Abstract
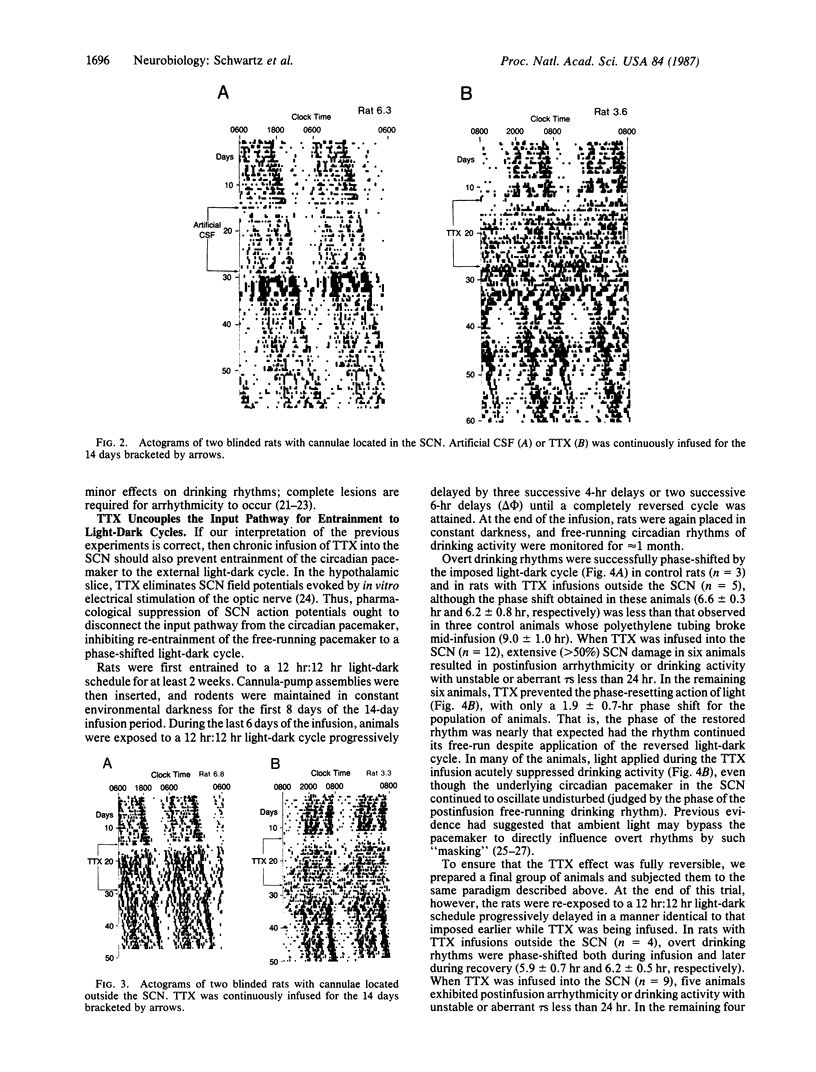
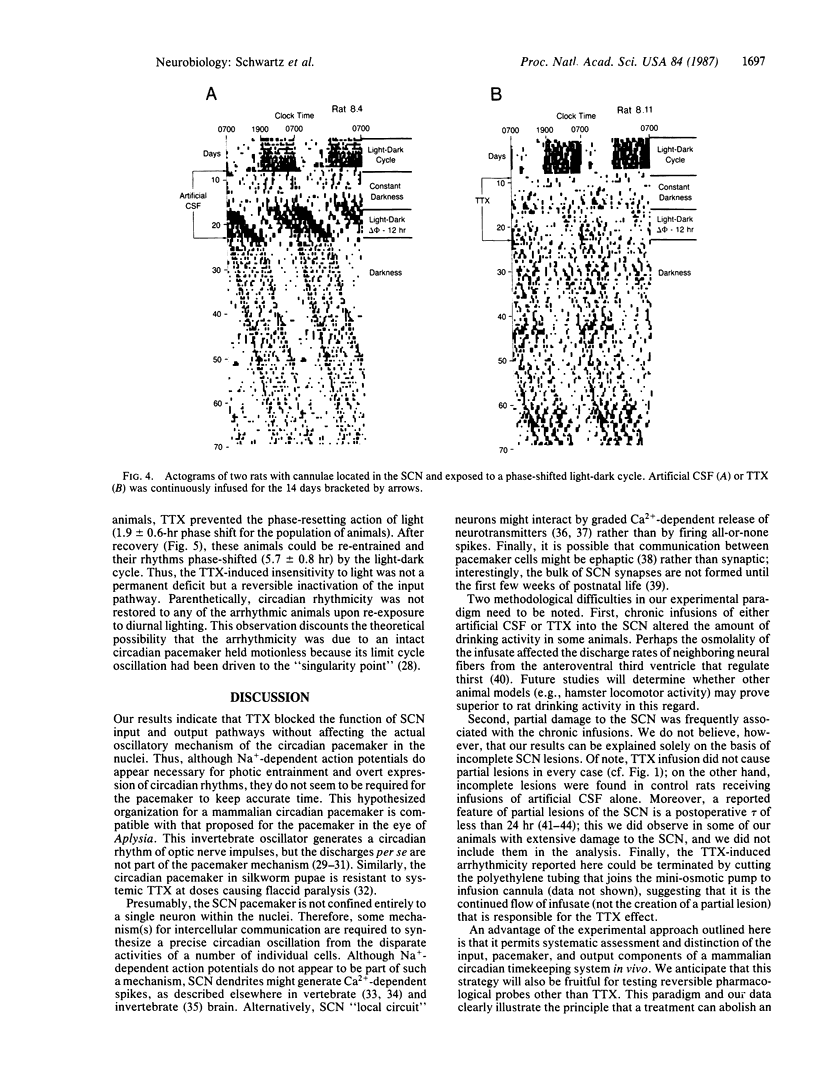
Tetrodotoxin was infused into the suprachiasmatic nuclei of unanesthetized and unrestrained rats continuously for 14 days. The internal timekeeping mechanism of the circadian pacemaker in the nuclei continued to oscillate unaffected by this treatment, although the toxin reversibly blocked function of both the input pathway for pacemaker entrainment and an output pathway for expression of the circadian drinking rhythm. Thus, Na+-dependent action potentials appear necessary for entrainment and expression of overt circadian rhythms, but they do not seem necessary for the pacemaker to keep accurate time. The experimental approach presented in this paper is useful because it allows systematic assessment and distinction of the input, pacemaker, and output components of a mammalian circadian timekeeping system in vivo.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson B., Rundgren M. Thirst and its disorders. Annu Rev Med. 1982;33:231–239. doi: 10.1146/annurev.me.33.020182.001311. [DOI] [PubMed] [Google Scholar]
- Carrow G. M., Calabrese R. L., Williams C. M. Architecture and physiology of insect cerebral neurosecretory cells. J Neurosci. 1984 Apr;4(4):1034–1044. doi: 10.1523/JNEUROSCI.04-04-01034.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dark J. Partial isolation of the suprachiasmatic nuclei: effects on circadian rhythms of rat drinking behavior. Physiol Behav. 1980 Dec;25(6):863–873. doi: 10.1016/0031-9384(80)90306-6. [DOI] [PubMed] [Google Scholar]
- Eskin A. Neurophysiological mechanisms involved in photo-entrainment of the circadian rhythm from the Aplysia eye. J Neurobiol. 1977 May;8(3):273–299. doi: 10.1002/neu.480080310. [DOI] [PubMed] [Google Scholar]
- Inouye S. T., Kawamura H. Persistence of circadian rhythmicity in a mammalian hypothalamic "island" containing the suprachiasmatic nucleus. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5962–5966. doi: 10.1073/pnas.76.11.5962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao C. Y. Tetrodotoxin, saxitoxin and their significance in the study of excitation phenomena. Pharmacol Rev. 1966 Jun;18(2):997–1049. [PubMed] [Google Scholar]
- Lenn N. J., Beebe B., Moore R. Y. Postnatal development of the suprachiasmatic hypothalamic nucleus of the rat. Cell Tissue Res. 1977 Mar 24;178(4):463–475. doi: 10.1007/BF00219568. [DOI] [PubMed] [Google Scholar]
