Abstract
Alternative splicing is a major mechanism for increasing the range of products encoded by the genome. We recently reported positive identification of the first alternative transcripts (AT) of fatty acid desaturase 3 (FADS3) and FADS2 in fetal and neonatal baboons. FADS3, a putative polyunsaturated fatty acid (PUFA) desaturase gene with no known function, has 7 AT that are expressed in at least twelve organs in an apparently constitutive manner. At least five of seven AT are expressed in several mammals and the chicken. FADS2, catalyzing 6 and 8 desaturation and having multiple PUFA substrates, has one AT that is missing two exons and portions of two others. Semi-quantitative expression estimates reveal at least 20-fold differential expression of FADS2 AT1 among neonatal baboon organs compared to 2-fold in the same organs for the classically spliced (CS) FADS2 transcript. Expression of four of the FADS3 AT, those with missing putatively active domains, is highly correlated among organs, suggesting coordinated coexpression. AT may serve as templates to generate protein isoforms or as signaling molecules, and their widespread detection and expression patterns suggest that they play an important role in PUFA biosynthesis.
Background
The interconversion of polyunsaturated fatty acids (PUFA) in mammals has long been known to be mediated by desaturation, elongation, and β-oxidation activities. Biosynthesis of long chain PUFA (LCPUFA) from the eighteen carbon precursors linoleic acid (LA, 18:2n-6) and linolenic acid (ALA, 18:3n-3) is especially important because of the prevalence of 18:2n-6 and 18:3n-3 in the human food supply.
Figure 1 is an outline of the currently accepted pathways for LCPUFA biosynthesis starting from 18:2n-6 and 18:3n-3, with genes and associated activities shown. In vertebrates, desaturation and elongation takes place in the endoplasmic reticulum (ER), apart from a report of 5-desaturase activity in the nucleus [1]. β-oxidation activity is found in the peroxisomes and mitochondria, including a critical step leading directly to 22:6n-3 biosynthesis from 24:6n-3 [2]. β-oxidation catalyzes chain shortening interconversion, while the fatty acid chain elongation system operating on PUFA via three genes (ELOVL2, 4, and 5) [3] chain lengthens.
Figure 1.
Biosynthesis of LCPUFA with emphasis on the role of FADS genes, as generally accepted. β-oxidation may chain shorten any LCPUFA. Not shown are 16:2n-6 and 16:3n-3 which can be elongated to 18:2n-6, and 18:3n-3, respectively.
PUFA desaturation activity is mediated by the Fatty Acid Desaturases (FADS) gene cluster located within a 100 kb region of the long arm of human chromosome 11q12-13.1, with the homologous genes located on mouse chromosome 19 [4]. FADS1, 2, and 3 consist of 12 exons and 11 introns, and include 3 conserved his box motifs and a cytochrome b5 motif. FADS1 [5] gene products were first established as having Δ5-desaturase activity catalyzing 20:3n-6 → 20:4n-6 (arachidonic acid, ARA) and 20:4n-3 → 20:5n-3 (eicosapentaenoic acid, EPA). FADS2 [6] gene products have Δ6-desaturase activity catalyzing 18:2n-6 → 18:3n-6 and 18:3n-3 → 18:4n-3; based on in vitro assays [7], Δ6-desaturation is generally considered the rate limiting step for LCPUFA biosynthesis. FADS3 [8] shares sequence homology with FADS1 and FADS2. Despite the congruence of sequence similarity, no function has emerged for FADS3.
Recent studies have detected associations between specific single nucleotide polymorphisms (SNP) in FADS genes and blood fatty acid levels in normal adults [9-11], adults with cardiovascular disease [12], pregnant and lactating women [13], and in attention-deficit/hyperactivity disorder (ADHD) [14]. More than 90% of SNPs that are associated with fatty acid concentrations are located in the untranslated regions, in introns, and in the intergene regions in CpG islands. Mechanisms that may be influenced by these non-coding regions are differential promoter binding and changes in methylation patterns that alter transcription.
