Abstract
Simultaneous contraction of agonist and antagonist muscles acting about a joint influences joint stiffness and stability. Although several studies have shown that reflexes in the muscle lengthened by a joint perturbation are modulated during co-contraction, little attention has been given to reflex regulation in the antagonist (shortened) muscle. The goal of the present study was to determine whether co-contraction gives rise to altered reflex regulation across the joint by examining reflexes in the muscle shortened by a joint perturbation. Reflexes were recorded from electromyographic activity in elbow flexors and extensors while positional perturbations to the elbow joint were applied. Perturbations were delivered during isolated activation of the flexor or extensor muscles as well as during flexor and extensor co-contraction. Across the group, the shortening reflex in the elbow extensor switched from suppression during isolated extensor muscle activation to facilitation during co-contraction. The shortening reflex in the elbow flexor remained suppressive during co-contraction but was significantly smaller compared to the response obtained during isolated elbow flexor activation. This response in the shortened muscle was graded by the level of activation in the lengthened muscle. The lengthening reflex did not change during co-contraction. These results support the idea that reflexes are regulated across multiple muscles around a joint. We speculate that the facilitatory response in the shortened muscle arises through a fast-conducting oligosynaptic pathway involving Ib interneurons.
Keywords: Stretch reflex, Co-contraction, Upper limb, Shortening reaction
Introduction
Simultaneous contraction of agonist and antagonist muscles acting on a common joint is a useful strategy to increase joint stiffness in response to environmental instabilities (Kornecki 1992; Osu et al. 2002) or while performing tasks that require a high degree of accuracy (Smith 1981; Enoka 1997; Selen et al. 2006). The ability to regulate the level of coactivation is necessary to interact successfully with the physical world; for example, in maintaining a constant arm position while opposing gravity or in prediction of upcoming external events that may compromise stability. The ability to influence joint mechanics through graded levels of co-contraction provides a neural means to increase limb stability. It has been demonstrated that feed-forward neuromotor pathways can compensate for changes in the mechanical properties of the environment (Milner and Cloutier 1998; Osu et al. 2002). Feedback pathways are also regulated to compensate for changes in environmental mechanics (Doemges and Rack 1992; Dietz et al. 1994; Perreault et al. 2008; Shemmell et al. 2009; Krutky et al. 2010), but it is unclear how the use of co-contraction influences feedback control about a joint, especially in muscles shortened by a perturbation.
Co-contraction of agonist and antagonist muscles can alter the reflex response in muscles stretched by a perturbation (Akazawa et al. 1983; Carter et al. 1993; Nielsen et al. 1994). There has been less extensive investigation of the effect of co-contraction on the reciprocal reflex occurring in the antagonist (shortened) muscle during a joint perturbation. If the altered gain of reflex pathways during co-contraction serves to coordinate multiple muscles across a joint, then one would expect a similar modulation of the stretch-elicited reflex in the antagonist muscle. One previous study has shown evidence of modulated responses in the biceps brachii (BB) muscle following elbow flexion perturbations applied during a ball-catching task (Lacquaniti et al. 1991). The normally inhibitory response in the BB switched to facilitation when the perturbation was applied at the time of ball impact. This time period coincided with co-contraction of elbow extensor and flexor muscles and provides some evidence of reflex modulation across the joint during changing demands in muscle activation. It is unclear, however, if the reflex modulation observed in the Lacquaniti study was specific to the ball-catching task and controlled directly, or if it was a result of the co-contraction that preceded the perturbation.
The aim of the present study was to investigate the effect of co-contraction on the reflex responses elicited in elbow flexor and extensor muscles by muscle shortening. Reflex responses were elicited during an isometric task at constant levels of muscle activity, to eliminate any transient changes in muscle and reflex activity. It was hypothesized that the imposition of a perturbation during co-contraction would result in a facilitation of muscle activity in both the lengthened and shortened muscles, whereas a suppression of activity in the shortened muscle would be observed when the same perturbation is applied during isolated activation of the shortened muscle. We also predicted that the level of activation of the antagonist muscle lengthened by the perturbation would influence the magnitude of response facilitation in the shortened muscle, such that greater levels of antagonist activation would give rise to greater facilitation of the shortened muscle.
