Abstract
Objectives
To quantify the effect of swallowing maneuvers on pharyngeal pressure events using high resolution manometry (HRM).
Methods
Seven subjects swallowed multiple, five ml water boluses in three different postural conditions: neutral, head turn, and chin tuck. Pressure and timing events were recorded with a 36-sensor HRM catheter. We analyzed the regions of the velopharynx and base of tongue for maximal pressure, rate of pressure increase, pressure gradient, and duration of pressure above baseline. In the region of the upper esophageal sphincter (UES), we analyzed duration of pressure declination, minimum pressure during opening, and maximum pressures before and after UES opening.
Results
Maneuvers did not have a significant effect on maximum pressure, rate of pressure increase, or pressure gradients in the velopharyngeal or tongue base regions. Duration of pressure above baseline was significantly longer in the velopharynx for head turn. Pre-swallow maximum UES pressure was significantly greater for neutral swallows compared to head turn and post-swallow maximum pressure was significantly lower for chin tuck. Both maneuvers appeared to prolong UES pressure declination duration, but neither reached significance.
Conclusions
HRM allows for optimal spatial and temporal resolution during recording of pressure events along the length of the pharynx and revealed previously undetected task-dependent pressure and timing differences during chin tuck and head turn in healthy adults. These maneuvers appear to influence the UES to a greater degree than the velopharynx and tongue base. Further studies designed to quantify the effect of other maneuvers and bolus consistencies on the generation of pharyngeal pressure events in both normal and disordered subjects may lead to hypothesis-driven, optimal, individualized, swallowing therapies.
Keywords: Pharyngeal pressure, swallowing maneuver, high resolution manometry, dysphagia
INTRODUCTION
The pharyngeal swallow is an event that orchestrates a complex series of muscle contractions to close air spaces in the oral cavity, nasopharynx, and glottis while simultaneously generating pressure gradients to move a bolus from the posterior oral cavity to the cervical esophagus. That is, movement of the bolus is a pressure-driven event. Multiple investigations on pharyngeal pressures during the swallow have been conducted using single, dual, or multi-array pressure sensors and have contributed to our understanding of pressure-modulated bolus movement (1 – 6). These studies employed catheters with one to four unidirectional sensors, usually with a posterior orientation. Since the pharynx is a relatively long, asymmetric, and moving structure, traditional manometry with a small number of unidirectional sensors cannot provide adequate spatial resolution, nor can it accommodate anatomic variations such as the length of the pharynx. Coupling traditional manometry with videofluorography has been standardized to some degree (7) and these combined studies generated much of the theory on bolus propulsion and movement (8,9). However, due to the technological limitations of traditional manometry, we primarily rely on visual observations made from videofluorography and make assumptions about pressure events during the pharyngeal swallow.
The advent of high resolution manometry (HRM) offers a drastically improved method to evaluate pressure during swallowing along the length of the entire pharynx and esophagus (10). Pioneering work with HRM in the pharyngoesophageal segment (PES) utilizing the “Dentsleeve”, a multi-channel, tightly spaced perfusion manometer (11), provided the background for this current study using modern, solid-state HRM through the entire pharynx. The most recent version of HRM (Sierra Scientific Instruments, Los Angeles CA), uses 36 sensor arrays spaced one centimeter apart and is capable of recording pressure in asymmetrical structures, offering the spatial and temporal resolution necessary to accurately capture rapidly changing pressures throughout the pharynx without concern for anatomic variation or moving structures. This allows for a comprehensive evaluation of the pharyngeal swallow. Analyzing pressures across the entire length of the pharynx should reveal additional and perhaps subtle findings that were previously undetectable using traditional manometry.
We used HRM to measure pressure events from the velopharynx to the cervical esophagus, with emphasis on the regions of the velopharynx, tongue base, and upper esophageal sphincter (UES). This was done with the head in the neutral position as well as during two compensatory maneuvers: head turn and chin tuck, as they are commonly employed clinical strategies (1,2,4,12,13) and have demonstrated alterations in pharyngeal and UES function (1,14,15). Head turn has been shown to redirect the bolus to the contralateral pyriform sinus (1,15) and chin tuck has been shown to widen the valleculae and narrow the aditus layrngis, reducing penetration depth into the larynx and trachea (16). These anatomic changes have been shown useful in many dysphagia patient populations (12,16,17). However, it remains unclear how these maneuvers affect swallowing physiology (5) and more specifically, pharyngeal pressures. By changing the dimensions of the pharynx, these maneuvers possibly alter pharyngeal pressure gradients, duration of pressure events, or both. We hypothesized that the chin tuck and head turn maneuvers would lead to changes in pressure-related events (maximum pressure, pressure gradients, duration of events). Specifically, we predicted altering the relative position of the larynx, pharyngeal walls, and tongue may lead to changes in tongue/pharyngeal driving force and UES opening, but little change at the level of the velopharynx.
MATERIALS AND METHODS
Equipment
A solid-state high resolution manometer was used for all data collection (ManoScan360 High Resolution Manometry System, Sierra Scientific Instruments, Los Angeles, CA). The manometric catheter has an outer diameter of 4 mm and 36 circumferential pressure sensors spaced 1 cm apart. Each sensor spans 2.5 mm and receives input from 12 circumferential sectors. These inputs are averaged and a mean pressure is recorded as the pressure detected by that individual sensor. The system is calibrated to record pressures between −20 and 600 mmHg with fidelity of 2 mmHg. Data were collected at a sampling rate of 50 Hz (ManoScan Data Acquisition, Sierra Scientific Instruments). Prior to calibration, the catheter was covered with a protective sheath to preserve sterility without the need to sterilize the catheter between uses (ManoShield, Sierra Scientific Instruments). The catheter was calibrated before each participant according to manufacturer specifications.
