transfected with empty and non-silencing control vectors were arbitrarily set at 100. B, MCF-7 cells were transfected with the indicated constructs as described for A, and 1 day later, cells were treated with actinomycin D (ActD; 5.0 μg/ml) for the indicated hours. Reverse transcription-PCR was carried out as described in the legend to Fig. 1A, and the amount of each of the DNA bands in the agarose gel was quantified by a densitometer. C–E, Western blot analyses monitoring expression of BCL-2 family members in C6 cells transiently transfected with empty or wtATF5-expressing (C) or dnATF5-expressing (D) vector or vector expressing non-silencing shRNA or ATF5 shRNA (E).
We next performed Western blot analysis to determine whether the BCL-2 protein was accordingly regulated by ATF5. Expression of wtATF5 significantly increased the BCL-2 protein level in C6 cells (Fig. 4C, upper panel), whereas expression of dnATF5 (D, upper panel) or an shRNA against ATF5 (E, upper panel) led to reduction in the BCL-2 protein in C6 cells. Similar results were obtained in experiments carried out using MCF-7 cells (data not shown). In addition, parallel Western blot analyses showed that, unlike BCL-2, several other BCL-2 family members, including MCL-1 and BCL-X, were not regulated by either wtATF5 or dnATF5 in C6 (Fig. 4, C–E) and MCF-7 (data not shown) cells. Together, these data indicate that BCL-2 is specifically transactivated by ATF5 in C6 and MCF-7 cells.
BCL-2 Is Required for the Prosurvival Function in C6 and MCF-7 Cells
To determine whether BCL-2 mediates ATF5-promoted cell survival, we first analyzed ATF5-dependent BCL-2 expression in C6 cells in response to SD and STS treatment. Although both SD and STS treatment, which down-regulated ATF5 in C6 cells (Fig. 1), reduced the BCL-2 promoter activity by >50% as determined by luciferase reporter assays (Fig. 5A, compare the first bar with the third and fifth bars), overexpression of ATF5 prevented BCL-2 promoter down-regulation caused by SD and STS treatment (compare the third and fourth bars with the fifth and sixth bars). In addition, ChIP analysis showed that endogenous ATF5 binding to the BCL-2 P2 promoter was significantly reduced in C6 cells in response to both SD and STS treatment (Fig. 5B). ATF5-dependent BCL-2 expression was further demonstrated using C6 cell lines stably expressing ATF5. As shown in Fig. 5C, whereas ectopic expression of ATF5 in the C6-FLAG-HA-ATF5 cell line elevated the basal level of ATF5 expression, BCL-2 expression was not altered in the unstressed condition (Fig. 5C, left panels), suggesting that BCL-2 was expressed steadily at the maximal level. Upon SD for 48 h, both endogenous ATF5 and BCL-2 were depleted in C6 and C6-pCIN4 cells. In contrast, substantial expression of BCL-2 was observed concomitantly with that of ectopic ATF5 in the C6-FLAG-HA-ATF5 cells (Fig. 5C, right panels). A similar expression pattern in BCL-2 protein levels was observed in these cells subjected to SD (Fig. 5D, Western blot inset, compare first, second, fourth, and fifth bars). Significantly, whereas expression of ectopic ATF5 blocked SD-induced cell death in the C6-ATF5 cells, infection of a retrovirus expressing BCL-2 shRNA in these cells reversed ATF5-dependent cell survival (Fig. 5D). We obtained similar results showing that transiently transfected BCL-2 shRNA reversed ATF5-promoted cell survival in C6 and MCF-7 cells subjected to SD or STS treatment (Fig. 5E and data not shown). These results suggest that BCL-2 is a downstream target of ATF5 and is required for ATF5-dependent cell survival in C6 glioma and MCF-7 breast cancer cells.
FIGURE 5.