Methods
Subjects
Fifteen individuals (age 20–57 years, 7 females) volunteered to participate in the study. Not all subjects participated in all experiments. All subjects were required to be neurologically intact and to have no muscular or orthopaedic limitations of the upper limb. Ethical approval for the study was received from the Northwestern University Institutional Review Board, and written informed consent was obtained prior to testing.
Equipment
Manipulandum
Subjects were seated comfortably with the trunk secured to an adjustable chair (Biodex, Shirley, NY) using padded straps. The subject’s right arm was positioned in the horizontal plane with the shoulder at 45° flexion and 90° abduction, the elbow joint at 90°, and the forearm fully pronated (Fig. 1). The angle of 90° was selected to be approximately in the middle of the voluntary range of motion. The upper arm was placed in a height-adjustable trough support to ensure a constant position of the shoulder joint. A fitted fibreglass cast extending from the fingers to the middle of the forearm was used to maintain the wrist joint in a neutral position and to attach the forearm to a linear actuator (Copley ThrustTube TB3806; Copley Controls, Canton, MA). A 10-cm steel plate located on the underside of the cast, centred at the wrist joint, was secured to the top surface of the actuator via a precision bearing that allowed rotation in the horizontal plane. The actuator was mounted at shoulder height on an adjustable aluminium frame and was oriented 45° from the midline, such that perturbations were applied in the horizontal plane in a direction orthogonal to forearm orientation. This resulted in flexion/extension motions primarily at the elbow joint. The actuator was instrumented with a linear encoder (RGH24; Renishaw, Gloucestershire, UK) to provide position information (resolution 1 μm) and was controlled by custom software developed using Matlab xPC (The Mathworks Co., Natick, MA).
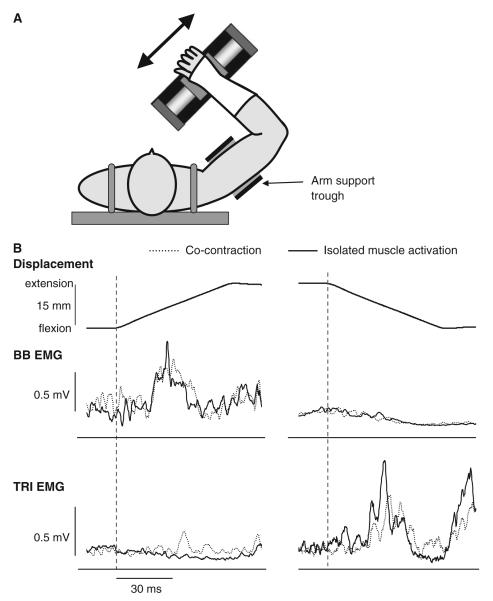
Fig. 1.
a Task set-up. The subject’s arm was positioned so that the shoulder was at 90° abduction and the forearm orthogonal to the actuator. The upper arm was secured in position using the trough support. Perturbations were delivered by the actuator in the directions of the arrow, resulting in flexion and extension movements at the elbow. b Reflex responses in an individual subject following elbow extension (left) and flexion (right) perturbations. Responses are shown for the biceps brachii (BB) and triceps brachii (TRI) muscles. Responses obtained during isolated activation of the target muscle are shown as the solid line. Responses obtained during co-contraction of the BB and TRI muscles are shown as the dotted line. The joint displacement for each condition is displayed above the reflex responses (elbow extension = positive displacement). The onset of the perturbation is shown as the vertical dashed line. In all conditions, muscle activation was at 5% maximum voluntary contraction (MVC). Each response is an average of 20 trials
Electromyography
Surface electromyographic (EMG) activity was recorded from the BB, brachioradialis (BRD), and the lateral head of triceps brachii (TRI) of the right arm. Standard skin preparation techniques were applied prior to the application of disposable dual electrodes (Noraxon USA Inc., AZ). Surface EMG recordings were ampliWed and conditioned using a Bortec AMT-8 (Bortec Biomedical Ltd, Canada) with high- and low-pass cut-off frequencies of 10 and 1,000 Hz, respectively. The resulting signals were anti-aliased filtered using 5th order Bessel filters with a cut-off frequency of 500 Hz and then sampled at 5 kHz for subsequent analysis.
