Abstract
Voltage-gated potassium channels are often assembled with accessory proteins which increases their functional diversity. KCNE proteins are small accessory proteins that modulate voltage-gated potassium (KV) channels. Although the functional effects of various KCNE proteins have been described, many questions remain regarding their assembly with the pore-forming subunits. For example, while previous experiments with some KV channels suggest that the association of the pore-subunit with the accessory subunits occurs co-translationally in the endoplasmic reticulum, it is not known whether KCNQ1 assembly with KCNE1 occurs in a similar manner to generate the medically important cardiac slow delayed rectifier current (IKs). In this study we used a novel approach to demonstrate that purified recombinant human KCNE1 protein (prKCNE1) modulates KCNQ1 channels heterologously expressed in Xenopus oocytes resulting in generation of IKs. Incubation of KCNQ1-expressing oocytes with cycloheximide did not prevent IKs expression following prKCNE1 injection. By contrast, incubation with brefeldin A prevented KCNQ1 modulation by prKCNE1. Moreover, injection of the trafficking-deficient KCNE1-L51H reduced KCNQ1 currents. Together, these observations indicate that while assembly of KCNE1 with KCNQ1 does not require co-translation, functional KCNQ1-prKCNE1 channels assemble early in the secretory pathway and reach the plasma membrane via vesicular trafficking.
Keywords: potassium channel, accessory subunit, channel assembly, protein purification, trafficking
Introduction
Voltage-gated potassium (KV) channels are often assembled as heteromultimeric complexes consisting of pore-forming (α) subunits and accessory proteins. Members of the KCNE family are small accessory proteins with a single-transmembrane domain that function to control or modulate KV channels in tissues such as the heart, cochlea, and small intestine.1,2 Heterologous expression experiments have demonstrated that KCNE proteins alter the properties of several KV channels,1–3 and that all KCNE proteins (KCNE1-KCNE5) interact with KCNQ1 (KV7.1), each yielding a distinct phenotype.4,5 Thus, the observed functional properties of KCNQ1, and other KV channels, vary depending on the associated KCNE subunit. KCNE1 affects several biophysical properties of KCNQ1 channels (i.e., increased whole-cell current density, slower activation, and activation at depolarized potentials) to generate the slow delayed rectifier current, IKs.6,7
Although many studies describe the effects of KCNEs on KV channel function, many questions remain regarding the structural biology of KCNE proteins and their assembly with α subunits. The structural determinants of KCNE1 modulation of KCNQ1 have been inferred by mutagenesis, chimera and chemical modification experiments,8–20 and recently the structure of full-length human KCNE1 has been obtained by solution NMR analysis.21 Nevertheless, questions remain regarding the timing and subcellular location for the assembly of KCNE proteins with the pore-forming subunits. Some studies suggest that KCNE1 and KCNQ1 can associate at the plasma membrane,22,23 but others propose that assembly occurs early in the secretory pathway.24,25 Previous experiments with other KV channels suggest that the association of α-subunit with the accessory subunits KVβ and KChAP occurs co-translationally.26, 27 Both KChAP27 and KVβ28 are soluble cytosolic proteins. However, KCNEs are integral membrane proteins29 and their assembly with KV channels may differ from that of cytoplasmic accessory proteins.
In this study we investigated KCNE1 and KCNQ1 assembly using a novel approach in which Xenopus oocytes expressing human KCNQ1 were injected with purified recombinant KCNE1 protein (prKCNE1). The injected protein modulated KCNQ1 function rapidly after protein injection (t½ ~3 h) as determined by the appearance of IKs at the plasma membrane. Modulation of KCNQ1 by injected prKCNE1 was not prevented by inhibiting new KCNQ1 synthesis with cycloheximide. However, modulation of KCNQ1 channels by prKCNE1 was inhibited by the secretory pathway blocker brefeldin A. Together these observations indicated that assembly of KCNE1 with KCNQ1 can occur independent of protein translation, and that functional KCNQ1-prKCNE1 channels assemble early in the secretory pathway then reach the plasma membrane through vesicular trafficking.
Results and Discussion
Time-course of prKCNE1 modulation of KCNQ1 channels
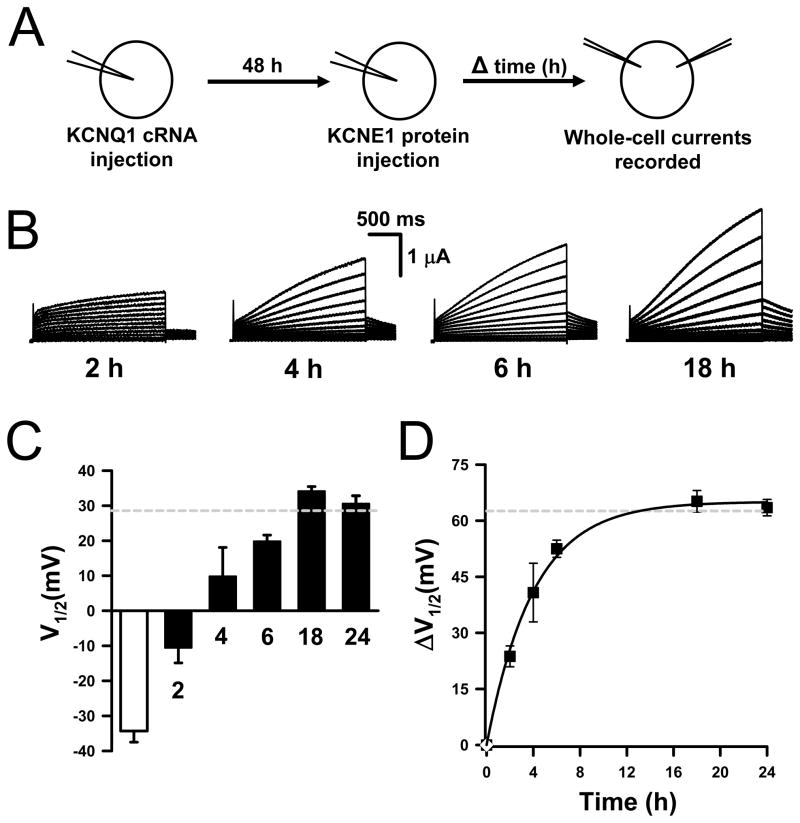
Our previous results demonstrated that purified recombinant human KCNE1 protein (prKCNE1) injected into Xenopus oocytes associates with KCNQ1 channels translated in vivo to generate IKs.30 In this study we have determined the time-course for the functional interaction of prKCNE1 with KCNQ1 following protein injection by monitoring the appearance of IKs currents. Figure 1B illustrates current traces recorded from KCNQ1-expressing oocytes at 2, 4, 6 or 18h following prKCNE1 injection. Addition of prKCNE1 slowed channel activation and increased the current magnitude in a time-dependent manner. We were unable to measure current prior to 2h post protein-injection because the oocytes had excessive leak. Figure 1C depicts the apparent half-maximal activation voltage (V½) determined for the whole-cell currents at each time point (solid bars). At the early time points (e.g., 2–4 h post prKCNE1 injection) isochronal activation curves from individual cells have variable shifts in V½ (note larger standard error for these time points in Fig. 1C) while whole-cell current recordings exhibit complex gating suggesting that these currents are carried by two channel populations: KCNQ1-alone and KCNQ1-KCNE1 channels (Fig. S1). The time-course for prKCNE1 functional interaction with KCNQ1 was determined by plotting the change in V½ ( V½) as compared with KCNQ1-only channels (Fig. 1D). The data were fit with a monoexponential function yielding an apparent t½ of ~3h (Fig. 1D). These results demonstrated that prKCNE1 solubilized in detergent micelles readily associates with endogenously processed KCNQ1 channels, and that most of the KCNQ1 channels at the plasma membrane appear fully modulated by prKCNE1 10–12h after protein injection. Moreover, these data also indicated that KCNQ1-KCNE1 assembly does not require the simultaneous translation of both proteins.