ATF5 expression in C6 and MCF-7 cells reverses BCL-2 down-regulation induced by SD and STS and suppresses SD- and STS-induced apoptotic cell death. A, luciferase reporter assay monitoring BCL-2 promoter activity in C6 cells transiently transfected with empty vector (Vector) or vector expressing wtATF5 and treated with SD and STS. B, QRT-PCR analysis of ChIP samples monitoring binding of endogenous ATF5 to the BCL-2 (P2) promoter in C6 cells subjected to SD (left panel) or STS treatment (right panel). The amount of the BCL-2 promoter in samples from untreated cells (with serum, without STS) was set at 100%. C, reverse transcription-PCR analysis monitoring abundance of transcripts of ATF5 and BCL-2 in the indicated C6 cell lines subjected to SD (without FBS). β-Actin was used as a control. D, C6-pCIN4 and C6-FLAG-HA-ATF5 cells infected with retroviruses expressing non-silencing shRNA (shRNA-NS) or BCL-2 shRNA were subjected to SD for 2 days. Apoptotic cells were scored as described in the legend to Fig. 2A. Inset, Western blot analysis monitoring expression of BCL-2 in the cells. E, MCF-7 cells transiently transfected with the indicated constructs were subjected to STS or mock (dimethyl sulfoxide (DMSO)) treatment for 2 days. Apoptotic cells of transfected (GFP-positive) cells were assessed as described in the legend to Fig. 2A. *, p < 0.01, statistical significance between the indicated cells.
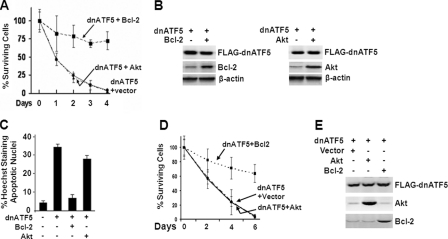
BCL-2 Mitigates the Apoptotic Effect Caused by Interference of ATF5 Function in C6 and MCF-7 Cells
If BCL-2 is a downstream target of ATF5 that mediates the prosurvival function of ATF5, it is expected that expression of BCL-2 would also block cell death induced by ATF5 loss of function. To test this possibility, we carried out survival and apoptotic analyses of C6 cells transiently transfected with a construct expressing dnATF5 and an empty or BCL-2-expressing construct. As shown in Fig. 6A, expression of dnATF5 led to a precipitous decrease in cell survival, but coexpression of BCL-2 inhibited dnATF5-induced cell death. In contrast, coexpression of AKT did not produce any discernible inhibition of dnATF5-induced cell death, despite robust AKT expression similar to BCL-2 (Fig. 6B). Apoptotic analysis of the same transfected C6 cells further showed that expression of dnATF5 evoked massive death, whereas coexpression of BCL-2 but not AKT blocked such death (Fig. 6C). Similar results were obtained using MCF-7 cells (Fig. 6, D and E, and data not shown). These data strongly support that BCL-2 is downstream of ATF5 and mediates the ATF5 prosurvival function.
FIGURE 6.
BCL-2 expression blocks death of C6 and MCF-7 cells induced by dnATF5. A, survival assay monitoring surviving C6 cells (GFP-positive) transfected with the indicated DNA constructs. B, Western blot analysis monitoring expression of FLAG-dnATF5, BCL-2, and AKT in C6 cells transfected with pLeGFP-FLAG-dnATF5 and empty vector (−) or vector expressing BCL-2 or AKT (+) as described for A. C, apoptotic analysis of C6 cells 2 days after transfection as described for A. D and E, survival (D) and Western blot (E) analyses performed as described for A and B, except that MCF-7 cells were used.