Protocol
A maximum voluntary contraction (MVC) of each muscle was recorded prior to the subjects being seated in front of the manipulandum. Visual feedback of EMG activity of the elbow flexor and extensor muscles was provided along with a target window (± 1% MVC) of activation for each muscle. To eliminate the influence of changing background EMG on response size (Matthews 1986), joint perturbations were delivered when EMG activity had been maintained within the target window for at least 100 ms. All displacements were ramp-and-hold position displacements provided at a velocity of 250 mm/s with a ramp duration of 60 ms (15 mm displacement; approximately 45°/s elbow rotation). A highly stiff environment (requiring no positional control) was adopted so that background muscle activation levels could be regulated while also delivering the same joint perturbation at the same joint angle in each condition. Twenty perturbations were delivered in each condition at random intervals of 3–6 s.
In the initial experiment (n = 15), elbow extension displacements were imposed while the level of activity in the BB and TRI muscles was manipulated. Three muscle activation conditions were investigated: isolated BB activation at 5% MVC, isolated TRI activation at 5% MVC, and BB and TRI co-activation at 5% MVC. In a subset of 8 subjects, we repeated the experimental protocol using elbow flexion perturbations. The same three muscle activation conditions were implemented.
To provide evidence that surface EMG cross-talk between muscles could not explain our findings, a control experiment was conducted in one subject in which fine wire EMG recordings were made from the BB and TRI muscles using intramuscular microelectrodes. A 31G needle was used to insert 50-μm double-bonded stablohm wires into the BB and TRI. The wires were double bonded in order to get differential signals. Intramuscular EMG signals were ampliWed using a Bortec AMT-8 (Bortec Biomedical Ltd, Canada) with high- and low-pass cut-off frequencies of 10 and 1,000 Hz, respectively. Surface EMG from the BB and TRI was recorded using the same techniques as described earlier and was sampled synchronously with the intramuscular EMG at 5 kHz. Using the same protocol as earlier, perturbations were delivered during isolated BB or TRI activation or during co-activation of BB and TRI at 5% MVC. Twenty reflex responses were collected following elbow extension and flexion perturbations in each muscle activation condition.
In a second experiment (n = 9), we investigated the effect of graded co-contraction of the antagonist (lengthened) muscle on the reflex response obtained in the shortened muscle. For these experiments, TRI and BRD were the target muscles. BRD is a synergist to BB in that it is an elbow flexor; however, it is a uniarticular muscle and is located in the forearm rather than the proximal upper limb. This reduced the potential influence of volume conduction from the TRI in the BRD responses. Twenty elbow joint perturbations were delivered both in flexion and in extension directions. Subjects maintained a contraction of 5% MVC in the muscle shortened by the displacement while varying levels of antagonist activation were speciWed (0, 2, 4, 5, 6, 10% MVC). The levels of muscle activation, which were presented in a random order, were chosen to provide a detailed examination of the effects of co-contraction at the elbow.
Data processing and analysis
EMG recordings were rectified and averaged in each condition prior to subsequent analysis. The onset of a reflex response in the target muscle was determined as the first point following perturbation onset at which the EMG activity was either greater or less than 3 standard deviations (SD) of the background muscle activation. Response size was measured as the integrated area of EMG activity in a 30-ms window following EMG response onset. A further 30-ms window of EMG activity was evaluated immediately prior to the perturbation to provide a measure of background muscle activation. To quantify reflex response size, background muscle activation was subtracted from the reflex response and the remainder expressed relative to the level of background activation. No consistent longer latency reflexes were observed across subjects following shortening perturbations, and therefore are not quantified.