Figure 1.
Time-course for prKCNE1 modulation of KCNQ1 channels.
(A) Schematic showing injection/incubation protocol. (B) Representative current traces recorded from KCNQ1-expressing oocytes at 2, 4, 6 and 18h following prKCNE1 injection. (C) V½ values for whole-cell currents recorded from oocytes injected with KCNQ1 cRNA (open bar) and KCNQ1 cRNA and prKCNE1 (solid bars). The dashed gray line denotes the V½ obtained for KCNQ1-KCNE1 channels translated by the oocytes. (D) Time-course for prKCNE1 functional interaction with KCNQ1. In this panel, the dashed line indicates ΔV½ between KCNQ1-only and KCNQ1-KCNE1 channels translated by the oocytes. N: KCNQ1 cRNA = 9; KCNQ1 + KCNE1 cRNA = 13; KCNQ1 + prKCNE1, 2h = 3; 4h = 5; 6h = 12; 18h = 11; 24h = 8.
IKs channel assembly does not require new KCNQ1 synthesis
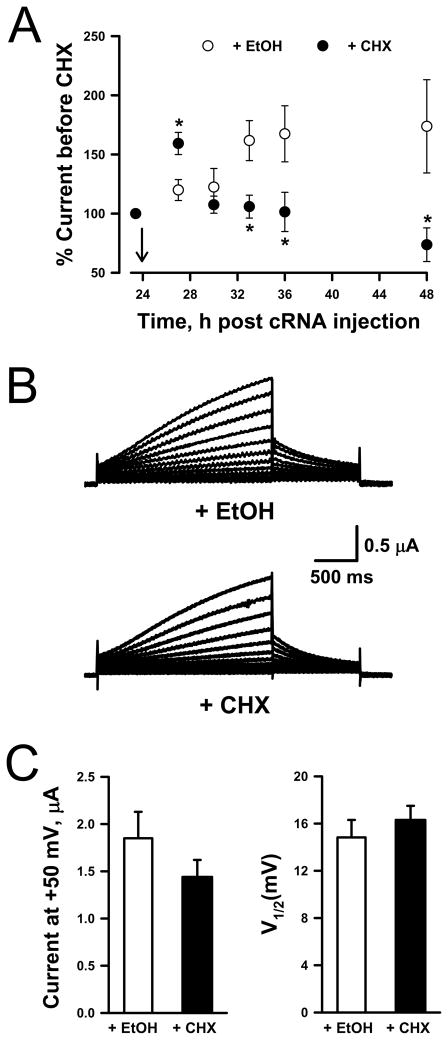
Previous experiments with other KV channels indicate that the association of α-subunit with the accessory subunits KVβ and KChAP occurs co-translationally while the nascent α-subunit is still attached to the ribosome complex.26,27 In order to test whether prKCNE1 associates with KCNQ1 during α-subunit translation, we inhibited protein synthesis with cycloheximide [CHX (50 μg/ml), Supplemental Results (Fig. S2) show that CHX prevented functional expression of KCNQ1 channels]. Block of KCNQ1 synthesis was inferred by the decrease in functional KCNQ1 expression following exposure to CHX. In these experiments, we delayed the application of CHX for 48h after KCNQ1 cRNA injection in order to obtain a measurable number of functional KCNQ1 channels at the plasma membrane. Current decrease is assumed to be caused by the removal of KCNQ1 channels from the plasma membrane by constitutive endocytosis coupled with disruption of channel synthesis. As illustrated in Fig. 2A, KCNQ1-expressing oocytes exposed to ethanol (EtOH, vehicle) exhibited steadily increasing current levels throughout the experiment phase (KCNQ1 expression has not peaked after 48h). In contrast, current amplitude recorded from KCNQ1-expressing oocytes treated with CHX initially rose but then progressively declined after ~6h incubation (Fig. 2A).
Figure 2.
Inhibition of protein synthesis does not prevent prKCNE1 assembly with KCNQ1.
(A) Whole-cell currents recorded at +50mV from oocytes injected with KCNQ1 and KCNE1 cRNAs expressed as a percentage of the current recorded prior to CHX (50 μg/ml) or EtOH treatment (N ≥ 5 for all time points). Arrow indicates when CHX or EtOH was added. (B) Representative whole-cell currents recorded from KCNQ1-expressing oocytes exposed to either EtOH (top traces) or CHX (bottom traces) for 6h, and then injected with prKCNE1 and further incubated in the presence of CHX or EtOH for an additional 8h. (C) Average currents recorded at +50mV (left panel) and average V½ values (right panel) obtained from the EtOH-treated (open bar, N = 9) or CHX-treated (solid bar, N = 11) oocytes illustrated in Fig. 2B.