The Cell Survival Requirement of ATF5 Correlates with the Ability of ATF5 to Regulate BCL-2 in Different Cell Types
Interfering with ATF5 function or expression causes death of glioma and breast cancer cells but not of several other types of cells, including non-transformed astrocytes and HBECs (9, 10). In light of our findings that BCL-2 is a transcription target of ATF5 and mediates the prosurvival function of ATF5 in C6 and MCF-7 cells, we hypothesized that in non-transformed rat astrocytes and HBECs, either BCL-2 is intransigent to ATF5 regulation, or down-regulation of BCL-2 by ATF5 loss of function does not affect cell survival. To test these possibilities, we infected rat astrocytes with an empty retrovirus or a retrovirus expressing wtATF5 or dnATF5 and determined the expression levels of the transcripts and proteins of ATF5 and BCL-2. As shown in Fig. 7A, although infection of retrovirus expressing ATF5 shRNA down-regulated and infection of retrovirus expressing ATF5 up-regulated the ATF5 transcript and protein in rat astrocytes as expected, they did not affect BCL-2 expression at either the mRNA or protein levels, in contrast to the ability of ATF5 to modulate expression of BCL-2 in C6 cells (Fig. 4). Similarly, we did not detect any changes in BCL-2 expression in response to expression of either wtATF5 or dnATF5 in HBECs (data not shown). To determine whether the inability of ATF5 to modulate BCL-2 expression in rat astrocytes and HBECs is the reason for these cells to be independent of ATF5 for survival, we carried out apoptotic analyses of astrocytes infected with retroviruses expressing shRNAs against ATF5 and BCL-2. As shown in Fig. 7B, although expression of BCL-2 shRNA led to a 3–4-fold increase in apoptotic death in the rat astrocytes, expression of ATF5 shRNA and control non-silencing shRNA did not elicit cell death. Parallel survival analyses further showed that expression of dnATF5 did not reduce cell survival in rat astrocytes, MEFs, and HBECs, whereas it led to precipitous cell death in C6 rat glioma and MCF-7 and T47D human breast cancer cells (Fig. 7, C and D). These results show that, unlike in the C6 and MCF-7 cells, BCL-2 is not regulated by ATF5 in non-transformed rat astrocytes and HBECs and suggest that the cell type-dependent prosurvival function of ATF5 is determined by cell type-specific regulation of BCL-2 by ATF5.
FIGURE 7.
ATF5 does not regulate BCL-2 expression in non-transformed rat astrocytes, MEFs, and HBECs where BCL-2 but not ATF5 is required for survival. A, reverse transcription-PCR (upper panels) and Western blot (lower panels) analyses monitoring expression of ATF5 and BCL-2 in rat astrocytes infected with empty retrovirus or retrovirus expressing non-silencing shRNA (shRNA-NS), ATF5 shRNA, or wtATF5. IB, immunoblot. B, rat astrocytes were infected with the indicated retroviruses as described for A. Apoptotic analysis of infected cells (GFP-positive) was performed as described in the legend to Fig. 2A after virus infection for the indicated days. C and D, C6 cells, rat astrocytes, and MEFs (C) or MCF-7 cells, T47D cells, and HBECs (D) were infected with retrovirus expressing dnATF5. Cell survival was monitored for 4 days as described in the legend to Fig. 6A.
DISCUSSION
Although ATF5 is critically involved, often in a cell type-dependent manner, in cell survival, proliferation, and differentiation, the mechanism by which ATF5 functions remains largely unknown. In this work, we have demonstrated that the prosurvival molecule BCL-2 is a downstream target of ATF5 and mediates the prosurvival function of ARF5 in C6 rat glioma and MCF-7 human breast cancer cells. ATF5 binds to the ARE site in the BCL-2 P2 promoter and activates BCL-2 expression in an ARE-dependent manner. In addition, we showed that expression of BCL-2 is intransigent to ATF5 activity in non-transformed rat astrocytes, MEFs, and HBECs, where expression of BCL-2 but not ATF5 is required for cell survival. Our findings identified BCL-2 as a novel and cell type-dependent downstream target of ATF5 and provide significant insight at to how ATF5 promotes cell survival in a cell type-dependent manner.