Another possibility are genome-wide alternative splicing (AS) events that yield alternative transcripts (AT) [15]. The majority of AT result from full or partial exon deletion or intron retention. AT may code for modified proteins with different specificities than the protein coded by the transcript resulting from classical splicing (CS), or serve some regulatory role. AT serve to expand the range of products for which a genome can code. It has been estimated that 90% of multi-exon genes have alternative transcripts. In a remarkable example of AS, the Drosophila gene Down syndrome cell adhesion molecule (Dscam) potentially generates over 38,000 transmembrane proteins of the immunoglobulin superfamily [16] that function in the development of neural circuitry, and specifically in dendritic self-avoidance [17]. Metabolic regulation of AT expression has also been reported. For instance, ABCA1, a gene required for lipid transport across the plasma membrane has at least three AT that are regulated in a tissue and diet specific manner [18].
Recently, we have reexamined various aspects of the pathways of LCPUFA biosynthesis, showing, for instance, that the FADS2 gene product catalyzes the Δ8-desaturation of 20:2n-6 → 20:3n-6 and 20:3n-3 → 20:4n-3 [19]. In the course of these molecular studies we discovered AT associated with FADS3 [20] and, most recently, FADS2 [21]. We discuss here salient aspects of these first reports of FADS AT and their possible implications for LCPUFA biosynthesis.
Table 1 outlines the number of transcripts, proteins, and activities reported for the mammalian FADS genes. FADS1 function is limited to Δ5-desaturation of the 20 carbon precursors of ARA and EPA. FADS2 has a much wider substrate spectrum. Its microsomal activity is about one quarter that of Δ5-desaturation [7], and it prefers n-3 to n-6 PUFA. It also shows small Δ8-desaturation activity toward 20 carbon PUFA in the presence of 18 carbon substrates [19]. Finally, FADS2 apparently mediates the biosynthesis of sapienic acid (16:1n-10), the most abundant unsaturated fatty acid on human skin, though the activity on 16:0 as a substrate is low [22]. FADS3 has no known substrates. Analogous PUFA desaturase genes reported in other species, including fish [23], C. elegans [24], and many plant species (e.g. [25]) have a much wider range of substrates and specificities than those of mammals.
Table 1.
Current knowledge of primate PUFA desaturases.
| Gene Transcript(s) | FADS1 | FADS2 | FADS3 |
|---|---|---|---|
| 1 | 2 | 8 (9?) | |
| Protein | Not characterized | ||
| Activities | Δ6-desaturation | Not identified | |
| 18:2n-6→18:3n-6 | |||
| 18:3n-3→18:4n-3 | |||
| Δ5-desaturation | 24:5n-3→24:6n-3 | ||
| 24:4n-6→24:5n-6 | |||
| 20:3n-6→20:4n-6 | 16:0→16:1n-10 | ||
| 20:4n-3→20:5n-3 | |||
| Δ8-desaturation | |||
| 20:2n-6→20:3n-6 | |||
| 20:3n-3→20:4n-3 | |||
FADS3 AT
We recently described the detection of 7 AT of FADS3 in 12 tissues of three month old baboons [20]. AT are numbered sequentially according to the order in which they were discovered. Figure 2 is an alignment of predicted amino acid sequences for the FADS3 CS and AT. AT1 and AT2 are missing exons 3 or 6, respectively. FADS3 AT3 has a truncated version of exon three; AT4 has truncated exons 1 and 3, and is missing exons 2 and 8; AT5 has truncated exons 1 and 4 and is missing exons 2 and 3, as well as retaining intron 8-9; AT6 skips the span from part of exon 4 through part of exon 9; AT7 skips part of exon 8 and all of exons 9 and 10. Predicted protein sequences indicate that AT1, AT3, and AT7 would code for shorter proteins; AT1 and AT3 retain all conserved motifs characteristic of PUFA desaturases (HPGG cytochrome b5 and three histidine repeats “HDLGH, HFQHH, QIEHH”). The deletion of part of exon 8 and all of exons 9-10 results in the loss of the last histidine repeat QIEHH in AT7. AT2, AT4, AT5, and AT6 would produce truncated proteins missing one or more motifs.
Figure 2.