We then tested the effect of blocking KCNQ1 synthesis on co-assembly with prKCNE1 by first treating KCNQ1-expressing oocytes (48h post cRNA injection) with either CHX or EtOH for 6h, sufficient time to observe inhibition of KCNQ1 synthesis (Fig. 2A). Both treatment groups were then injected with prKCNE1 and further incubated with CHX or EtOH for 8h. This period allows for consistently observing KCNQ1 modulation following prKCNE1 injection (Fig. 1). Figure 2B illustrates representative whole-cell currents recorded from KCNQ1-expressing oocytes treated with CHX or EtOH and injected with prKCNE1. Whole-cell currents measured at +50mV and V½ values were similar for both treatments [Fig. 2C, CHX (solid bars), EtOH (empty bars)]. Longer incubations with CHX or EtOH yielded similar results (24h post protein injection, data not shown). These results show that incubation of KCNQ1-expressing oocytes with the protein-synthesis inhibitor CHX did not prevent expression of IKs following prKCNE1 injection. This observation strongly suggests that correct assembly of functional KCNQ1-KCNE1 channel complexes does not require co-translation of the two proteins in this system. The post-translational assembly of KCNE1 with KCNQ1 suggests that these proteins could associate outside the ER.
This is a distinct mechanism than the co-translational assembly of the voltage-gated K+ channel KV1.2 with the accessory subunits KVβ and KChAP.26,27 This difference may be due to the accessory protein. Both KChAP27 and KVβ28 are soluble cytosolic proteins, while KCNE1 is a trans-membrane protein.29 Interestingly, the assembly of the α-subunit of the voltage-gated rat brain Na+ channel with its accessory subunit β2, another transmembrane accessory protein,31 also occurs post-translationally.32 Whether co-translation-independent assembly of KCNQ1 with KCNE1 is subunit specific or a property shared by all KCNE proteins is unresolved.
KCNQ1 modulation by prKCNE1 requires vesicular trafficking
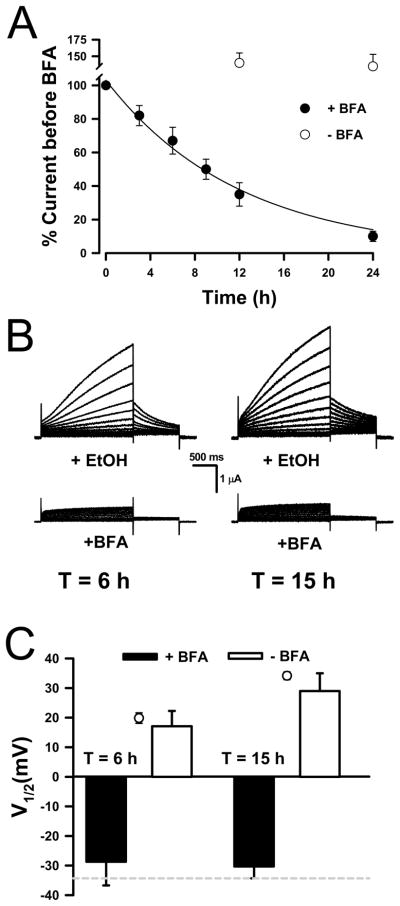
Previous studies demonstrated that proteins injected into Xenopus oocytes reach many compartments including the plasma membrane.33–35 We envisioned two possible pathways for prKCNE1 to reach the plasma membrane: simple diffusion from the cytoplasm to the plasma membrane, or vesicular trafficking following the insertion of prKCNE1 into the endoplasmic reticulum (ER) or Golgi membranes. Brefeldin A (BFA) is a fungal metabolite that blocks anterograde trafficking of secretory vesicles from the ER and causes the movement of Golgi membranes into the ER.36 BFA was demonstrated to inhibit the insertion of newly synthesized channel proteins [ENaC37 and ROMK38] into the plasma membrane of Xenopus oocytes. BFA (5μM) also prevents KCNQ1/KCNE1 complexes from reaching the plasma membrane (Supplemental Results, Fig. S3). Figure 3A shows that BFA exposure decreased whole-cell currents recorded from oocytes injected with KCNQ1 and KCNE1 cRNAs. Current decrease occurred as early as ~3h after BFA exposure, and did not occur with EtOH (vehicle) treatment alone. These results also indicated that KCNQ1-KCNE1 channels were removed from the plasma membrane with a t½ ~9h. (Fig. 3A, solid line). Interestingly, a turnover rate of ~11h was reported for KCNQ1-KCNE1 channels in COS-7 cells.39
Figure 3.
Modulation of KCNQ1 by prKCNE1 requires vesicular trafficking.
(A) Whole-cell currents recorded at +50mV following BFA (5 μM) or EtOH treatment from oocytes injected with KCNQ1 and KCNE1 cRNAs expressed as percentage of the current recorded prior to treatment (N ≥ 5 for all time points. (B) Representative whole-cell currents recorded from KCNQ1-expressing oocytes exposed to either EtOH (top traces) or BFA (bottom traces) for 3h, then injected with prKCNE1 and further incubated for 6 or 15h. (C) V½ calculated from the normalized current data recorded after 6 and 15h incubations. The dashed line in the bottom panel is the V½ calculated for KCNQ1-only channels and the open symbols are the V½ values for currents recorded from KCNQ1-expressing oocytes 6 and 15h after prKCNE1 injection under control conditions. N 6 for both conditions at each time point.
To test whether prKCNE1 reaches the plasma membrane by vesicular trafficking through the secretory pathway, KCNQ1-expressing oocytes (48h post cRNA injection) were first exposed to BFA or EtOH (vehicle) for 3h, sufficient time to detect the block of vesicle delivery to the plasma membrane (Fig. 3A). The BFA- or EtOH-treated oocytes were then injected with prKCNE1 and further incubated in either BFA or EtOH for 6 or 15h. These time intervals were selected because KCNQ1 modulation by prKCNE1 was evident at 6h post protein injection (Fig. 1) and 15h should be sufficient time for prKCNE1 to diffuse to the plasma membrane (typical oocyte radius = 0.5mm). Figure 3B depicts representative whole-cell currents recorded from KCNQ1-expressing oocytes exposed to either EtOH (top traces) or BFA (bottom traces) and injected with prKCNE1. Figure 3C indicates that V½ values for currents recorded at 6 and 15h time points were similar to the values obtained under control conditions at 6 and 18h post prKCNE1 injection (○, from Fig. 1C). By contrast, the V½ values obtained after prKCNE1 injection into KCNQ1-expressing oocytes incubated in BFA were not different from oocytes injected only with KCNQ1 cRNA (dotted line). Longer incubations gave identical results (24h, N = 3, data not shown). In some oocytes, BFA was removed after the 24h incubations and currents recorded ~18h later, but only non-modulated KCNQ1 currents were detected in the prKCNE1-injected oocytes. In summary, exposure to BFA prevented the appearance of IKs following prKCNE1 injection.