Data collection
Four males and three females, aged 20.9 ± 2.1 years (19 – 25), participated in this study with the approval of the Institutional Review Board of the University of Wisconsin-Madison. All subjects were without swallowing, neurological, or gastrointestinal disorders. Participants were instructed not to eat for four hours and not drink liquids for two hours prior to testing to avoid any potential confounding effect of satiety.
Topical 2% viscous lidocaine was applied to the nasal passages with a cotton swab and participants gargled a solution of 4% lidocaine (1 to 2 cc) for several seconds. The manometric catheter was lubricated with 2% viscous lidocaine to ease passage of the catheter through the pharynx. Once the catheter was positioned within the pharynx, participants sat quietly for 5–10 minutes to adjust to the catheter prior to performing swallowing tasks.
The following tasks were performed 5 times with a 5 milliliter (ml) water bolus: neutral swallow, head turn (to the side ipsilateral to catheter placement), and chin tuck. Each bolus was delivered to the oral cavity via syringe. Participants were trained on each swallow task prior to the placement of the catheter. A total of 15 swallows were analyzed for each participant. A 5 ml bolus was selected as it is commonly employed in research paradigms and also represents a volume similar to the restricted volumes many dysphagic patients are limited to as a component of aspiration management.
Data analysis
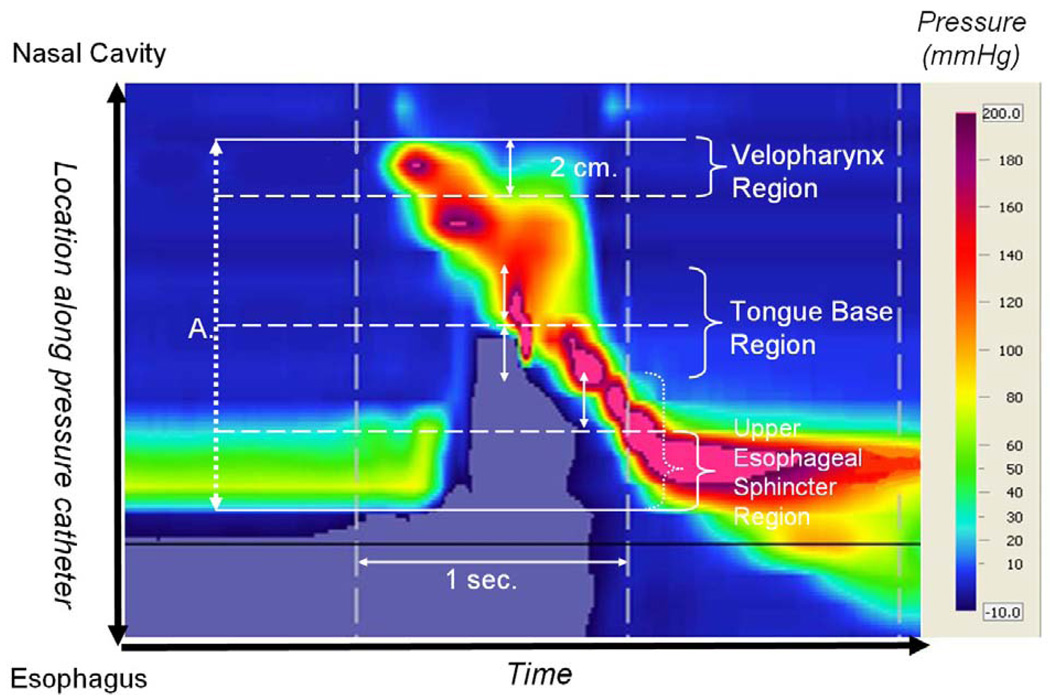
Pressure and timing data were extracted using ManoView software (Sierra Scientific Instruments). The regions of interest were defined manometrically (figure 1). The velopharynx was defined as the region of swallow-related pressure change just proximal to the area of continuous nasal cavity quiescence and extending two centimeters. The tongue base region was defined as the area of swallow related pressure change with a high pressure zone identified approximately midway between the nasopharynx and the UES, with its epicenter at the high pressure point and extending two centimeters proximal and distal to that point. The UES region was defined as the midpoint of stable high pressure just distal (rostral) to the baseline low esophageal pressure zone, extending to a point of low esophageal pressure distally and low baseline pharyngeal pressure proximally. It is important to note that during swallowing, this anatomic area is mobile along the catheter, moving rostrally as much as 4 cm.
Figure 1.
Spatiotemporal plot of 5 ml swallow in the head turn position. A. Total catheter length within pharynx (rostral boundary is the nasopharynx and caudal boundary is the upper esophageal sphincter (UES) (12 cm in this subject).
Mean and standard deviation values were recorded for maximum pressure, rate of pressure increase, pressure gradient, and duration of pressure above baseline in the regions of the velopharynx and tongue base. Rate of pressure increase was calculated by subtracting baseline pressure from maximum pressure and dividing by the time lapse between these points. Pressure gradients were measured by determining the sensor at which maximum pressure within a region occurred and then determining the pressure recorded in sensors one and two cm downstream (toward the esophagus) at the same time-point. Duration of pressure above baseline within a region was defined as the time duration between the onset of pressure escalation and its return to or below baseline using the single senor where maximum pressure was recorded. Minimum pressure during UES opening as well as maximum pressures proceeding and succeeding UES opening were also recorded. The time lapse between these pressure peaks is termed UES opening time (figure 2). Total swallow duration was defined as the time lapse between onset of velopharyngeal pressure rise and the post-swallow UES pressure peak.