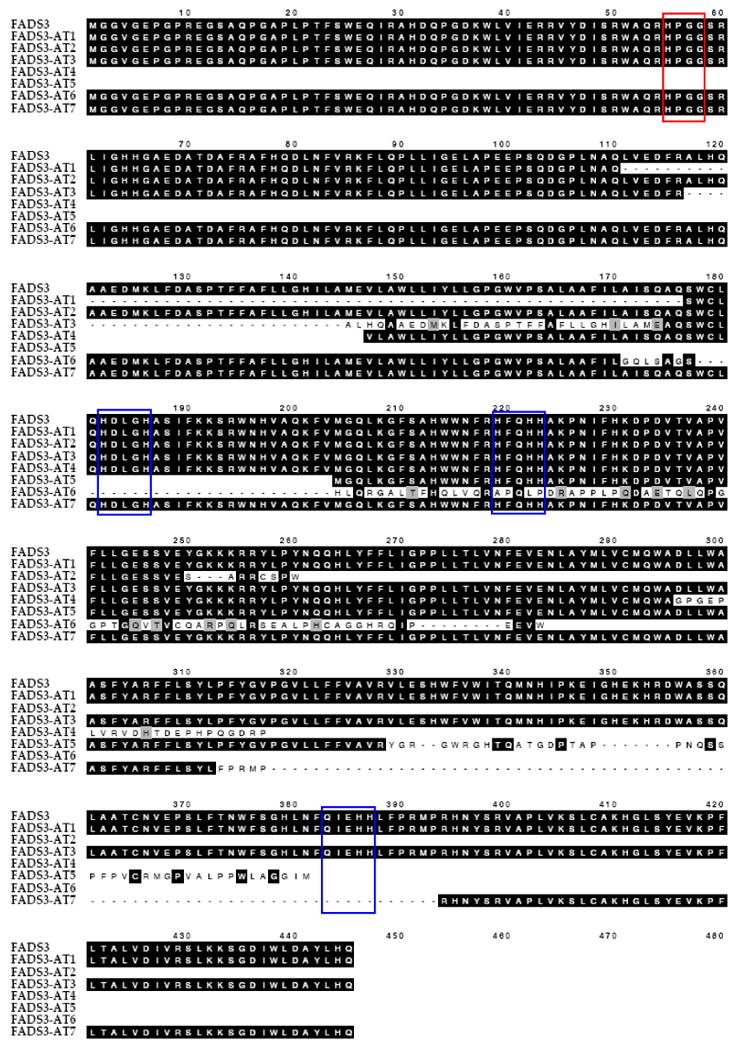
Alignment of Baboon FADS3 alternative transcripts using MacVector software and ClustalW alignment. Well conserved motifs common for desaturases are depicted in boxes. The “HPGG” characteristic of Cytochrome b5 is boxed in red, whereas three conserved histidine motifs “HDLGH, HFQHH and QIEHH” are shown in blue boxes.
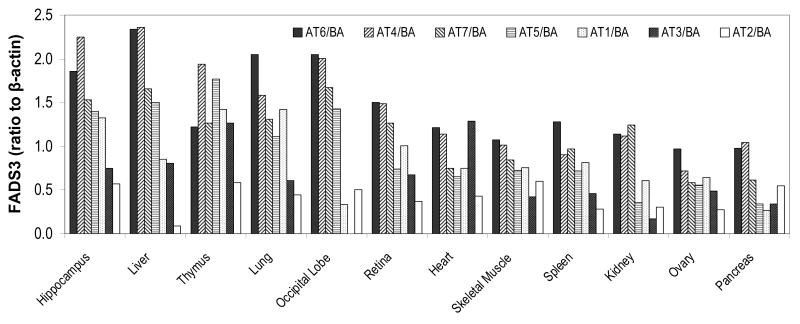
The FADS3 AT were cloned into yeast (Saccharomyces cerevisiae) and mammalian cells, but showed no activity toward a variety of PUFA substrates (our unpublished data, 2009). Further studies were carried out to detect FADS3 AT expression in baboon tissue and cultured human neuroblastoma cells using AT-specific PCR primers bridging deleted parts of exons. Remarkably, all seven AT, were detected in all baboon tissues and in human cells [20]. Figure 3 shows expression relative to β-actin for 12 tissues. Summed expression of all AT was highest in hippocampus and more than double that in pancreas; summed expression in all tissues was highest for AT6, and more than triple that of AT2. Correlation analysis of these data was performed using JMP 7.0 (SAS Institute, Cary, NC, USA) for the 21 possible pairs of 7 different AT. Results are shown in Table 2 for the significant correlations (p<0.05). Six of the seven correlations are among FADS3 AT4, 5, 6, and 7. These findings imply coordinated expression of these AT in tissues, and also imply that expression of the other AT are independent. FADS4,5,6,and 7 putatively code for truncated proteins that, individually, are all missing one or two of the four conserved motifs, and all are missing the QIEHH at the 3′ end. It is tempting to speculate that simultaneous translation of all these ATs would code for proteins that could bind to form one or more functional desaturases, possibly to enable activity toward multiple substrates or for purposes of regulation.