Statistical analysis
In the first experiment, paired Student’s t tests were used to compare reflex response size and latency in the BB and TRI muscles between conditions where the target muscle was pre-activated at the same level. Specifically, we compared BB response size between isolated BB activation and BB-TRI co-activation, and compared TRI response size between isolated TRI activation and BB-TRI co-activation. For the second experiment, a one-way repeated measures ANOVA was used to investigate the influence of the level of antagonist muscle activation on reflex response size in the target (shortened) muscle. The level of significance for all statistical analyses was set at P < 0.05. Results are reported as mean ± 1 SD.
Results
In line with our hypothesis, reflex responses in the muscle shortened by the joint perturbation were modulated by the activation state of the antagonist muscle. This modulation was more prominent for the TRI compared to the BB. Figure 1 shows the EMG responses in the BB and TRI of an individual subject following elbow extension (left) and flexion (right) perturbations. In this subject, clear facilitatory responses were elicited in the muscle lengthened by the perturbation both during isolated activation and co-contraction. In elbow flexion perturbations, the BB was suppressed during isolated activation and co-contraction. In contrast, following elbow extension perturbations, an EMG suppression was elicited in the TRI when only the TRI was pre-activated, but the response in the TRI was facilitatory when BB and TRI were co-activated prior to the joint perturbation. These findings are reflected in the group results shown in Fig. 2.
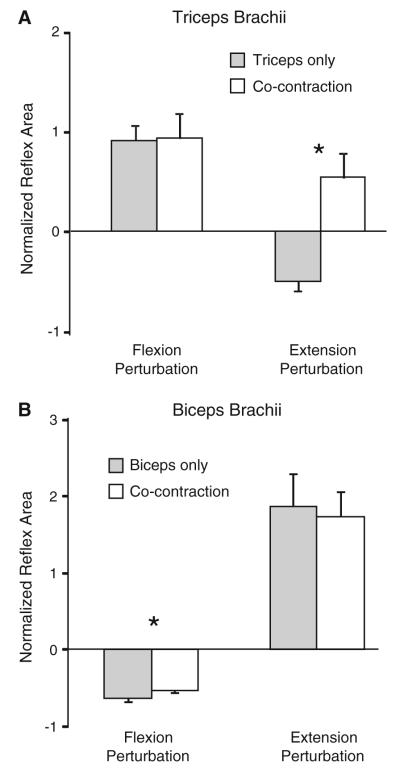
Fig. 2.
Group data (n = 15) showing reflex response size for the triceps (a) and biceps (b) muscles during elbow extension and flexion perturbations. Response size is shown as (reflex response area – background area)/background area. Filled bars indicate response size when the target muscle was pre-activated in isolation. Empty bars indicate response size when the BB and TRI were co-activated prior to the perturbation. Error bars represent one standard error of the mean. Asterisks indicates a significant difference between isolated muscle pre-activation and co-activation (P < 0.05)
TRI reflex response
Following elbow extension perturbations (TRI shortening) delivered during isolated pre-activation of the TRI, a suppression of the ongoing TRI EMG was evident in 14 of 15 subjects. When the same perturbation was delivered during co-contraction of BB and TRI, a facilitatory response was seen in TRI in nine of 15 subjects. All except one of the remaining six subjects demonstrated less EMG suppression following perturbations applied during co-contraction. This resulted in a mean facilitation of the response, compared to background EMG, in TRI during co-activation that was significantly larger than that elicited during isolated TRI pre-activation (P = 0.004; Fig. 2a). Background TRI EMG levels (P = 1) and the perturbation characteristics were equivalent for these two conditions. The mean latency of the EMG suppression in the TRI elicited with isolated TRI activation was 35 ± 6 ms. The latency of the facilitatory response elicited during TRI and BB co-activation was 29 ± 8 ms, which was a significantly shorter onset than the EMG suppression (P = 0.04; Fig. 3a).
Fig. 3.