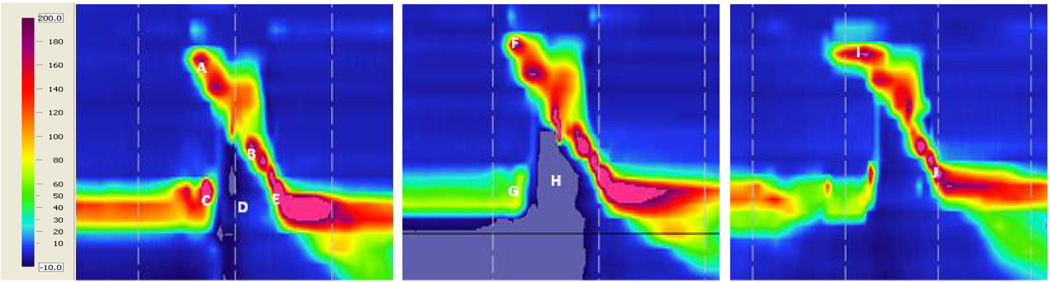
Figure 2.
Spatiotemporal plots of neutral (left), head turn (middle), and chin tuck (right) swallows from one subject. Catheter position is on the y-axis, time is on the x-axis, and pressure is indicated by the color scale. A – velopharyngeal pressure; B – tongue base pressure; C – pre-swallow UES pressure peak; D – UES relaxation pressure; E – post-swallow UES pressure peak; (UES opening time is the time duration between D and E); F – increased duration of velopharyngeal pressure above baseline during head turn; G – decreased pre-swallow UES pressure during head turn; H – further subatmospheric UES relaxation pressure during head turn; I – increased duration of velopharyngeal pressure above baseline during chin tuck; J – decreased post-swallow UES pressure peak during chin tuck.
SigmaPlot 11.0 software was employed for statistical analyses. Mean values recorded during head turn and chin tuck were compared to those recorded for neutral swallows using two-tailed paired t-tests. Shapiro-Wilk and Levene’s tests were used to determine normality and equal variance, respectively. If data did not meet the statistical assumptions for parametric testing, a Wilcoxon-Mann-Whitney rank sum test was performed. A significance level of α = 0.05 was determined a-priori.
RESULTS
Figure 2 provides sample spatiotemporal plots for the three tasks from a single subject and a group data summary is provided in table 1.
Table 1.
Summary data. Values are presented as mean ± standard deviation.
| Neutral | Head turn | Chin tuck | ||||
|---|---|---|---|---|---|---|
| Region | Parameter | Mean | Mean | P-value | Mean | P-value |
| Velopharynx | Pmax (mmHg) | 169 ± 50 | 170 ± 41 | 0.914 | 163 ± 57 | 0.688 |
| Closure Duration (s) | 0.79 ± 0.13 | 0.91 ± 0.22 | 0.016* | 0.85 ± 0.15 | 0.182 | |
| Rise rate (mmHg/s) | 865 ± 202 | 848 ± 241 | 0.889 | 805 ± 247 | 0.545 | |
| Tongue base | Pmax (mmHg) | 306 ± 163 | 276 ± 94 | 0.938 | 252 ± 77 | 0.392 |
| High Pressure Duration (s) | 0.67 ± 0.16 | 0.75 ± 0.25 | 0.276 | 0.62 ± 0.19 | 0.4 | |
| Rise Rate (mmHg/s) | 1576 ± 598 | 1793 ± 1856 | 0.757 | 1239 ± 438 | 0.297 | |
| UES | Ppre(mmHg) | 227 ± 100 | 118 ± 60 | 0.017* | 154 ± 118 | 0.216 |
| Ppost(mmHg) | 239 ± 78 | 256 ± 72 | 0.497 | 196 ± 56 | 0.037* | |
| Opening Duration (s) | 0.85 ± 0.16 | 0.93 ± 0.21 | 0.18 | 0.89 ± 0.22 | 0.454 | |
| Pmin(mmHg) | −5 ± 9 | −10 ± 14 | 0.211 | 0 ± 8 | 0.256 | |
| Pharynx | Total Swallow Duration (s) | 1.03 ± 0.16 | 1.02 ± 0.20 | 0.907 | 0.99 ± 0.18 | 0.616 |
Asterisks denote significant p-values
Velopharynx
Maneuvers did not have a significant effect on maximum velopharyngeal pressure (head turn: t = −0.113, df = 6, p = 0.914; chin tuck: t = 0.422, df = 6, p = 0.688). Rate of velopharyngeal pressure rise was not significantly different from neutral for either head turn (t = −0.398; df = 6; p = 0.705) or chin tuck (t = 1.220; df = 6; p = 0.268), though it was discernibly lower for chin tuck. Duration of velopharyngeal pressure above baseline was significantly longer for the head turn (W = 28; T+ = 28; T− = −0; Z = 2.375; p = 0.016) and discernibly longer for the chin tuck (t = −1.511; df = 6; p = 0.182).
Tongue base
Tongue base maximum pressure was highest in the neutral position and no significant difference was found with head turn or chin tuck, (head turn: W = 2, T+ = 15, T− = − 13, Z = 0.169, p = 0.938; chin tuck: t = 0.922, df = 6, p = 0.392). Rate of pressure rise at the tongue base was not significantly different compared to neutral for either head turn (t = −0.761; df = 6; p = 0.476) or chin tuck (t = −0.079; df = 6; p = 0.946). And duration of tongue base pressure above baseline was longer for head turn (t = −1.198; df = 6; p = 0.276) and shorter for chin tuck (t = 0.906; df = 6; p = 0.4), though neither difference was significant.