Figure 3.
Expression of the seven FADS3 AT in 12 tissues of a 12 week old baboon. Data are arranged left to right in order of total expression relative to β-actin. Gels were scanned and quantified manually using ImageJ <http://rsbweb.nih.gov/ij/>.
Table 2.
Significant correlations between AT in various tissues.1
| r2 | P | |
|---|---|---|
| AT7 – AT4 | 0.79 | 0.0001 |
| AT5 – AT4 | 0.77 | 0.0002 |
| AT7 – AT6 | 0.74 | 0.0003 |
| AT4 – AT6 | 0.68 | 0.0010 |
| AT5 – AT7 | 0.55 | 0.0055 |
| AT5 – AT6 | 0.46 | 0.0150 |
| AT3 – AT1 | 0.37 | 0.0352 |
Calculated with JMP 7.0 <http://www.jmp.com/>, “Multivariate Methods >Multivariate” menu and activation of “pairwise correlations” to obtain p values.
FADS2 AT
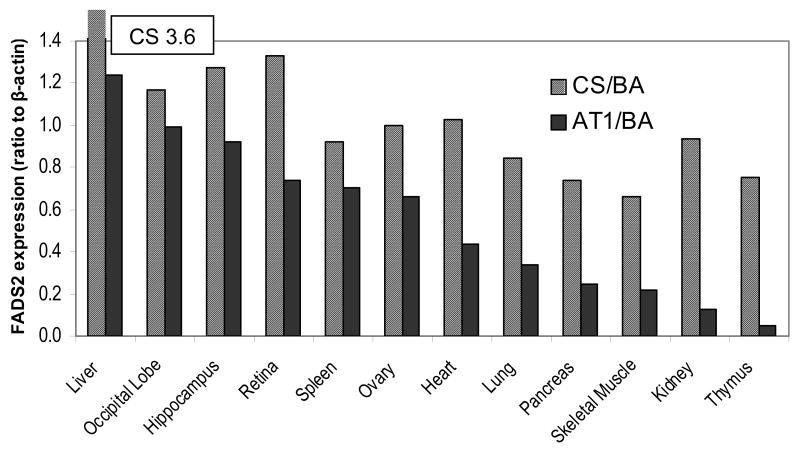
Figure 4 presents quantitative data on the expression of FADS2 CS and AT1 in the same twelve organs of baboon neonate as in Figure 3. Liver expression of FADS2 CS is 4-fold that of the average expression of the other organs. The range of expression in the other organs is 2-fold, from the highest in occipital lobe (cerebral cortex) to the lowest in skeletal muscle. In contrast, the range for FADS2 AT1 in these same tissues varies over a factor of 20, from highest again in occipital lobe to the lowest in thymus. The dramatic difference in expression suggests a difference in function and independent regulation.
Figure 4.
Expression of the FADS2 classically spliced (CS) transcript and AT1 in various tissues of a 12 week old baboon. The y-axis is plotted with liver FADS2 CS off-scale because it is four-fold the average of the others. The range of expression for FADS2 AT1 is much greater among the 11 tissues (other than liver) than for the CS.
Preliminary observations on species conservation of FADS AT
The conservation of alternatively spliced transcript variants of FADS AT was examined in several animal species, as shown in Figure 5. Using the baboon-specific primers [21] for each of the FADS AT, we performed RT-PCR analysis using liver cDNA from mouse, pig, dog, fox, chicken and horse. At least five of the seven FADS3 AT and FADS2 AT1 are reproducible in many species. These PCR products have not yet been confirmed by sequencing, but are consistent with the hypothesis that FADS AT perform some functionally important role, encoding proteins or performing a regulatory role.
Figure 5.