Although the results indicate that vesicular trafficking was required for prKCNE1 and KCNQ1 to form functional IKs channels at the plasma membrane, these results do not determine whether prKCNE1 associates with KCNQ1 in an intracellular compartment (i.e., ER or Golgi) and the assembled channel complex reaches the plasma membrane by vesicular trafficking, or whether prKCNE1 is transported by vesicular trafficking to the plasma membrane where it associates with KCNQ1 channels that are already present. KCNE1 and KCNQ1 assembly has been proposed to occur at either the plasma membrane22,23,39 or the ER.24,25 Previous studies demonstrated that proteins injected into Xenopus oocytes reach many compartments including the plasma membrane.33–35 If the injected KCNE1 protein could form functional IKs channels by associating with KCNQ1 in a post-ER/Golgi compartment (i.e., plasma membrane), we would observed IKs-like currents following injection of prKCNE1 protein into KCNQ1-expressing oocytes treated with BFA. However, only KCNQ1 currents were recorded following BFA treatment suggesting that functional assembly of prKCNE1 with KCNQ1 at the plasma membrane does not occur.
BFA block of KCNQ1 modulation by prKCNE1 demonstrates that vesicular traffic, not direct fusion to the plasma membrane, is required for the appearance of functional prKCNE1-KCNQ1 channels. This observation suggests that functional assembly of prKCNE1 with KCNQ1 occurs early in the secretory pathway (i.e., ER or Golgi). This is a similar pathway to that reported for the Shaker K+ channel and accessory subunits. Experiments by Nagaya and Papazian demonstrated that Shaker K+ channel α subunits assemble with KVβ accessory subunits in the ER, and together are transported to the Golgi apparatus in route to the plasma membrane.40
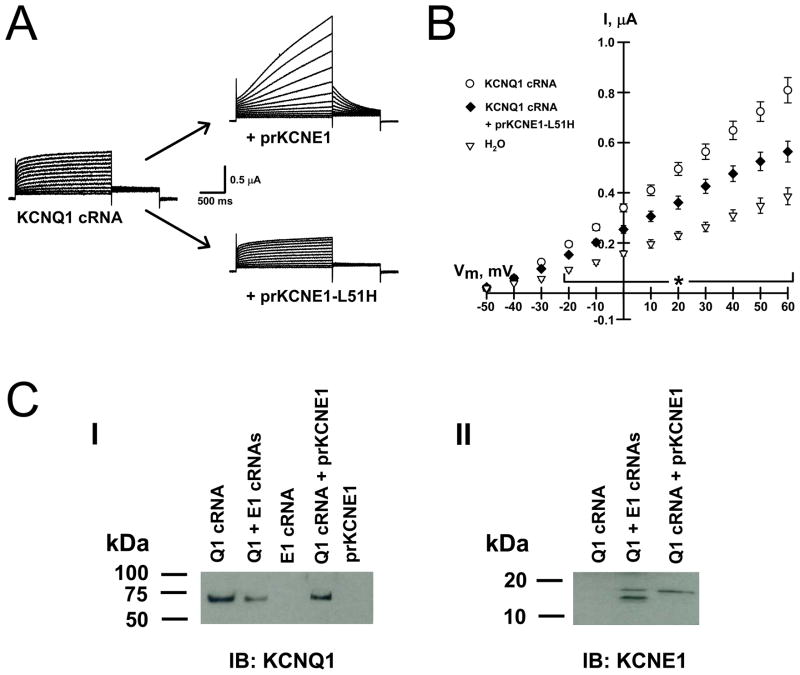
In order to further localize the site of prKCNE1-KCNQ1 assembly we injected KCNQ1-expressing oocytes with the non-dominant, trafficking-deficient KCNE1-L51H mutant protein.24 This mutant KCNE1 does not reach the plasma membrane, it associates with KCNQ1 in the ER and prevents the channel from reaching the plasma membrane, thus reducing KCNQ1 currents without altering the kinetics.24,41 Figure 4A illustrates whole-cell currents and current-voltage relationships obtained from KCNQ1-expressing oocytes before and ~18h after injecting either prKCNE1 (upper trace) or prKCNE1-L51H (lower trace). Injection of prKCNE1 into KCNQ1-expressing oocytes yielded IKs currents, while introduction of prKCNE1-L51H reduced current amplitude without altering the kinetics.
Figure 4.
Intracellular processing of prKCNE1.
(A) Average whole-cell currents from oocytes expressing KCNQ1 alone (N = 8) and those injected with prKCNE1 (N = 7) or prKCNE1-L51H (N = 10). (B) Average current-voltage relationships from oocytes injected with water (▽, N = 8), KCNQ1 cRNA (○, N = 8) or KCNQ1 cRNA + prKCNE1-L51H (◆, N = 10). Injection of KCNE1-L51H reduced KCNQ1 currents by ~40% when compared to LMPG injection. * = P < 0.02 between the 3 treatments, one-way ANOVA. (C) Panel I, membrane preparations probed with anti-KCNQ1. Panel II, membrane preparations probed with anti-KCNE1.
The results with the trafficking-deficient KCNE1-L51H suggest that KCNQ1-prKCNE1 assembly may occur in the ER. Experiments carried out with KCNQ1 and KCNE1 in HEK-293,24 CHO and COS-7 cells25 support the notion that KCNE1-KCNQ1 assembly occurs early in the secretory pathway, most likely in the ER. KCNE1 has a tetrapeptide motif (KKLE) positioned in a cytosolic juxtamembrane domain that resembles known ER retention signals for type I membrane proteins.42 This raises the intriguing possibility that KCNE1/KCNQ1 co-assembly masks this retention signal and allows the assembled complex to exit from the ER.