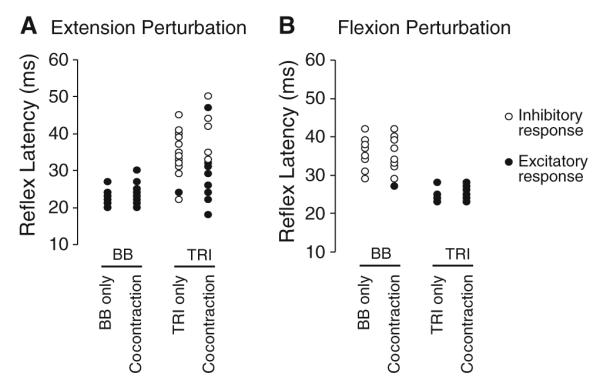
Group data showing reflex response latency for the biceps (BB) and triceps (TRI) muscles during elbow extension (a) and flexion (b) perturbations. Response latencies are shown for each individual subject. Filled circles represent excitatory responses; open circles represent inhibitory responses. Responses are shown during isolated BB/TRI activation as well as co-contraction. Note the delayed latency of the inhibitory responses in the TRI compared to the excitatory response
Following elbow flexion perturbations (TRI lengthening), a facilitatory response was evident in the TRI during isolated TRI activation and during co-contraction. The size of these facilitatory responses was not different between muscle activation conditions (P = 0.6). Response latency was 24 ± 2 and 26 ± 2 ms in isolated TRI activation and co-contraction, respectively (P = 0.1; Fig. 3b).
BB reflex response
Following elbow flexion perturbations (BB shortening) a reflex suppression was elicited in the BB. In support of our hypothesis, the extent of suppression was slightly but consistently reduced during co-contraction compared to isolated BB activation (P = 0.007; Fig. 2b). The latency of the reflex suppression was 35 ± 4 ms during isolated BB activation and 35 ± 5 ms during BB and TRI co-contraction ± (P = 0.8; Fig. 3b).
When the BB was pre-activated in isolation, a large facilitatory response was elicited in BB following elbow extension perturbations (BB lengthening; Fig. 2b). The average latency of this response was 23 ± 2 ms (Fig. 3a). During co-contraction, reflex response size and latency (23 ± 3 ms) was not different from isolated BB activation (both P > 0.2).
Intramuscular responses
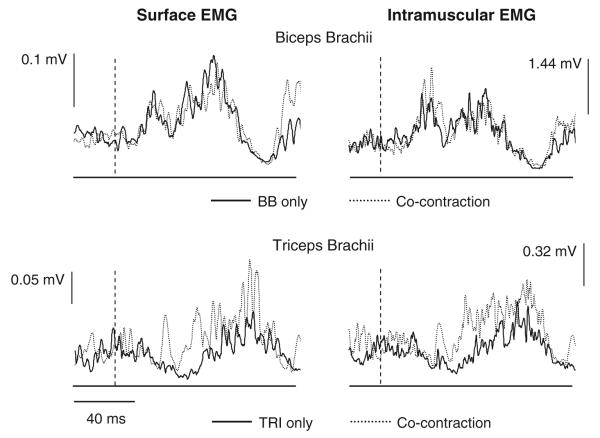
In one subject, we used intramuscular EMG recordings to reduce the likelihood of cross-talk in the recorded responses. The responses seen in the surface and intramuscular recordings for this subject were comparable and followed the previous group results (Fig. 4). The shortening reflex response in the TRI was altered depending on the level of activation in the BB muscle. During isolated TRI activation, EMG suppression is present following an elbow extension perturbation (TRI shortening). When co-activated with BB, a short-latency facilitatory response can be seen. The stretch reflex response elicited in BB during the same perturbations was similar between isolated BB pre-activation and co-contraction with TRI.
Fig. 4.