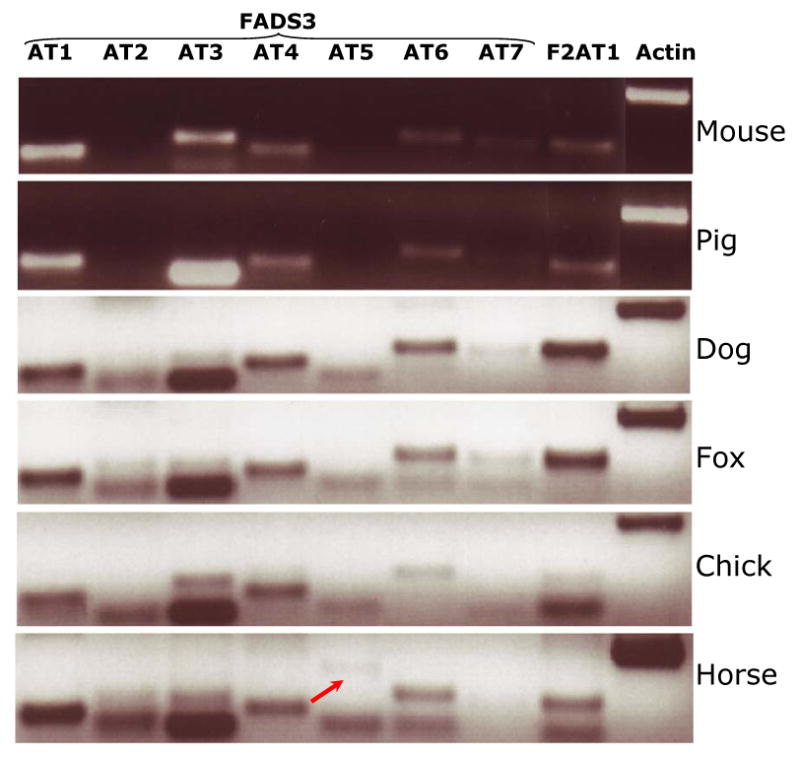
Expression of FADS3 AT and FADS2 AT1 in five mammalian species and in the chick, analyzed using baboon-specific primers. All but FADS3 AT2 and FADS3 AT5 are expressed in most of the species. FADS3 AT5 is detected in horse.
Some investigators of desaturase function have suggested the existence of at least two different 6-desaturases as a possible explanation of the findings of functional studies [26, 27]. To our knowledge, isozymes of a Δ6-desaturase have been described only in the arachidonic acid producing fungus Mortierella sp. and not for other organisms [4, 28]. In previous work, we and others have also suggested that specific LCPUFA synthases would explain results that are not easily accounted for by the accepted LCPUFA synthesis pathways [29, 30]. Although a function has yet to emerge for FADS3 and for the various AT that we have described, they represent a putative molecular basis on which alternative regulation and LCPUFA biosynthetic pathways could be based.
Hypothetically, FADS AT as mediators of LCPUFA biosynthesis or regulation could have implications for establishing variability in individual and population LCPUFA dietary requirements. Among the few studies that have reported on stable isotope tracers in humans, at least one in preterm infants showed wide variability to form 22:6n-3 and 20:4n-6 [31]. Differential expression of AT among individuals in response to diets could imply differential LCPUFA requirements. For instance, some populations subsisting on diets that provide only 18:2n-6 and 18:3n-3 as PUFA may require supplementary LCPUFA because of low expression of putative AT-related LCPUFA synthetic proteins, while others might synthesize sufficient LCPUFA from precursors. Group differences of this type have been suggested [32] but no compelling data has been presented. The novel findings of FADS AT suggest that investigations to elucidate molecular mechanisms and nutritional factors regulating their expression are required to elucidate factors mediating human PUFA desaturation.
Acknowledgments
This work was supported by a seed grant from the Cornell Vertebrate Genomics Program. The authors are grateful to Françoise Vermeylen for assistance with statistics and Holly Reardon for helpful discussions.