- Llinás R., Yarom Y. Properties and distribution of ionic conductances generating electroresponsiveness of mammalian inferior olivary neurones in vitro. J Physiol. 1981 Jun;315:569–584. doi: 10.1113/jphysiol.1981.sp013764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason W. T., Leng G. Complex action potential waveform recorded from supraoptic and paraventricular neurones of the rat: evidence for sodium and calcium spike components at different membrane sites. Exp Brain Res. 1984;56(1):135–143. doi: 10.1007/BF00237449. [DOI] [PubMed] [Google Scholar]
- Moore R. Y. Organization and function of a central nervous system circadian oscillator: the suprachiasmatic hypothalamic nucleus. Fed Proc. 1983 Aug;42(11):2783–2789. [PubMed] [Google Scholar]
- Mosko S. S., Moore R. Y. Neonatal suprachiasmatic nucleus lesions: effects on the development of circadian rhythms in the rat. Brain Res. 1979 Mar 23;164:17–38. doi: 10.1016/0006-8993(79)90003-9. [DOI] [PubMed] [Google Scholar]
- Narahashi T. Chemicals as tools in the study of excitable membranes. Physiol Rev. 1974 Oct;54(4):813–889. doi: 10.1152/physrev.1974.54.4.813. [DOI] [PubMed] [Google Scholar]
- Nunez A. A., Stephan F. K. The effects of hypothalamic knife cuts on drinking rhythms and the estrus cycle of the rat. Behav Biol. 1977 Jun;20(2):224–234. doi: 10.1016/s0091-6773(77)90786-6. [DOI] [PubMed] [Google Scholar]
- Pickard G. E., Turek F. W. The suprachiasmatic nuclei: two circadian clocks? Brain Res. 1983 Jun 6;268(2):201–210. doi: 10.1016/0006-8993(83)90486-9. [DOI] [PubMed] [Google Scholar]
- Reppert S. M., Schwartz W. J. Maternal coordination of the fetal biological clock in utero. Science. 1983 May 27;220(4600):969–971. doi: 10.1126/science.6844923. [DOI] [PubMed] [Google Scholar]
- Reppert S. M., Schwartz W. J. The suprachiasmatic nuclei of the fetal rat: characterization of a functional circadian clock using 14C-labeled deoxyglucose. J Neurosci. 1984 Jul;4(7):1677–1682. doi: 10.1523/JNEUROSCI.04-07-01677.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T., Kawamura H. Circadian rhythms in multiple unit activity inside and outside the suprachiasmatic nucleus in the diurnal chipmunk (Eutamias sibiricus). Neurosci Res. 1984 Feb;1(1):45–52. doi: 10.1016/0168-0102(84)90029-4. [DOI] [PubMed] [Google Scholar]
- Schwartz W. J., Davidsen L. C., Smith C. B. In vivo metabolic activity of a putative circadian oscillator, the rat suprachiasmatic nucleus. J Comp Neurol. 1980 Jan 1;189(1):157–167. doi: 10.1002/cne.901890109. [DOI] [PubMed] [Google Scholar]