UES
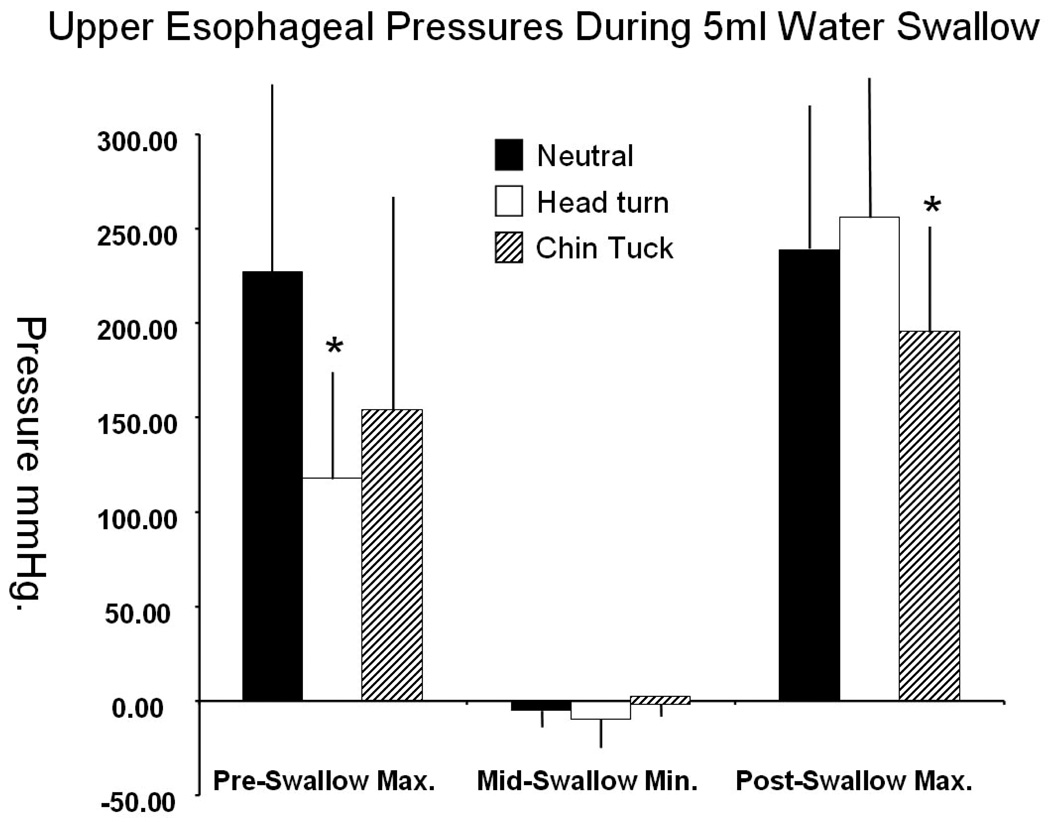
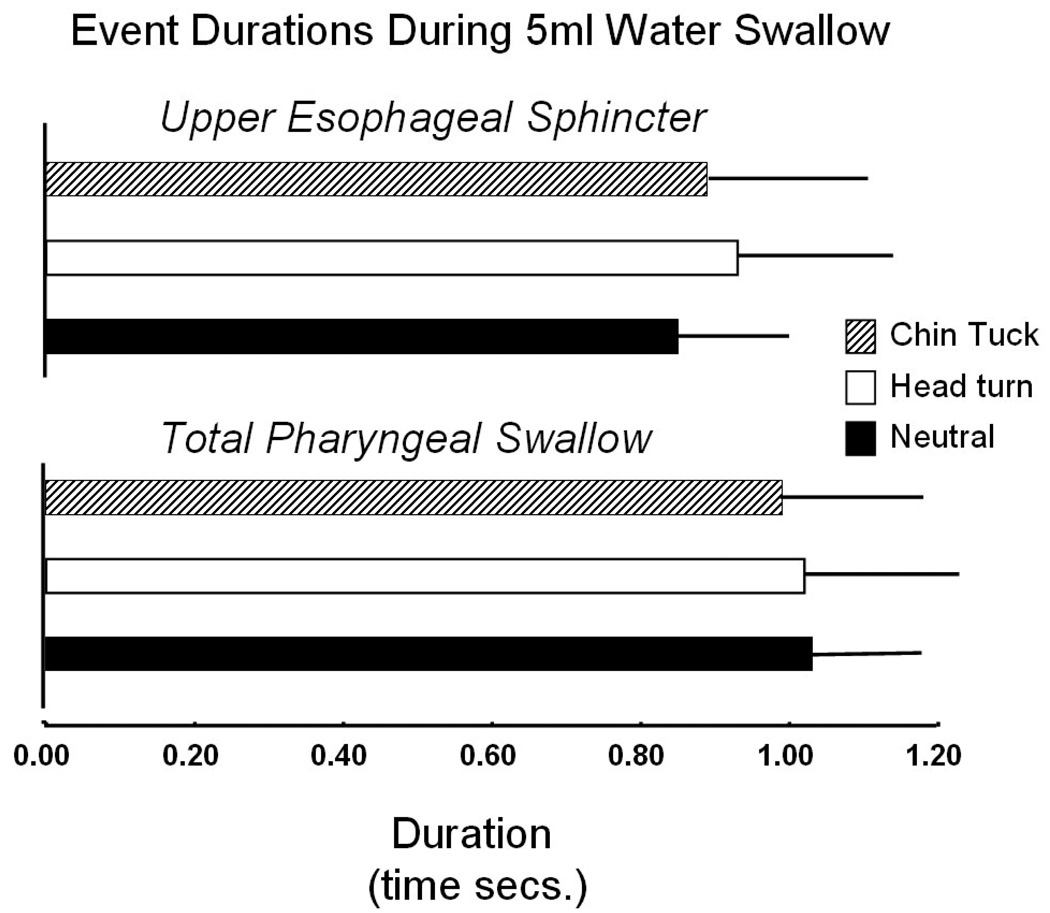
Pre-swallow peak UES pressure was significantly greater for neutral swallows compared to head turn (t = 3.275; df = 6; p = 0.017). This pressure was also higher for neutral swallows compared to chin tuck, but this difference was not significant (t = 1.384; df = 6; p = 0.216). Chin tuck exhibited significantly lower post-swallow peak UES pressure compared to neutral (t = 2.662; df = 6; p = 0.037), and there was no discernible trend between neutral and head turn (t = −0.723; df = 6; p = 0.497). Minimum pressure during UES opening was lower for head turn (t = 1.401; df = 6; p = 0.211) and higher for chin tuck (t = −1.254; df = 6; p = 0.256) (figure 3), but these differences were not significant. Both maneuvers appeared to increase UES opening time, but neither increase was significant (head turn: t = −1.157, df = 6, p = 0.18; chin tuck: t = −0.800, df = 6, p = 0.454) (figure 4).