Our results are compatible with previous data demonstrating that ATF5 levels decrease dramatically in HeLa and FL5.12 lymphocytes cells after withdrawal of trophic support that evokes apoptotic death (8) and that ATF5 loss of function causes massive apoptotic death in various glioma and breast cancer cells, including C6 and MCF-7 cells (9, 10). Indeed, our results indicated that ATF5 expression is down-regulated in C6 and MCF-7 cells subjected to SD and STS (Fig. 1) and that overexpression of ATF5 reverses the loss of BCL-2 expression and blocks apoptotic cell death evoked by SD and STS (Figs. 5C and 6). Consistent with BCL-2 as a mediator of the prosurvival function of ATF5 in these cells, overexpression of BCL-2 blocks death of C6 and MCF-7 cells induced by ATF5 interference (Fig. 6), and knockdown of BCL-2 abolishes ATF5-promoted cell survival (Fig. 5, D and E).
Our studies and several other laboratories provided strong support for the claim that ATF5 functions in a cell- and tissue type-dependent manner. First, ATF5 seems to regulate different BCL-2 family members in different types of cells. It has been recently shown that ATF5 regulates the anti-apoptotic BCL-2 family member MCL-1, but not BCL-2, in the mouse malignant glioma cell line GL261 (26). The same report also showed a loss of ATF5 and MCL-1 in the glioma stem cell line GS9-6 when induced to differentiate into astrocytes (26). We examined the expression levels of MCL-1S, MCL-1L, and BCL-X in addition to BCL-2 in C6 or MCF-7 cells and did not observe any change in protein expression of MCL-1S, MCL-1L, or BCL-X in response to coexpression of ATF5 or dnATF5 (Fig. 4, D–F). Second, whereas this study and several other reports (8–10) demonstrated that ATF5 expression is required for survival of a number of cancer cells, including HeLa, glioma, and breast cancer cells, it was reported that ATF5 is down-regulated in cell lines and primary tumor samples of hepatocellular carcinoma and that re-expression of ATF5 in hepatocellular carcinoma installs a G2-M phase blockade (25). Our recent study confirmed that ATF5 expression inhibits cell proliferation of Hep3B cells.3 Third, in contrast to a cell cycle inhibitory role of ATF5 in hepatocellular carcinoma cells, ATF5 expression was implicated in maintaining the undifferentiated and proliferating state in slowly dividing neuroprogenitor cells, and down-regulation of ATF5 permits cell differentiation (5–7). Therefore, our findings identifying ATF5 as a cell type-specific regulator of BCL-2 significantly modified our current view on the cell type-specific functions of ATF5 and advanced our understanding of the mechanisms by which ATF5 works.
Intriguingly, the ARE in the BCL-2 P2 promoter is only 30 nucleotides downstream of the NRE that is ∼1.3 kb in length and whose most potent regions are close to the ARE (Fig. 3A). The NRE is a major regulatory site in the BCL-2 promoter and is subject to developmental and cell type-dependent regulation. For instance, p53 represses the BCL-2 NRE, whereas the retinoblastoma protein activates the BCL-2 NRE synergistically with the transcription factor AP-2 in epithelial cells but not NIH3T3 mesenchymal cells (20, 21). Consistent with NRE and ARE together playing critical roles in developmental and cell type-dependent regulation of BCL-2, both the NRE- and ARE-binding sites are encompassed within the DNA hypersensitivity sites in the BCL-2 promoter. Significantly, these hypersensitivity sites are regulated in sync with expression levels of BCL-2 during B cell development. The hypersensitivity sites are prominent in REH pre-B cells in which BCL-2 is highly activated and are lost in DHL9 mature B cells in which BCL-2 is not expressed (17, 18). Because ATF5 is highly expressed in neuroprogenitor cells and dramatically down-regulated in mature neurons, astrocytes, and oligodendrocytes (5–7), current evidence supports the view that ATF5 controls, at least in part, the differential regulation of BCL-2 in undifferentiated and differentiated cells that include the C6 glioma/astrocytes and MCF-7/HBECs we used. One mechanism for ATF5 to exert developmental and cell type-dependent regulation of BCL-2 is that ARE-bound ATF5 modifies the function of the adjacent NRE or NRE-interacting factors so that cell type-specific BCL-2 expression is achieved. Alternatively, ATF5 binding to ARE as an activating regulator may simply bypass the upstream NRE and activate the BCL-2 expression directly. The two models are not mutually exclusive.