Surface (left) and intramuscular (right) electromyographic (EMG) recordings from the biceps brachii (BB) and triceps brachii (TRI) muscles following elbow extension perturbations. The perturbations were all 250 mm/s and 15 mm. The top traces are from the BB muscle; the lower are from the TRI muscle. The intramuscular and surface EMG recordings are the average of the same 20 trials. Responses are shown for both isolated activation of the target muscle (solid lines) and co-contraction of BB and TRI (dotted lines). The dashed lines indicate the onset of the joint perturbation. Note the presence of an excitatory response in surface and intramuscular recordings of the TRI during co-contraction
Graded antagonist co-activation
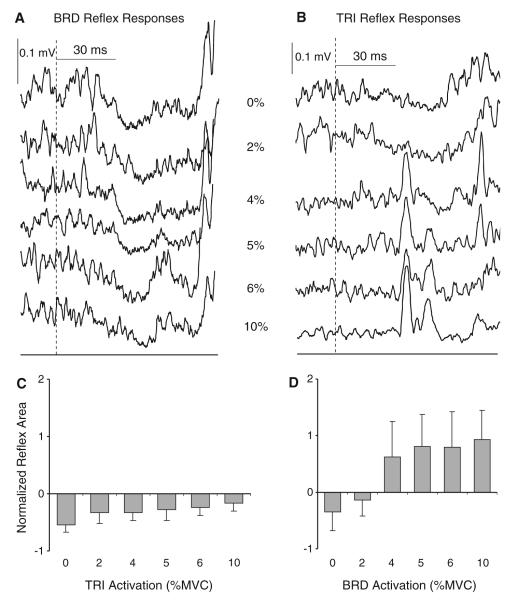
In this experiment, the level of activation of the lengthened muscle was manipulated while activation in the shortened muscle was set at 5% MVC. Following our hypothesis, responses in the muscle shortened by the perturbation were influenced by the level of co-activation of the antagonist muscle. Figure 5 shows an individual subject and group averages for elbow flexion and extension perturbations. During elbow flexion perturbations, responses in the BRD remained suppressive at all levels of TRI co-activation. The ANOVA revealed a significant effect of TRI activation level, reflecting a reduction in the extent of suppression as TRI activation increased (F5,40 = 5.5; P = 0.001; Fig. 5a). Responses in the TRI following elbow extension perturbations were more variable between subjects. Overall, inhibitory reflex suppression was elicited at low levels of BRD activation and then switched to facilitation when the BRD was activated at 4% MVC and above. This was confirmed by a significant effect of BRD activation level on the size of the TRI reflex response (F5,40 = 4.7; P = 0.04; Fig. 5b).
Fig. 5.
Example individual responses (a, b) and group averages (n = 9) of normalized reflex response size (c, d) in the brachioradialis (BRD) and triceps brachii (TRI) muscles during graded activation of the antagonist muscle. Responses in the BRD are shown on the left (a, c) and responses in TRI are shown on the right (b, d). The target muscle was always shortened by the perturbation and was always at 5% of maximum voluntary contraction (MVC). The centre column of a and b indicates the level of activation (% MVC) of the antagonist muscle for each trace. The onset of the joint perturbation is indicated by the dashed line. Error bars represent one standard error of the mean
Discussion
The reduction in TRI EMG during shortening, which may arise from reflex inhibition or disfacilitation, was reversed to facilitation during co-contraction, similar to the findings of Lacquaniti et al. (1991). Inhibitory shortening responses in elbow flexor muscles also were reduced during co-contraction but did not reverse to significant facilitation. For both elbow flexors and extensors, modulation of the reflex in the shortened muscle was graded following the level of activation of the antagonist. These findings show that increased facilitatory reflexes are elicited during co-contraction and support our hypothesis that reflexes are regulated across muscles rather than on a muscle-specific basis. Our results suggest that the normal reflex suppression elicited during muscle shortening is augmented by activity from the lengthened antagonist, and add to the growing literature demonstrating that net behaviour of segmental reflexes can be altered in different environments (Akazawa et al. 1983; Perreault et al. 2008) and by the required response to the perturbation (Hammond 1956; Crago et al. 1976; Colebatch et al. 1979; Rothwell et al. 1980; Dietz et al. 1990; Bawa and Sinkjaer 1999; Lewis et al. 2006; Pruszynski et al. 2008).