Figure 3.
Maximum and minimum pressures at the UES region associated with 5 ml water swallow during each swallow task. Mean and Standard deviations with * identifying significant difference relative to Neutral head position (α = 0.05).
Figure 4.
Duration of UES opening (top) and duration of the total pharyngeal swallow (bottom)
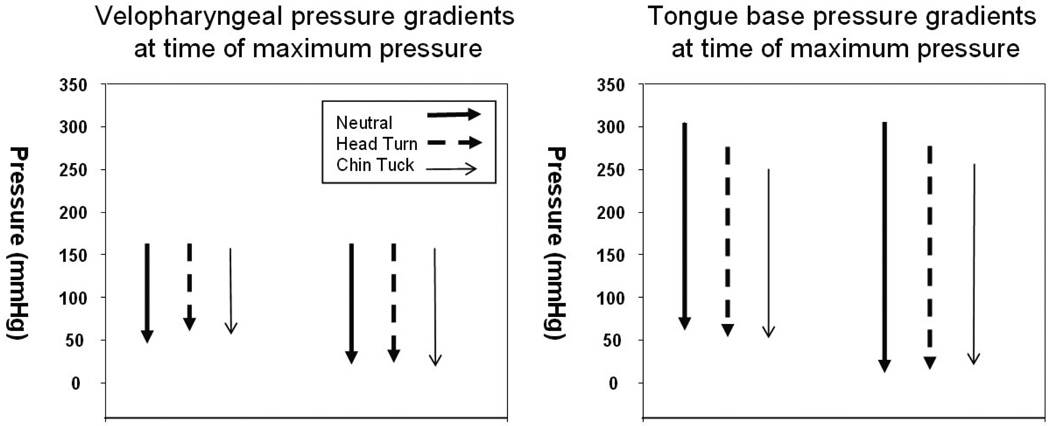
Pressure gradients
No differences were found in one centimeter pressure gradients at the level of the velopharynx or tongue base when comparing neutral swallow to head turn (velopharynx: t = 0.487, df = 6, p = 0.644; tongue base: t = 0.305, df = 6, p = 0.770) and chin tuck (velopharynx: t = 0.740, df = 6, p = 0.487; tongue base: W = 2, T+ = 15, T− = −13, Z = 0.169, df = 6, p = 0.938). There were also no differences for two centimeter gradients for either the head turn (velopharynx: t = −0.044, df = 6, p = 0.966; tongue base: W = 2, T+ = 15, T− = −13, Z = 0.169, df = 6, p = 0.938) or chin tuck (velopharynx: t = 0.271, df = 6, p = 0.796; tongue base: W = 0, T+ = 14, T− = −14, Z = 0, df = 6, p = 1.000). There was, however, an interesting group mean shift of the tongue base gradients from neutral to the postures with lower mean maximum pressure and lower caudal pressures preserving a stable gradient (figure 4, table 2).
Table 2.
Pressure gradients in velopharynx and tongue base at 1 and 2 cm. Max – 1 cm is equal to maximum pressure in the region minus the pressure one sensor downstream at the same time. Max – 2 cm is equal to maximum pressure minus the pressure two sensors downstream.
| Neutral | Head turn | Chin tuck | ||||
|---|---|---|---|---|---|---|
| Region | Gradient | Mean | Mean | P-value | Mean | P-value |
| Velopharynx | Max – 1 cm | 121 ± 58 | 114 ± 56 | 0.644 | 108 ± 60 | 0.487 |
| Max – 2 cm | 149 ± 48 | 150 ± 43 | 0.966 | 146 ± 55 | 0.796 | |
| Tongue base | Max – 1 cm | 242 ± 177 | 223 ± 109 | 0.770 | 206 ± 86 | 0.938 |
| Max – 2 cm | 293 ± 166 | 260 ± 102 | 0.938 | 233 ± 82 | 1.000 | |
Total swallow duration was not significantly affected by head turn (t = 0.122, df = 6, p = 0.907) and chin tuck (t = 0.529, df = 6, p = 0.616).
DISCUSSION
Maneuver-dependent differences in pharyngeal pressures were observed primarily in the UES, not in the velopharynx or tongue base. Thus, our hypotheses were partially supported as we anticipated that altering the relative position of the larynx, pharyngeal walls, and tongue may lead to pressure changes in the pharynx at the level of the tongue base and UES, but little change to the velopharynx. However, the results do not completely support our assumptions that the velopharynx is insulated from the changes in anatomic relationship which occur with postural maneuvers, as durational changes were noted. Somewhat contrary to our predictions for the region of the tongue base, maximal tongue base pressures were the highest for the neutral position, followed by head turn and the lowest for the chin tuck. There was no change in rate of pressure rise and there was no change in absolute pressure gradient, but an interesting shift in the entire gradient values toward lower pressures with both of the maneuvers appeared.