In summary, we have established that BCL-2 is a downstream target of ATF5 and that this mechanism is responsible for cancer cell survival in C6 and MCF-7 cells. Our findings highlight the growing evidence that ATF5 regulates cancer cell survival in a cell type-specific manner.
Acknowledgments
We thank J. Angelastro, C. Barnstable, L. Boxer, J. Connor, J. Goldman, and L. Greene for providing valuable reagents for this study. We also thank Y. Xu for technical assistance.
This work was supported in part by grants from the American Cancer Society and United States Department of Defense.
Xijun Liu, Xinyuan Liu, and David X. Liu, unpublished data.
- CREB
- cAMP response element-binding protein
- CRE
- cAMP response element(s)
- ARE(s)
- ATF5-specific response element
- NRE
- negative regulatory element(s)
- dn
- dominant-negative
- HBEC
- human breast epithelial cell
- SD
- serum deprivation
- STS
- staurosporine
- MEF
- mouse embryonic fibroblast
- QRT
- quantitative real-time.
REFERENCES
- 1. Hai T. W., Liu F., Coukos W. J., Green M. R. (1989) Genes Dev. 3, 2083–2090 [DOI] [PubMed] [Google Scholar]
- 2. Li G., Li W., Angelastro J. M., Greene L. A., Liu D. X. (2009) Mol. Cancer Res. 7, 933–943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Peters C. S., Liang X., Li S., Kannan S., Peng Y., Taub R., Diamond R. H. (2001) J. Biol. Chem. 276, 13718–13726 [DOI] [PubMed] [Google Scholar]
- 4. Yamazaki T., Ohmi A., Kurumaya H., Kato K., Abe T., Yamamoto H., Nakanishi N., Okuyama R., Umemura M., Kaise T., Watanabe R., Okawa Y., Takahashi S., Takahashi Y. (2010) Life Sci. 87, 294–301 [DOI] [PubMed] [Google Scholar]
- 5. Angelastro J. M., Ignatova T. N., Kukekov V. G., Steindler D. A., Stengren G. B., Mendelsohn C., Greene L. A. (2003) J. Neurosci. 23, 4590–4600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Angelastro J. M., Mason J. L., Ignatova T. N., Kukekov V. G., Stengren G. B., Goldman J. E., Greene L. A. (2005) J. Neurosci. 25, 3889–3899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mason J. L., Angelastro J. M., Ignatova T. N., Kukekov V. G., Lin G., Greene L. A., Goldman J. E. (2005) Mol. Cell Neurosci. 29, 372–380 [DOI] [PubMed] [Google Scholar]
- 8. Persengiev S. P., Devireddy L. R., Green M. R. (2002) Genes Dev. 16, 1806–1814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Angelastro J. M., Canoll P. D., Kuo J., Weicker M., Costa A., Bruce J. N., Greene L. A. (2006) Oncogene 25, 907–916 [DOI] [PubMed] [Google Scholar]
- 10. Monaco S. E., Angelastro J. M., Szabolcs M., Greene L. A. (2007) Int. J. Cancer 120, 1883–1890 [DOI] [PubMed] [Google Scholar]
- 11. Chipuk J. E., Moldoveanu T., Llambi F., Parsons M. J., Green D. R. (2010) Mol. Cell 37, 299–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Harris M. H., Thompson C. B. (2000) Cell Death Differ. 7, 1182–1191 [DOI] [PubMed] [Google Scholar]