Potential mechanisms contributing to the excitatory shortening response
Co-contraction was found to decrease the suppression of ongoing activity, or at times even cause facilitation, in the shortened muscle. We speculate that the neural pathway associated with the increased facilitation or decreased suppression in the shortened muscle involves either a force-sensitive Ib pathway or reduced reciprocal inhibition. Neurophysiological evidence of disynaptic facilitatory reciprocal connections between antagonist muscles was first provided by Laporte and Lloyd (1952) in the cat hindlimb. Short-latency facilitatory responses between antagonist lower limb muscles also have been reported in humans with congenital spasticity (Gottlieb et al. 1982; Myklebust et al. 1982) and spinal cord injury (Crone et al. 2003; Xia and Rymer 2005). In our study, the latency of the facilitatory response elicited when the triceps was shortened during voluntary co-contraction was approximately 4-5 ms longer than the facilitatory short-latency reflex elicited during muscle stretch and 8 ms shorter than the latency of the EMG suppression elicited when the muscle was shortened during isolated activation. Due to the shorter EMG rise time in facilitatory responses, our method of determining onset latency may have resulted in a bias towards shorter latency estimates of onset in facilitatory responses compared to suppression of EMG. However, this factor cannot account for the 4-5 ms difference in latency between the two facilitatory responses. The reciprocal facilitatory response during co-contraction is therefore unlikely to involve monosynaptic connections from Ia afferents. One possible mechanism is the activation of Ib afferents in the lengthened muscle. A Ib reciprocal facilitation pathway was outlined almost 30 years ago from studies of cat spinal cord circuitry (Jankowska et al. 1981). In humans, Katz et al. (1991) reported a modest reciprocal facilitation between elbow flexor and extensor muscles that followed the normal, stronger reciprocal inhibitory response. They attributed this facilitation to a force-sensitive Ib pathway from the conditioned (triceps) to the test (biceps) muscle. A similar Ib-mediated reciprocal facilitation may occur during co-contraction, contributing to the excitatory response evident in the shortened muscle in our study. In the lower limb, cutaneous input has been shown to modulate Ib inhibition at rest to facilitation during muscle activity (Pierrot-Deseilligny et al. 1982). Altered cutaneous input during co-contraction may, therefore, have contributed to the modulation of our reflex responses. The force-dependent nature of the response during graded activation of the antagonist would be consistent with a Ib mechanism. Interestingly, Berardelli and Hallett (1984) reported an excitatory reflex response in the tibialis anterior muscle following ankle dorsi-flexion perturbations that was graded with activation of the plantar-flexors. Although the latency of this response was longer than that seen in our study, the findings are comparable.
Reduced excitability of Ia inhibitory interneurons during co-contraction also could contribute to the observed responses. Activation of Ia inhibitory interneurons serves to inhibit the antagonist muscle during tasks requiring isolated activation (Jankowska et al. 1976). It has been speculated that modulation of Ia interneuron excitability during co-contraction may arise through reduced descending excitation (Nielsen et al. 1993; Xia and Rymer 2005) or facilitation of Renshaw cells (Nielsen and Pierrot-Deseilligny 1996), which have inhibitory connections to Ia interneurons (Hultborn et al. 1971). Additionally, an increase in presynaptic inhibition of Ia interneurons (Enriquez-Denton et al. 2000) or mutual inhibition from antagonist Ia interneurons (Hultborn et al. 1976) is possible. While these mechanisms may contribute to the reduced suppression observed in the elbow flexors during co-contraction, they alone are unlikely to be responsible for the facilitatory responses observed during shortening of the elbow extensors, although a depression of Ia reciprocal inhibition may contribute to the emergence of Ib facilitation. It also cannot be discounted that some of the reflex modulation may be mediated by the C3/4 propriospinal interneuronal system (Pierrot-Deseilligny 1996), which has been shown to be altered during co-contraction compared to isolated muscle activation (Nicolas et al. 2001).
The reflex modulation during co-contraction was more prominent in elbow extensor compared to flexor muscles. In the two elbow flexor muscles examined, there was less suppression evident during co-contraction, but the reflex response did not reverse to facilitation. This may reflect a differential regulation of flexor and extensor control in the upper limb, although it would be interesting to determine whether the shortening reflex in the flexors switched to facilitation at higher levels of antagonist activation.