We next tested whether prKCNE1 is glycosylated as it progresses through the secretory pathway. Figure 4C illustrates that KCNQ1 protein was only detected after KCNQ1 cRNA injection (panel I) and that KCNE1 was only detected in oocytes injected with either KCNE1 cRNA or prKCNE1 (panel II). After immunodetection using a KCNE1 antibody, 2 bands are observed when KCNE1 was expressed from cRNA but only one band was detected in membranes from oocytes injected with prKCNE1 (panel II, solid arrows). The observed band for prKCNE1 has a slightly higher apparent mass than KCNE1 translated from cRNA because of the His6-containing purification tag (2 glycines and 6 histidines) in prKCNE1 that adds ~1kDa. This insertion does not affect KCNQ1-KCNE functional interaction.30 Treatment with the amidase PNGase-F reduced the heavier KCNE1 band but had no effect on prKCNE1 suggesting that the recombinant protein does not become glycosylated (data not shown). Interestingly, prKCNE1 does not appear to be glycosylated, as treatment with PNGase-F did not reduce the apparent molecular size. This would not be surprising, as N-glycosylation is believed to be a co-translational process.43 An alternative explanation is that only prKCNE1 that enters the early secretory pathway and associates with KCNQ1 is properly glycosylated,25 and this protein represents a very small fraction of the total amount injected. This would be reasonable as our calculations indicate that ~0.025% of the injected prKCNE1 forms functional complexes with KCNQ1 channels at the plasma membrane. These calculations are based on the whole-cell current amplitude measured after prKCNE1 injection (2μA at +50mV), the single-channel conductance for IKs channels (8pS), the estimated open probability (0.5), a subunit stoichiometry of two 2 prKCNE1 molecules per IKs channel, and ~8ng prKCNE1 injected per oocyte.
Conclusion
In summary, our results strongly suggest that purified recombinant KCNE1 protein associates with KCNQ1 channels early in the secretory pathway, possibly in the ER, and that the complex is then transported to the plasma membrane by vesicular trafficking. Our results do not exclude that prKCNE1 reaches other compartments including the plasma membrane, or that prKCNE1 and KCNQ1 may associate at the plasma membrane. However, our results indicate that these proteins do not assemble into functional IKs channels at this location. Our results also demonstrated that KCNE1 and KCNQ1 do not require co-translation for proper assembly. This is a different mechanism than that observed for assembly of KV channels with cytoplasmic accessory subunits. The observation that these channel proteins can assemble post-translationally raises interesting questions about how specific KV-KCNE complexes are formed in tissues known to express more than one type of KCNE and KV channel protein.2,44–46 The use of recombinant KCNE proteins and KV channels expressed in Xenopus oocytes may assist in answering these important questions.
Methods
Functional analysis
Complementary DNAs encoding KCNQ1 and KCNE1 were constructed in plasmid vectors pSP64T and pRc/CMV, respectively, as previously described.12 Briefly, Xenopus laevis oocytes (stage V–VI) were microinjected with either sterile water (control) or cRNA that was transcribed in vitro from EcoRI (pSP64T-KCNQ1) or XbaI (pRc/CMV-KCNE1) digested linear DNA templates using Sp6 or T7 RNA polymerase and the mMessage mMachine transcription system (Ambion Inc, Austin, USA). Injected oocytes were incubated at 18°C for 48–96 h in Leibovitz’s media (Invitrogen, Carlsbad, USA) diluted 1:1 with water and supplemented with penicillin (150 units/ml) and streptomycin (150 μg/ml).
Whole-cell currents were recorded at room temperature (RT) 1–4d after injections with an OC-725B amplifier (Warner Instruments Corp., Hamden, USA). Oocytes were bathed at RT (22–25°C) in a modified ND96 solution (in mM: 96 NaCl, 4 KCl, 2 MgCl2, 0.1 CaCl2, 5 HEPES, pH 7.6, ~200 mosmol/kg). Cycloheximide (CHX) and brefeldin A (BFA) stocks were dissolved in ethanol (EtOH). Currents were recorded using Clampex 7 (Molecular Devices Corp., Sunnyvale, USA), filtered at 500Hz and digitized at 2kHz. Data were analyzed and plotted using Clampex, SigmaPlot 2000 (SPSS Science, Chicago, USA), SigmaStat (SPSS Science) and Origin 7.0 (OriginLab, Northampton, USA) softwares. Current-voltage and normalized isochronal voltage–activation relationships were obtained by measuring current at 2s during depolarizing pulses between −50 and +60 mV from a holding potential of −80mV. The normalized isochronal data were fit with a Boltzmann function of the form: 1/{1 + exp[(V − V1/2)/kv]}, where V1/2 is the apparent half-maximal activation voltage and kv is the slope factor. At early time points (e.g., 2, 4 hours) after prKCNE1 injection when there may be mixed channel populations, single Boltzmann fits serve to approximate an average V1/2. Oocytes with base-line currents larger than those measured for water-injected oocytes (typically −0.10 μA at −80mV) were considered leaky and not used.
Overexpression, purification and incorporation of prKCNE1 and prKCNE1-L51H into Xenopus oocytes
The expression, purification and reconstitution of recombinant human KCNE1 protein have been previously described.30 Briefly, the cDNA for recombinant human His6-tagged KCNE1 was expressed in bacteria, solubilized from inclusion bodies, purified on nickel-NTA agarose resin and eluted as detergent micelles in 0.2% 1-myristoyl-2-hydroxy-sn-glycero-3-[phosphor-rac-(1-glycerol)] (LMPG, Avanti Polar Lipids Inc., Alabaster, USA).
Oocytes were first injected with KCNQ1 cRNA (6 ng) and incubated for 24 or 48 h. Following incubation, KCNQ1-expressing oocytes were injected with 10 nl of protein-containing (0.8 mg/ml) or protein-free detergent micelles
Biochemical analysis
KCNQ1-expressing oocytes (48h post cRNA injections) were injected with prKCNE1 and harvested ~18h later. KCNQ1 and KCNE1 cRNA-injected oocytes were harvested 64–70 h post cRNA injections. Oocyte membranes were prepared using a previously published method47 and western blotting was performed as described previously.48,49 Immunoblots for KCNE1 proteins were performed overnight at 4°C with anti-KCNE1 (1:200; Alomone Labs, Jerusalem, Israel), and for KCNQ1 proteins for 1h at room temperature with a goat anti-KCNQ1 (1:200; Santa Cruz Biotechnology, Inc., Santa Cruz, USA). PNGase-F treatment was performed according to the supplier’s instructions (New England Biolabs, Ipswich, USA).
Chemicals were from SIGMA (St. Louis, USA) unless otherwise stated. All experimental conditions were tested in oocytes from at least three frogs. Data are represented as means ± SEM, and in some figures error bars are smaller than the symbols. The numbers of oocytes used in experiments (N) are provided in the figure legends. Statistical significance was determined using unpaired Student’s t-test or one-way ANOVA, (pairwise comparisons analyzed with the Tukey Test).
Supplementary Material
Figure S1. Isochronal activation curves 4 h after prKCNE1 injection.