- 13. Hockenbery D. M., Zutter M., Hickey W., Nahm M., Korsmeyer S. J. (1991) Proc. Natl. Acad. Sci. U.S.A. 88, 6961–6965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Novack D. V., Korsmeyer S. J. (1994) Am. J. Pathol. 145, 61–73 [PMC free article] [PubMed] [Google Scholar]
- 15. Heckman C. A., Mehew J. W., Boxer L. M. (2002) Oncogene 21, 3898–3908 [DOI] [PubMed] [Google Scholar]
- 16. Heckman C. A., Wheeler M. A., Boxer L. M. (2003) Oncogene 22, 7891–7899 [DOI] [PubMed] [Google Scholar]
- 17. Seto M., Jaeger U., Hockett R. D., Graninger W., Bennett S., Goldman P., Korsmeyer S. J. (1988) EMBO J. 7, 123–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Young R. L., Korsmeyer S. J. (1993) Mol. Cell. Biol. 13, 3686–3697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chen H. M., Boxer L. M. (1995) Mol. Cell. Biol. 15, 3840–3847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Miyashita T., Harigai M., Hanada M., Reed J. C. (1994) Cancer Res. 54, 3131–3135 [PubMed] [Google Scholar]
- 21. Decary S., Decesse J. T., Ogryzko V., Reed J. C., Naguibneva I., Harel-Bellan A., Cremisi C. E. (2002) Mol. Cell. Biol. 22, 7877–7888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pascual M., Gómez-Lechón M. J., Castell J. V., Jover R. (2008) Drug Metab. Dispos. 36, 1063–1072 [DOI] [PubMed] [Google Scholar]
- 23. Wang H., Lin G., Zhang Z. (2007) Cell Biol. Int. 31, 1309–1315 [DOI] [PubMed] [Google Scholar]
- 24. Wei Y., Jiang J., Sun M., Chen X., Wang H., Gu J. (2006) Biochem. Biophys. Res. Commun. 339, 591–596 [DOI] [PubMed] [Google Scholar]
- 25. Gho J. W., Ip W. K., Chan K. Y., Law P. T., Lai P. B., Wong N. (2008) Cancer Res. 68, 6743–6751 [DOI] [PubMed] [Google Scholar]
- 26. Sheng Z., Li L., Zhu L. J., Smith T. W., Demers A., Ross A. H., Moser R. P., Green M. R. (2010) Nat. Med. 16, 671–677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Garbe J. C., Holst C. R., Bassett E., Tlsty T., Stampfer M. R. (2007) Cell Cycle 6, 1927–1936 [DOI] [PubMed] [Google Scholar]
- 28. Liu D. X., Greene L. A. (2001) Neuron 32, 425–438 [DOI] [PubMed] [Google Scholar]
- 29. Belmokhtar C. A., Hillion J., Ségal-Bendirdjian E. (2001) Oncogene 20, 3354–3362 [DOI] [PubMed] [Google Scholar]
- 30. Bertrand R., Solary E., O'Connor P., Kohn K. W., Pommier Y. (1994) Exp. Cell Res. 211, 314–321 [DOI] [PubMed] [Google Scholar]
- 31. Ma Q., Li X., Vale-Cruz D., Brown M. L., Beier F., LuValle P. (2007) J. Cell. Biochem. 101, 477–487 [DOI] [PubMed] [Google Scholar]
- 32. Ji L., Mochon E., Arcinas M., Boxer L. M. (1996) J. Biol. Chem. 271, 22687–22691 [DOI] [PubMed] [Google Scholar]
- 33. Cole R., Vellis J. (2001) Protocols for Neural Cell Culture (Fedoroff S., Richardson A. eds) 3rd Ed., pp. 117–127, Humana Press Inc., Totowa, NJ [Google Scholar]