- Schwartz W. J., Gainer H. Suprachiasmatic nucleus: use of 14C-labeled deoxyglucose uptake as a functional marker. Science. 1977 Sep 9;197(4308):1089–1091. doi: 10.1126/science.887940. [DOI] [PubMed] [Google Scholar]
- Schwartz W. J., Reppert S. M., Eagan S. M., Moore-Ede M. C. In vivo metabolic activity of the suprachiasmatic nuclei: a comparative study. Brain Res. 1983 Sep 5;274(1):184–187. doi: 10.1016/0006-8993(83)90538-3. [DOI] [PubMed] [Google Scholar]
- Shibata S., Liou S. Y., Ueki S. Development of the circadian rhythm of neuronal activity in suprachiasmatic nucleus of rat hypothalamic slices. Neurosci Lett. 1983 Dec 30;43(2-3):231–234. doi: 10.1016/0304-3940(83)90193-3. [DOI] [PubMed] [Google Scholar]
- Shibata S., Oomura Y., Hattori K., Kita H. Responses of suprachiasmatic nucleus neurons to optic nerve stimulation in rat hypothalamic slice preparation. Brain Res. 1984 Jun 4;302(1):83–89. doi: 10.1016/0006-8993(84)91287-3. [DOI] [PubMed] [Google Scholar]
- Shibata S., Shiratsuchi A., Liou S. Y., Ueki S. The role of calcium ions in circadian rhythm of suprachiasmatic nucleus neuron activity in rat hypothalamic slices. Neurosci Lett. 1984 Nov 23;52(1-2):181–184. doi: 10.1016/0304-3940(84)90371-9. [DOI] [PubMed] [Google Scholar]
- Stephan F. K., Nunez A. A. Elimination of circadian rhythms in drinking, activity, sleep, and temperature by isolation of the suprachiasmatic nuclei. Behav Biol. 1977 May;20(1):1–61. doi: 10.1016/s0091-6773(77)90397-2. [DOI] [PubMed] [Google Scholar]
- Stephan F. K., Zucker I. Circadian rhythms in drinking behavior and locomotor activity of rats are eliminated by hypothalamic lesions. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1583–1586. doi: 10.1073/pnas.69.6.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson A. M. Slow, regular discharge in suprachiasmatic neurones is calcium dependent, in slices of rat brain. Neuroscience. 1984 Nov;13(3):761–767. doi: 10.1016/0306-4522(84)90094-0. [DOI] [PubMed] [Google Scholar]
- Underwood H., Groos G. Vertebrate circadian rhythms: retinal and extraretinal photoreception. Experientia. 1982 Sep 15;38(9):1013–1021. doi: 10.1007/BF01955345. [DOI] [PubMed] [Google Scholar]
- Van den Pol A. N. The hypothalamic suprachiasmatic nucleus of rat: intrinsic anatomy. J Comp Neurol. 1980 Jun 15;191(4):661–702. doi: 10.1002/cne.901910410. [DOI] [PubMed] [Google Scholar]
- Williams C. M. Tetrodotoxin: a nonlethal paralytic agent for insects. Science. 1968 Apr 26;160(3826):444–444. doi: 10.1126/science.160.3826.444-a. [DOI] [PubMed] [Google Scholar]