The absence of more notable changes at the tongue base region should not be interpreted as a universal effect as it will be important to evaluate this feature in a disordered subject population. Further, we studied this in a small number of participants and a small sample size can lead to beta error in results interpretation. However, in another study, a similar lack of effect during chin tuck was reported in hypopharyngeal intra-bolus pressure and duration in patients with moderate to severe dysphagia (5). In contrast, head rotation has also been shown to increase hypopharyngeal (valleculae and pyriform sinus) pressure when the head is rotated toward the pyriform sinus occupied by the catheter, but decrease when the head is rotated away from the catheter (15). The bolus was directed away from the side of head rotation which would infer that the intra-bolus pressure was best recorded with head rotation away from the catheter (15). It should be noted that this study used a unidirectional sensor, and thus their high pressure findings may be more related to catheter position rather than true intra-bolus pressures. In the current study, we recorded circumferential pressures, which eliminates directionality of pressure recording and this may account for the discrepancy in hypopharyngeal pressures among studies.
Perhaps of most interest were our observed differences in the duration of pressure events. The duration of time above baseline pressures for the velopharyngeal region was significantly longer for head turn and discernibly longer for the chin tuck. These durations coincide with longer time of minimum pressure (opening time) in the UES, which is not surprising as the velopharyngeal port would usually remain sealed during UES opening to facilitate bolus flow toward the esophagus. This infers either a passive or active interaction between these two distant pharyngeal sites during swallow. However, even as individual components of the swallow showed durational change, the total time of swallow was unaffected (table 1). Duration of tongue base pressure above baseline was longer for head turn, but actually shorter for chin tuck, although these differences were not significant. It would be interesting to evaluate this in a disordered population, as we speculate that some changes to the duration of tongue base activity may be vulnerable to disease processes that affect tongue strength and timing.
Pressure and duration effects were observed in the UES region for both maneuvers. Pre-swallow maximum pressures were the greatest in the neutral position and a head turn led to a significant pressure decrease, with pre swallow mean maximum pressures averaging 227 mmHg in the neutral position and 118 mmHg with head turn. This is consistent with the study of normal subjects by Logemann et al., where an 18 mmHg mean drop in UES pressure was noted with head rotation (1). Our post-swallow pressures were greater with the head turn and significantly lower with the chin tuck as compared to neutral. Both maneuvers increased the duration of UES opening, though this measure did not reach statistical significance. These findings are in accord with previous work using a sleeve catheter in the UES (1). According to radiographic studies, both head turn and chin tuck alter cricopharyngeal position, facilitating UES opening and bolus passage into the esophagus (15,16). As such, these maneuvers may have caused passive changes in UES physiology via structural change.
Our data roughly correspond with those presented in previous studies using conventional manometry (table 3), with a few key differences. Maximum pharyngeal pressure, defined in this study as tongue base pressure, was higher than in previous studies (3,5,18). This could be attributed to HRM measuring pressure across a greater length of the pharynx than traditional manometry, as more sensors allow for increased measurement precision. Additionally, HRM may detect higher pressures in asymmetric regions which are undetectable using unidirectional manometers. UES opening time, defined in this study as the time between the pre-swallow pressure peak and post-swallow pressure peak, was longer than UES relaxation time reported elsewhere (3,5). We chose to use a peak to peak definition as it reflects the entire period of sphincter opening including the times of transition from closed to open and open to closed. Others have used the points of pressure decrease and increase equal to half the UES baseline pressure (14), pressure curve analysis to identify transition points between bolus and contractile pressures (19), or the time of low pressure between the high pressure peaks produced by a single recording catheter placed at the level of the UES baseline high pressure zone, the ‘M’ wave (8,18). As we were focusing on intrasubject changes, we believe our method was simple, reliable, and appropriate for the research questions. Further, our ability to select a group of sensors covering the entire UES region, as opposed to a single sensor somewhere within the region, most likely accounts for these differences.
Table 3.
Data presented by other manometric studies.
| Study | Pmax (mmHg) |
Duration (ms) |
UES relaxation (ms) |
UES Pmax (mmHg) |
UES Prelaxed (mmHg) |
VP Pmax (mmHg) |
|---|---|---|---|---|---|---|
| Boden, neutral3 | 178 ± 18 | 656 ± 59 | 509 ± 24 | 509 ± 24 | - | - |
| Bulow, neutral6 | 255 ± 24 | 487 ± 22 | - | - | - | - |
| Bulow, chin tuck6 | 193 ± 16 | 421 ± 25 | 610 ± 29 | 233 ± 21 | 0 ± 1 | - |
| Takasaki, neutral, male21 | 183 ± 84 | - | - | 236 ± 79 | - | 163 ± 95 |
| Takasaki, neutral, female21 | 167 ± 65 | - | - | 243 ± 87 | - | 125 ± 43 |
| Butler, neutral male18 | 140 | - | −5 | - | - | - |
| Butler, neutral female18 | 152 | - | −2 | - | - | - |
Pmax = peak pharyngeal contraction pressure; Duration = duration of pharyngeal contraction; UES Pmax = peak upper esophageal sphincter pressure; UES relaxation = duration of upper esophageal sphincter opening; UES Prelaxed = upper esophageal sphincter pressure during opening; VP Pmax = peak velopharyngeal pressure.