Abbreviations
- LCPUFA
long chain polyunsaturated fatty acids
- EPA
eicosapentaenoic acid
- ARA
arachidonic acid
- DHA
docosahexaenoic acid
- FAME
fatty acid methyl ester
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ves-Losada A, Brenner RR. Fatty acid delta 5 desaturation in rat liver cell nuclei. Mol Cell Biochem. 1995;142:163–170. doi: 10.1007/BF00928937. [DOI] [PubMed] [Google Scholar]
- 2.Sprecher H, Luthria DL, Mohammed BS, et al. Reevaluation of the pathways for the biosynthesis of polyunsaturated fatty acids. J Lipid Res. 1995;36:2471–2477. [PubMed] [Google Scholar]
- 3.Jakobsson A, Westerberg R, Jacobsson A. Fatty acid elongases in mammals: Their regulation and roles in metabolism. Prog Lipid Res. 2006;45:237–249. doi: 10.1016/j.plipres.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 4.Nakamura MT, Nara TY. Structure, function, and dietary regulation of delta6, delta5, and delta9 desaturases. Ann Rev Nutr. 2004;24:345–376. doi: 10.1146/annurev.nutr.24.121803.063211. [DOI] [PubMed] [Google Scholar]
- 5.Cho HP, Nakamura M, Clarke SD. Cloning, expression, and fatty acid regulation of the human delta-5 desaturase. J Biol Chem. 1999;274:37335–37339. doi: 10.1074/jbc.274.52.37335. [DOI] [PubMed] [Google Scholar]
- 6.Cho HP, Nakamura MT, Clarke SD. Cloning, expression, and nutritional regulation of the mammalian delta-6 desaturase. J Biol Chem. 1999;274:471–477. doi: 10.1074/jbc.274.1.471. [DOI] [PubMed] [Google Scholar]
- 7.Su HM, Brenna JT. Simultaneous measurement of desaturase activities using stable isotope tracers or a nontracer method. Anal Biochem. 1998;261:43–50. doi: 10.1006/abio.1998.2706. [DOI] [PubMed] [Google Scholar]
- 8.Marquardt A, Stohr H, White K, et al. Cdna cloning, genomic structure, and chromosomal localization of three members of the human fatty acid desaturase family. Genomics. 2000;66:175–183. doi: 10.1006/geno.2000.6196. [DOI] [PubMed] [Google Scholar]
- 9.Schaeffer L, Gohlke H, Muller M, et al. Common genetic variants of the fads1 fads2 gene cluster and their reconstructed haplotypes are associated with the fatty acid composition in phospholipids. Hum Mol Genet. 2006;15:1745–1756. doi: 10.1093/hmg/ddl117. [DOI] [PubMed] [Google Scholar]
- 10.Rzehak P, Heinrich J, Klopp N, et al. Evidence for an association between genetic variants of the fatty acid desaturase 1 fatty acid desaturase 2 (fads1 fads2) gene cluster and the fatty acid composition of erythrocyte membranes. Br J Nutr. 2008:1–7. doi: 10.1017/S0007114508992564. [DOI] [PubMed] [Google Scholar]
- 11.Gieger C, Geistlinger L, Altmaier E, et al. Genetics meets metabolomics: A genome-wide association study of metabolite profiles in human serum. PLoS Genet. 2008;4:e1000282. doi: 10.1371/journal.pgen.1000282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Malerba G, Schaeffer L, Xumerle L, et al. Snps of the fads gene cluster are associated with polyunsaturated fatty acids in a cohort of patients with cardiovascular disease. Lipids. 2008;43:289–299. doi: 10.1007/s11745-008-3158-5. [DOI] [PubMed] [Google Scholar]
- 13.Xie L, Innis SM. Genetic variants of the fads1 fads2 gene cluster are associated with altered (n-6) and (n-3) essential fatty acids in plasma and erythrocyte phospholipids in women during pregnancy and in breast milk during lactation. J Nutr. 2008;138:2222–2228. doi: 10.3945/jn.108.096156. [DOI] [PubMed] [Google Scholar]
- 14.Brookes KJ, Chen W, Xu X, et al. Association of fatty acid desaturase genes with attention-deficit/hyperactivity disorder. Biol Psychiatry. 2006;60:1053–1061. doi: 10.1016/j.biopsych.2006.04.025. [DOI] [PubMed] [Google Scholar]
- 15.Gilbert W. Why genes in pieces? Nature. 1978;271:501. doi: 10.1038/271501a0. [DOI] [PubMed] [Google Scholar]