(A) Whole-cell current recorded from a KCNQ1-expressing oocyte 4 h post prKCNE1 injection. The current exhibits rapid activation followed by a slower activation to larger current amplitude, suggesting that the current is carried by 2 channel populations: KCNQ1-alone and KCNQ1-KCNE1 channels. (B) Normalized isochronal activation curve obtained for the same oocyte. The lines indicate the average Boltzmann fits for currents recorded from oocytes injected with KCNQ1 cRNA (N= 9, solid line) and KCNQ1 + KCNE1 cRNAs (N = 13, gray dashed line). Together, the data indicate that during the early time points following prKCNE1 injection (2 and 4 h) two populations of KCNQ1 channels exist at the plasma membrane, modulated and non-modulated channels.
Figure S2. Cycloheximide (CHX, 50 μg/ml) inhibits KCNQ1 synthesis.
Currents recorded at +50mV from oocytes injected with water (N = 7) or KCNQ1 and KCNE1 cRNAs and then exposed to CHX (N = 6) or EtOH (N = 6) within 15 min after cRNA injection. Currents were also recorded from oocytes injected with KCNQ1 and KCNE1 cRNAs after 24 h incubation in control media (N = 5). The currents recorded from +EtOH and control oocytes were significantly different from water and +CHX oocytes (P < 0.05), and there was no difference between the water and +CHX oocytes (one-way ANOVA). Exposure to CHX immediately following KCNQ1 cRNA injection abolished current expression; recorded currents were similar to those measured from water-injected oocytes. In contrast, cRNA-injected oocytes exposed to EtOH expressed currents similar to non-treated cRNA-injected oocytes
Figure S3. Brefeldin A (BFA, 5μM) prevents IKs expression.
Currents recorded at +50 mV from oocytes injected with water (N = 6) or KCNQ1 and KCNE1 cRNAs then immediately exposed to BFA (N = 8) or EtOH (N = 8) for 24 h. Currents were also recorded 24 h after BFA (N = 6) or EtOH (N = 4) removal. * = P < 0.05 (Student’s t-test). Exposure to BFA completely prevented IKs expression implying that functional KCNQ1/KCNE1 complexes failed to reach the plasma membrane, and that this effect was not due to non- specific toxic effects.
Acknowledgments
This work was supported by grants from the Vanderbilt Discovery Grant Program (C.G.V.) and DK061359 (C.G.V.), HL077188 (A.L.G.) and DC00716 (C.R.S.) from the National Institutes of Health.
Abbreviations
- KV
voltage-gated potassium channel
- prKCNE1
purified human KCNE1 protein
- IKs
slow delayed rectifier current
- ER
endoplasmic reticulum
- CHX
cycloheximide
- BFA
brefeldin A
References
- 1.Li Y, Um SY, McDonald TV. Voltage-gated potassium channels: regulation by accessory subunits. Neuroscientist. 2006;12:199–210. doi: 10.1177/1073858406287717. [DOI] [PubMed] [Google Scholar]
- 2.McCrossan ZA, Abbott GW. The MinK-related peptides. Neuropharmacology. 2004;47:787–821. doi: 10.1016/j.neuropharm.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 3.Abbott GW, Goldstein SAN, Sesti F. Do all voltage-gated potassium channels use MiRPs? Circulation Research. 2001;88:981–983. doi: 10.1161/hh1001.091869. [DOI] [PubMed] [Google Scholar]
- 4.Bendahhou S, Marionneau C, Haurogne K, Larroque MM, Derand R, Szuts V, Escande D, Demolombe S, Barhanin J. In vitro molecular interactions and distribution of KCNE family with KCNQ1 in the human heart. Cardiovasc Res. 2005;67:529–538. doi: 10.1016/j.cardiores.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 5.Lundquist AL, Manderfield LJ, Vanoye CG, Rogers CS, Donahue BS, Chang PA, Drinkwater DC, Murray KT, George AL., Jr Expression of multiple KCNE genes in human heart may enable variable modulation of I(Ks) J Mol Cell Cardiol. 2005;38:277–287. doi: 10.1016/j.yjmcc.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 6.Barhanin J, Lesage F, Guillemare E, Fink M, Lazdunski M, Romey G. K(V)LQT1 and lsK (minK) proteins associate to form the IKs cardiac potassium current. Nature. 1996;384:78–80. doi: 10.1038/384078a0. [DOI] [PubMed] [Google Scholar]
- 7.Sanguinetti MC, Curran ME, Zou A, Shen J, Spector PS, Atkinson DL, Keating MT. Coassembly of K(V)LQT1 and minK (IsK) proteins to form cardiac IKs potassium channel. Nature. 1996;384:80–83. doi: 10.1038/384080a0. [DOI] [PubMed] [Google Scholar]
- 8.Takumi T, Moriyoshi K, Aramori I, Ishii T, Oiki S, Okada Y, Ohkubo H, Nakanishi S. Alteration of channel activities and gating by mutations of slow ISK potassium channel. J Biol Chem. 1991;266:22192–22198. [PubMed] [Google Scholar]
- 9.Ben Efraim I, Shai Y, Attali B. Cytoplasmic and extracellular IsK peptides activate endogenous K+ and Cl- channels in Xenopus oocytes. Evidence for regulatory function. J Biol Chem. 1996;271:8768–8771. doi: 10.1074/jbc.271.15.8768. [DOI] [PubMed] [Google Scholar]
- 10.Romey G, Attali B, Chouabe C, Abitbol I, Guillemare E, Barhanin J, Lazdunski M. Molecular mechanism and functional significance of the MinK control of the KvLQT1 channel activity. J Biol Chem. 1997;272:16713–16716. doi: 10.1074/jbc.272.27.16713. [DOI] [PubMed] [Google Scholar]
- 11.Tai KK, Goldstein SA. The conduction pore of a cardiac potassium channel. Nature. 1998;391:605–608. doi: 10.1038/35416. [DOI] [PubMed] [Google Scholar]
- 12.Tapper AR, George AL., Jr MinK subdomains that mediate modulation of and association with KvLQT1. J Gen Physiol. 2000;116:379–390. doi: 10.1085/jgp.116.3.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lerche C, Seebohm G, Wagner CI, Scherer CR, Dehmelt L, Abitbol I, Gerlach U, Brendel J, Attali B, Busch AE. Molecular impact of MinK on the enantiospecific block of IKs by chromanols. British J Pharm. 2000;131:1503–1506. doi: 10.1038/sj.bjp.0703734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Melman YF, Doménech A, de la Luna S, McDonald TV. Structural determinants of KvLQT1 control by the KCNE family of proteins. J Biol Chem. 2001;276:6439–6444. doi: 10.1074/jbc.M010713200. [DOI] [PubMed] [Google Scholar]