Intersubject variability probably partially accounts for some of the non-significant findings as we identified a wide range of pressure events even in this small set of normal healthy swallowers. It is likely that this variability is a characteristic of human swallowing and will need to be considered when working with dysphagia patients. Subtle disruptions in a unique swallow pattern will lead to a dysphagia which may be difficult to characterize with isolated pressure recordings. It is possible that these maneuvers would indeed facilitate functional pressure changes in disordered individuals with abnormal pressures.
Although topical anesthesia was used in this study to diminish participant discomfort, we do not believe it significantly altered swallow physiology with regard to our measurements. Omitting this in pilot experiments led to increased gagging and resting UES pressure, confounding data collection. As swallowing is a sensorimotor phenomenon, impairing afferent nerves in the pharynx could potentially alter swallow physiology. However, mechanoreceptors in the pharynx (deep to the mucosa) are largely responsible for modulating swallow physiology (20) and these fibers were probably not affected by our topical anesthetic. Additionally, the oral mucosa was minimally affected, and afferent information from this area is also important to swallow modulation. We believe that the trade-off for increased comfort at the expense of short-term pain/temperature afferent alteration improved the reliability of our data.
HRM provides the opportunity to evaluate pressure gradients during swallowing. However, even with high resolution capabilities, our definition of “pressure gradient” is simplified to a measure of difference in pressure along the course of known fluid movement. In a stricter sense, pressure gradient is defined as a vector quantity of a three dimensional scalar field where every point in the field has an assignable magnitude value, in this case pressure, and a determinable vector influenced by pressure of adjacent points within the field. To a certain extent, we are able to assume information about the pressure in two of the three directions due to boundary conditions at the walls of the pharynx, leaving only one direction for change to occur (the pharyngo-esophageal conduit where the HRM catheter is located). This assumption breaks down in the dysfunctional swallow where sphincteric failure at the oral cavity, nasopharynx, and larynx can modify these simplified boundary conditions.
It is also important to recognize that HRM fails to measure any of the pressure events that occur in the oral cavity during the swallow as the moving bolus and its surrounding pressure events reach the catheter with established momentum. Thus, we must keep in mind that we are measuring many of the important swallow related pressures, but not all of them.
This study examined pressure and timing measures using a small water bolus (5 ml). It would be useful to examine these same parameters in different bolus volumes and consistencies in a larger cohort of both normal and disordered subjects. With larger volumes, pressure changes may be found at the tongue base region; however, the identified changes at the UES level appear to support the use of these maneuvers regardless of the absence of an observed change in the “tongue driving force”. Though significant differences could be observed for some parameters across swallowing tasks, increasing sample size in the future may reveal more evident trends. Trends may also be more apparent in patients with dysphagia, for whom maneuvers are often necessary to restore functional swallowing. Exploring the effects of other maneuvers such as effortful swallow, Mendelsohn’s maneuver, or supraglottic swallow may also be valuable, as these maneuvers will have different effects on pharyngeal pressure patterns. Performing an objective, quantitative evaluation of various maneuvers in a variety of bolus volumes and consistencies may allow for selection of an optimal maneuver for use by individual dysphasic patients.
CONCLUSION
High resolution manometry allows us to collect pressure and timing data with optimal spatial and temporal resolution along the length of the pharynx and is a useful tool to measure variations associated with pharyngeal swallow physiology. Two swallowing maneuvers, head turn and chin tuck, were evaluated using HRM with an emphasis on the pressure events in the regions of the velopharynx, base of tongue, and upper esophageal sphincter in healthy subjects. The measurement of new parameters and improved precision of HRM revealed previously undetected, task-dependent manometric differences. These maneuvers appear to influence the upper esophageal sphincter to a greater degree than the velopharynx and tongue base. We are indentifying a considerable degree of individual variation in swallow-related pressures even within our small and relatively uniform group of subjects. This uniqueness is most likely a normal and important quality of human swallow and will need to be better characterized with additional studies. In addition, further studies designed to quantify the effect of other maneuvers, bolus consistencies and therapies on the generation of pharyngeal pressures, pressure gradients and timing of pressure events in dysphasic patients may lead to the development of optimal, individualized, hypothesis-based swallow rehabilitation strategies.
Figure 5.
Gradients in velopharynx (A) and tongue base (B) for each swallowing task. Measurements were recorded at the maximum pressure within a region (max), and the pressure at the same time point one (1 cm) and two (2 cm) sensors downstream. Min represents the baseline pressure recorded at the same sensor as the maximum pressure.
ACKNOWLEDGEMENTS
This research was supported by a grant from the Department of Surgery of the University of Wisconsin School of Medicine and Public Health. Thank you to Dr. Glen Leverson for statistical consulting and the laboratory of Dr. Jack Jiang for personnel and technical support.