- 16.Hattori D, Demir E, Kim HW, et al. Dscam diversity is essential for neuronal wiring and self-recognition. Nature. 2007;449:223–227. doi: 10.1038/nature06099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matthews BJ, Kim ME, Flanagan JJ, et al. Dendrite self-avoidance is controlled by dscam. Cell. 2007;129:593–604. doi: 10.1016/j.cell.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 18.Singaraja RR, James ER, Crim J, et al. Alternate transcripts expressed in response to diet reflect tissue-specific regulation of abca1. J Lipid Res. 2005;46:2061–2071. doi: 10.1194/jlr.M500133-JLR200. [DOI] [PubMed] [Google Scholar]
- 19.Park WJ, Kothapalli KS, Lawrence P, et al. An alternate pathway to long-chain polyunsaturates: The fads2 gene product delta8-desaturates 20:2n-6 and 20:3n-3. J Lipid Res. 2009;50:1195–1202. doi: 10.1194/jlr.M800630-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park WJ, Kothapalli KS, Reardon HT, et al. Novel fatty acid desaturase 3 (fads3) transcripts generated by alternative splicing. Gene. 2009 doi: 10.1016/j.gene.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park WJ, Reardon HT, Tyburczy C, et al. Alternative splicing generates a novel fads2 alternative transcript in baboons. Mol Biol Rep. 2009 doi: 10.1007/s11033-009-9750-9. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ge L, Gordon JS, Hsuan C, et al. Identification of the delta-6 desaturase of human sebaceous glands: Expression and enzyme activity. J Invest Dermatol. 2003;120:707–714. doi: 10.1046/j.1523-1747.2003.12123.x. [DOI] [PubMed] [Google Scholar]
- 23.Hastings N, Agaba MK, Tocher DR, et al. Molecular cloning and functional characterization of fatty acyl desaturase and elongase cdnas involved in the production of eicosapentaenoic and docosahexaenoic acids from alpha-linolenic acid in atlantic salmon (salmo salar) Mar Biotechnol (NY) 2004;6:463–474. doi: 10.1007/s10126-004-3002-8. [DOI] [PubMed] [Google Scholar]
- 24.Spychalla JP, Kinney AJ, Browse J. Identification of an animal omega-3 fatty acid desaturase by heterologous expression in arabidopsis. Proc Natl Acad Sci U S A. 1997;94:1142–1147. doi: 10.1073/pnas.94.4.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sayanova O, Haslam R, Venegas Caleron M, et al. Cloning and characterization of unusual fatty acid desaturases from anemone leveillei: Identification of an acyl-coenzyme a c20 delta5-desaturase responsible for the synthesis of sciadonic acid. Plant Physiol. 2007;144:455–467. doi: 10.1104/pp.107.098202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bourre JM, Piciotti M. Delta-6 desaturation of alpha-linolenic acid in brain and liver during development and aging in the mouse. Neurosci Lett. 1992;141:65–68. doi: 10.1016/0304-3940(92)90335-5. [DOI] [PubMed] [Google Scholar]
- 27.Marzo I, Alava MA, Pineiro A, et al. Biosynthesis of docosahexaenoic acid in human cells: Evidence that two different delta 6-desaturase activities may exist. Biochim Biophys Acta. 1996;1301:263–272. doi: 10.1016/0005-2760(96)00051-3. [DOI] [PubMed] [Google Scholar]
- 28.Sakuradani E, Shimizu S. Gene cloning and functional analysis of a second delta 6-fatty acid desaturase from an arachidonic acid-producing mortierella fungus. Biosci Biotechnol Biochem. 2003;67:704–711. doi: 10.1271/bbb.67.704. [DOI] [PubMed] [Google Scholar]
- 29.Infante JP, Huszagh VA. Analysis of the putative role of 24-carbon polyunsaturated fatty acids in the biosynthesis of docosapentaenoic (22:5n-6) and docosahexaenoic (22:6n-3) acids. FEBS Lett. 1998;431:1–6. doi: 10.1016/s0014-5793(98)00720-0. [DOI] [PubMed] [Google Scholar]
- 30.Infante JP, Tschanz CL, Shaw N, et al. Straight-chain acyl-coa oxidase knockout mouse accumulates extremely long chain fatty acids from alpha-linolenic acid: Evidence for runaway carousel-type enzyme kinetics in peroxisomal beta-oxidation diseases. Mol Genet Metab. 2002;75:108–119. doi: 10.1006/mgme.2001.3279. [DOI] [PubMed] [Google Scholar]
- 31.Uauy R, Mena P, Wegher B, et al. Long chain polyunsaturated fatty acid formation in neonates: Effect of gestational age and intrauterine growth. Pediatr Res. 2000;47:127–135. doi: 10.1203/00006450-200001000-00022. [DOI] [PubMed] [Google Scholar]
- 32.Gibson RA, Sinclair AJ. Are eskimos obligate carnivores? Lancet. 1981;1:1100. doi: 10.1016/s0140-6736(81)92263-7. [DOI] [PubMed] [Google Scholar]