- 15.Kurokawa J, Motoike HK, Kass RS. TEA+-sensitive KCNQ1 constructs reveal pore-independent access to KCNE1 in assembled IKs channels. J Gen Physiol. 2001;117:43–52. doi: 10.1085/jgp.117.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tapper AR, George AL., Jr Location and orientation of minK within the IKs potassium channel complex. J Biol Chem. 2001;276:38249–38254. doi: 10.1074/jbc.M103956200. [DOI] [PubMed] [Google Scholar]
- 17.Melman YF, Krumerman A, McDonald TV. A single transmembrane site in the KCNE-encoded proteins controls the specificity of KvLQT1 channel gating. J Biol Chem. 2002;277:25187–25194. doi: 10.1074/jbc.M200564200. [DOI] [PubMed] [Google Scholar]
- 18.Chen HJ, Sesti F, Goldstein SAN. Pore- and state-dependent cadmium block of IKs channels formed with MinK-55C and wild-type KCNQ1 subunits. Biophys J. 2003;84:3679–3689. doi: 10.1016/S0006-3495(03)75097-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Melman YF, Um SY, Krumerman A, Kagan A, McDonald TV. KCNE1 binds to the KCNQ1 pore to regulate potassium channel activity. Neuron. 2004;42:927–937. doi: 10.1016/j.neuron.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 20.Panaghie G, Tai KK, Abbott GW. Interaction of KCNE subunits with the KCNQ1 K+ channel pore. J Physiol. 2006;570:455–467. doi: 10.1113/jphysiol.2005.100644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kang C, Tian C, Sonnichsen FD, Smith JA, Meiler J, George AL, Jr, Vanoye CG, Kim HJ, Sanders CR. Structure of KCNE1 and implications for how it modulates the KCNQ1 potassium channel. Biochemistry. 2008;47:7999–8006. doi: 10.1021/bi800875q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grunnet M, Jespersen T, Rasmussen HB, Ljungstrom T, Jorgensen NK, Olesen SP, Klaerke DA. KCNE4 is an inhibitory subunit to the KCNQ1 channel. J Physiol. 2002;542:119–130. doi: 10.1113/jphysiol.2002.017301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Poulsen AN, Klaerke DA. The KCNE1 beta-subunit exerts a transient effect on the KCNQ1 K+ channel. Biochem Biophys Res Commun. 2007;363:133–139. doi: 10.1016/j.bbrc.2007.08.146. [DOI] [PubMed] [Google Scholar]
- 24.Krumerman A, Gao X, Bian JS, Melman YF, Kagan A, McDonald TV. An LQT mutant minK alters KvLQT1 trafficking. Am J Physiol. 2004;286:C1453–C1463. doi: 10.1152/ajpcell.00275.2003. [DOI] [PubMed] [Google Scholar]
- 25.Chandrasekhar KD, Bas T, Kobertz WR. KCNE1 subunits require co-assembly with K+ channels for efficient trafficking and cell surface expression. J Biol Chem. 2006;281:40015–40023. doi: 10.1074/jbc.M604398200. [DOI] [PubMed] [Google Scholar]
- 26.Shi G, Nakahira K, Hammond S, Rhodes KJ, Schechter LE, Trimmer JS. Beta subunits promote K+ channel surface expression through effects early in biosynthesis. Neuron. 1996;16:843–852. doi: 10.1016/s0896-6273(00)80104-x. [DOI] [PubMed] [Google Scholar]
- 27.Wible BA, Yang Q, Kuryshev YA, Accili EA, Brown AM. Cloning and expression of a novel K+ channel regulatory protein, KChAP. J Biol Chem. 1998;273:11745–11751. doi: 10.1074/jbc.273.19.11745. [DOI] [PubMed] [Google Scholar]
- 28.Scott VE, Rettig J, Parcej DN, Keen JN, Findlay JB, Pongs O, Dolly JO. Primary structure of a beta subunit of alpha-dendrotoxin-sensitive K+ channels from bovine brain. Proc Natl Acad Sci U S A. 1994;91:1637–1641. doi: 10.1073/pnas.91.5.1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takumi T, Ohkubo H, Nakanishi S. Cloning of a membrane protein that induces a slow voltage-gated potassium current. Science. 1988;242:1042–1045. doi: 10.1126/science.3194754. [DOI] [PubMed] [Google Scholar]
- 30.Tian C, Vanoye CG, Kang C, Welch RC, Kim HJ, George AL, Jr, Sanders CR. Preparation, functional characterization, and NMR studies of human KCNE1, a voltage-gated potassium channel accessory subunit associated with deafness and long QT syndrome. Biochemistry. 2007;46:11459–11472. doi: 10.1021/bi700705j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Isom LL, Ragsdale DS, De Jongh KS, Westenbroek RE, Reber BF, Scheuer T, Catterall WA. Structure and function of the beta 2 subunit of brain sodium channels, a transmembrane glycoprotein with a CAM motif. Cell. 1995;83:433–442. doi: 10.1016/0092-8674(95)90121-3. [DOI] [PubMed] [Google Scholar]
- 32.Schmidt JW, Catterall WA. Biosynthesis and processing of the alpha subunit of the voltage-sensitive sodium channel in rat brain neurons. Cell. 1986;46:437–444. doi: 10.1016/0092-8674(86)90664-1. [DOI] [PubMed] [Google Scholar]
- 33.Ivorra I, Fernández A, Gal B, Aleu J, González-Ros JM, Ferragut JA, Morales A. Protein orientation affects the efficiency of functional protein transplantation into the Xenopus oocyte membrane. J Membr Biol. 2002;185:117–127. doi: 10.1007/s00232-001-0118-x. [DOI] [PubMed] [Google Scholar]
- 34.Le Cahérec F, Bron P, Verbavatz JM, Garret A, Morel G, Cavalier A, Bonnec G, Thomas D, Gouranton J, Hubert JF. Incorporation of proteins into (Xenopus) oocytes by proteoliposome microinjection: functional characterization of a novel aquaporin. J Cell Sci. 1996;109 ( Pt 6):1285–1295. doi: 10.1242/jcs.109.6.1285. [DOI] [PubMed] [Google Scholar]