Grant Support: This research was supported by a grant from the University of Wisconsin School of Medicine and Public Health, Department of Surgery
REFERENCES
- 1.Logemann JA, Kahrilas PJ, Kobara M, Vakil NB. The benefit of head rotation on pharyngoesophageal dysphagia. Arch Phys Med Rehabil. 1989 Oct;70(10):767–771. [PubMed] [Google Scholar]
- 2.Lazarus C, Logemann JA, Song CW, Rademaker AW, Kahrilas PJ. Effects of voluntary maneuvers on tongue base function for swallowing. Folia Phoniatr Logop. 2002 Jul–Aug;54(4):171–176. doi: 10.1159/000063192. [DOI] [PubMed] [Google Scholar]
- 3.Boden K, Hallgren A, Witt Hedstrom H. Effects of three different swallow maneuvers analyzed by videomanometry. Acta Radiol. 2006 Sep;47(7):628–633. doi: 10.1080/02841850600774043. [DOI] [PubMed] [Google Scholar]
- 4.Hind JA, Nicosia MA, Roecker EB, Carnes ML, Robbins J. Comparison of effortful and noneffortful swallows in healthy middle-aged and older adults. Arch Phys Med Rehabil. 2001 Dec;82(12):1661–1665. doi: 10.1053/apmr.2001.28006. [DOI] [PubMed] [Google Scholar]
- 5.Bulow M, Olsson R, Ekkberg O. Supraglottic swallow, effortful swallow, and chin tuck did not alter hypopharyngeal intrabolus pressure in patients with pharyngeal dysfunction. Dysphagia. 2002;17:197–201. doi: 10.1007/s00455-002-0050-y. [DOI] [PubMed] [Google Scholar]
- 6.Bulow M, Olsson R, Ekkberg O. Videomanometric analysis of supraglottic swallow, effortful swallow, and chin tuck in healthy volunteers. Dysphagia. 1999;14:67–72. doi: 10.1007/PL00009589. [DOI] [PubMed] [Google Scholar]
- 7.Salassa JR, DeVault KR, McConnel FMS. Proposed catheter standards for pharyngeal manofluorography (videomanometry) Dysphagia. 1998;13:105–110. doi: 10.1007/PL00009553. [DOI] [PubMed] [Google Scholar]
- 8.Olsson R, Nilsson H, Ekberg O. Simultaneous videoradiography and computerized pharyngeal manometry-videomanometry. Acta Radiol. 1994 Jan;35(1):30–34. [PubMed] [Google Scholar]
- 9.Olsson R, Nilsson H, Ekberg O. Simultaneous videoradiography and pharyngeal solid state manometry (videomanometry) in 25 nondysphagic volunteers. Dysphagia. 1995;10(1):36–41. doi: 10.1007/BF00261278. [DOI] [PubMed] [Google Scholar]
- 10.Fox M, Bredenoord A. Oesophageal high-resolution manometry: moving from research into clinical practice. Gut. 2008;57:405–423. doi: 10.1136/gut.2007.127993. [DOI] [PubMed] [Google Scholar]
- 11.Williams RB, Pal A, Brasseur JG, Cook IJ. Space-time pressure structure of pharyngoesophageal segment during swallowing. Am J Physiol Gastrointest Liver Physiol. 2001;281:G1290–G1300. doi: 10.1152/ajpgi.2001.281.5.G1290. [DOI] [PubMed] [Google Scholar]
- 12.Lewin JS, Herbert TM, Putnam JB, Jr, DuBrow RA. Experience with the chin tuck maneuver in postesophagectomy aspirators. Dysphagia. 2001 Summer;16(3):216–219. doi: 10.1007/s00455-001-0068-6. [DOI] [PubMed] [Google Scholar]
- 13.Kahrilas PJ, Logemann JA, Lin S, Ergun GA. Pharyngeal clearance during swallowing: a combined manometric and videofluroscopic study. Gastroenterology. 1992 Jul;103(1):128–136. doi: 10.1016/0016-5085(92)91105-d. [DOI] [PubMed] [Google Scholar]
- 14.Castell JA, Castell DO. Modern solid state computerized manometry of the pharyngoesophageal segment. Dysphagia. 1993;8:270–275. doi: 10.1007/BF01354550. [DOI] [PubMed] [Google Scholar]
- 15.Ohmae Y, Ogura M, Kitahara S, Karaho T, Inouye T. Effects of head rotation on pharyngeal function during normal swallow. Ann Otol Rhinol Laryngol. 1998;107(4):344–348. doi: 10.1177/000348949810700414. [DOI] [PubMed] [Google Scholar]
- 16.Bulow M, Olsson R, Ekkberg O. Videomanometric analysis of supraglottic swallow, effortful swallow, and chin tuck in patients with pharyngeal dysfunction. Dysphagia. 2001;16:190–195. doi: 10.1007/s00455-001-0065-9. [DOI] [PubMed] [Google Scholar]
- 17.Logemann JA. Evaluation and Treatment of Swallowing Disorders. 2nd edition. Pro-Ed Austin, TX: 1998. [Google Scholar]
- 18.Butler SG, Stuart A, Castell D, Russel GB. Effects of age, gender, bolus condition, viscosity, and volume on pharyngeal and upper esophageal sphincter pressure and temporal measurements during swallowing. J Speech Lang Hear Res. 2009 Feb;52:240–253. doi: 10.1044/1092-4388(2008/07-0092). [DOI] [PubMed] [Google Scholar]
- 19.Ghosh SK, Pandolfino JE, Zhang Q, Jarosz A, Kahrilas PJ. Deglutitive upper esophageal sphincter relaxation: a study of 75 volunteer subjects using solid-state high-resolution manometry. Am J Physiol Gastrointest Liver Physiol. 2006 Sep;291(3):G525–G531. doi: 10.1152/ajpgi.00081.2006. [DOI] [PubMed] [Google Scholar]
- 20.Ali GN, Cook IJ, Laundl TM, Wallace KL, De Carle DJ. Influence of altered tongue contour and position on deglutitive pharyngeal and UES function. Am J Physiol. 1997 Nov;273(5 Pt 1):G1071–G1076. doi: 10.1152/ajpgi.1997.273.5.G1071. [DOI] [PubMed] [Google Scholar]
- 21.Takasaki K, Umeki H, Enatsu K, Tanaka F, et al. Investigation of pharyngeal swallowing function using high-resolution manometry. Laryngoscope. 2008 Oct;118(10):1729–1732. doi: 10.1097/MLG.0b013e31817dfd02. [DOI] [PubMed] [Google Scholar]