- 35.Morales A, Aleu J, Ivorra I, Ferragut JA, Gonzalez-Ros JM, Miledi R. Incorporation of reconstituted acetylcholine receptors from Torpedo into the Xenopus oocyte membrane. Proc Natl Acad Sci U S A. 1995;92:8468–8472. doi: 10.1073/pnas.92.18.8468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Klausner RD, Donaldson JG, Lippincott-Schwartz J. Brefeldin A: insights into the control of membrane traffic and organelle structure. J Cell Biol. 1992;116:1071–1080. doi: 10.1083/jcb.116.5.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shimkets RA, Lifton RP, Canessa CM. The activity of the epithelial sodium channel is regulated by clathrin-mediated endocytosis. J Biol Chem. 1997;272:25537–25541. doi: 10.1074/jbc.272.41.25537. [DOI] [PubMed] [Google Scholar]
- 38.Zeng WZ, Babich V, Ortega B, Quigley R, White SJ, Welling PA, Huang CL. Evidence for endocytosis of ROMK potassium channel via clathrin-coated vesicles. Am J Physiol Renal Physiol. 2002;283:F630–F639. doi: 10.1152/ajprenal.00378.2001. [DOI] [PubMed] [Google Scholar]
- 39.Jiang M, Xu X, Wang Y, Toyoda F, Liu XS, Zhang M, Robinson RB, Tseng GN. Dynamic partnership between KCNQ1 and KCNE1 and influence on cardiac IKs current amplitude by KCNE2. J Biol Chem. 2009;284:16452–16462. doi: 10.1074/jbc.M808262200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nagaya N, Papazian DM. Potassium channel alpha and beta subunits assemble in the endoplasmic reticulum. J Biol Chem. 1997;272:3022–3027. doi: 10.1074/jbc.272.5.3022. [DOI] [PubMed] [Google Scholar]
- 41.Bianchi L, Shen Z, Dennis AT, Priori SG, Napolitano C, Ronchetti E, Bryskin R, Schwartz PJ, Brown AM. Cellular dysfunction of LQT5-minK mutants: abnormalities of IKs, IKr and trafficking in long QT syndrome. Hum Mol Genet. 1999;8:1499–1507. doi: 10.1093/hmg/8.8.1499. [DOI] [PubMed] [Google Scholar]
- 42.Ellgaard L, Helenius A. Quality control in the endoplasmic reticulum. Nat Rev Mol Cell Biol. 2003;4:181–191. doi: 10.1038/nrm1052. [DOI] [PubMed] [Google Scholar]
- 43.Cheung JC, Reithmeier RA. Scanning N-glycosylation mutagenesis of membrane proteins. Methods. 2007;41:451–459. doi: 10.1016/j.ymeth.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 44.Pourrier M, Schram G, Nattel S. Properties, expression and potential roles of cardiac K+ channel accessory subunits: MinK, MiRPs, KChIP, and KChAP. Journal of Membrane Biology. 2003;194:141–152. doi: 10.1007/s00232-003-2034-8. [DOI] [PubMed] [Google Scholar]
- 45.Trimmer JS, Rhodes KJ. Localization of voltage-gated ion channels in mammalian brain. Annu Rev Physiol. 2004;66:477–519. doi: 10.1146/annurev.physiol.66.032102.113328. [DOI] [PubMed] [Google Scholar]
- 46.O’Grady SM, Lee SY. Molecular diversity and function of voltage-gated (Kv) potassium channels in epithelial cells. Int J Biochem Cell Biol. 2005;37:1578–1594. doi: 10.1016/j.biocel.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 47.Gage SD, Kobertz WR. KCNE3 truncation mutants reveal a bipartite modulation of KCNQ1 K+ channels. J Gen Physiol. 2004;124:759–771. doi: 10.1085/jgp.200409114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Manderfield LJ, George AL., Jr KCNE4 can co-associate with the IKs (KCNQ1-KCNE1) channel complex. FEBS J. 2008;275:1336–1349. doi: 10.1111/j.1742-4658.2008.06294.x. [DOI] [PubMed] [Google Scholar]
- 49.Vanoye CG, Welch RC, Daniels MA, Manderfield LJ, Tapper AR, Sanders CR, George AL., Jr Distinct subdomains of the KCNQ1 S6 segment determine channel modulation by different KCNE subunits. J Gen Physiol. 2009;134:207–217. doi: 10.1085/jgp.200910234. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Isochronal activation curves 4 h after prKCNE1 injection.
(A) Whole-cell current recorded from a KCNQ1-expressing oocyte 4 h post prKCNE1 injection. The current exhibits rapid activation followed by a slower activation to larger current amplitude, suggesting that the current is carried by 2 channel populations: KCNQ1-alone and KCNQ1-KCNE1 channels. (B) Normalized isochronal activation curve obtained for the same oocyte. The lines indicate the average Boltzmann fits for currents recorded from oocytes injected with KCNQ1 cRNA (N= 9, solid line) and KCNQ1 + KCNE1 cRNAs (N = 13, gray dashed line). Together, the data indicate that during the early time points following prKCNE1 injection (2 and 4 h) two populations of KCNQ1 channels exist at the plasma membrane, modulated and non-modulated channels.
Figure S2. Cycloheximide (CHX, 50 μg/ml) inhibits KCNQ1 synthesis.
Currents recorded at +50mV from oocytes injected with water (N = 7) or KCNQ1 and KCNE1 cRNAs and then exposed to CHX (N = 6) or EtOH (N = 6) within 15 min after cRNA injection. Currents were also recorded from oocytes injected with KCNQ1 and KCNE1 cRNAs after 24 h incubation in control media (N = 5). The currents recorded from +EtOH and control oocytes were significantly different from water and +CHX oocytes (P < 0.05), and there was no difference between the water and +CHX oocytes (one-way ANOVA). Exposure to CHX immediately following KCNQ1 cRNA injection abolished current expression; recorded currents were similar to those measured from water-injected oocytes. In contrast, cRNA-injected oocytes exposed to EtOH expressed currents similar to non-treated cRNA-injected oocytes
Figure S3. Brefeldin A (BFA, 5μM) prevents IKs expression.
Currents recorded at +50 mV from oocytes injected with water (N = 6) or KCNQ1 and KCNE1 cRNAs then immediately exposed to BFA (N = 8) or EtOH (N = 8) for 24 h. Currents were also recorded 24 h after BFA (N = 6) or EtOH (N = 4) removal. * = P < 0.05 (Student’s t-test). Exposure to BFA completely prevented IKs expression implying that functional KCNQ1/KCNE1 complexes failed to reach the plasma membrane, and that this effect was not due to non- specific toxic effects.