Altered segmental and descending control during co-contraction
There is evidence for altered neural control at segmental levels to facilitate co-contraction. The stretch reflex amplitude elicited in the lengthened muscle is potentiated during co-contraction at high levels compared to isolated activation (Akazawa et al. 1983; Nielsen et al. 1994). This occurs despite an increase in presynaptic inhibition of Ia afferents (Nielsen and Kagamihara 1993). In addition, recurrent inhibition is increased and reciprocal inhibition is reduced, allowing co-activation to be achieved without the normal reflex mechanisms present that serve to ensure reciprocal activation. We found that the reflex response in the muscle lengthened by the perturbation did not show any difference during co-contraction. Both Carter et al. (1993) and Nielsen et al. (1994) reported that the stretch reflex and joint stiffness were relatively smaller than expected during low levels of muscle co-contraction, indicating that these alterations in segmental pathways are not a simple process. It is difficult to compare the levels of muscle activation between studies given the different joints and recording techniques adopted. The background level of activation in our study (5% MVC) is likely to be towards the lower end of co-contraction levels in relation to these other studies. This level of co-contraction may be sufficient to modulate the response in the shortened muscle but not the muscle lengthened by the joint perturbation.
There is also evidence of altered descending activation of motoneurons from higher centres during co-contraction. From both animal and human studies, it has been suggested that there is a separate population of co-contraction specific corticospinal neurons in the motor cortex (Humphrey and Reed 1983; Nielsen and Kagamihara 1993; Johannsen et al. 2001). Others have proposed an oligosynaptic descending pathway that diverges at segmental levels to innervate agonist–antagonist pairs (Humphrey 1982). Both lines of evidence are suggestive of an alternative descending control strategy from supraspinal centres that facilitates tasks that require co-contraction.
Potential confounds
It could be argued that the facilitatory reflexes seen during co-contraction reflect cross-talk from antagonist muscles. In a few participants, Miscio et al. (2001) reported a short-latency excitatory response in a wrist flexor muscle following shortening by a wrist flexion displacement. These short-latency facilitatory responses were accredited to volume conduction, or cross-talk, from the stretch reflex response in the wrist extensors. There are several reasons why we believe our short-latency facilitatory shortening responses were not due to cross-talk. First, we recorded a facilitatory reflex response using intramuscular electrodes in one subject. Fine wire electrodes have less exposure to signals from distant sources and have a substantially reduced level of cross-talk compared to surface EMG (Solomonow et al. 1994). The intramuscular EMG recordings in our subject were almost identical to surface EMG and displayed a clear facilitatory peak in the TRI following elbow extension perturbations applied during co-contraction. Second, the latency of the facilitatory response in the shortened TRI was, on average, 7 ms longer than the latency of the facilitatory response in the lengthened BB. This timing does not Wt with a volume conducted response across the upper arm. Third, we observed co-contraction related reflex modulation in BRD, which spans a different section of the upper limb from the elbow extensors, and therefore the influence of volume conduction is much reduced.
It is also possible that the facilitatory shortening response arose through vibration of the upper limb during the perturbation that was transmitted to the shortened muscle, eliciting activation of Ia afferents (Lance and Degail 1965). We do not believe that this occurred for two reasons. Firstly, the same perturbation delivered during isolated activation of the shortened muscle did not elicit a facilitatory response. Secondly, during co-contraction the facilitatory response graded with the level of activation of lengthened muscle. Neither of these findings is consistent with a vibration-induced reflex. Finally, interpretation of EMG activity, in absolute values, to infer change in size of segmental or descending responses may be misleading because of amplitude cancellation of the signal. However, even when cancellation exists, the relationship between average rectified EMG and ensemble motoneuron Wring increases in a monotonic manner (Day and Hulliger 2001). This monotonic relationship makes it very unlikely that amplitude cancellation would account for the changes in sign we have reported or even the progressive trends with increasing activation level.
Conclusions and functional implications
The observed changes in reflex size and direction indicate that reciprocal inhibition can be over-ridden during co-contraction, where it would be counter to the voluntary drive. Co-contraction is often employed in tasks requiring fine positional control of the joint (Smith 1981; Milner and Cloutier 1993; Enoka 1997; Selen et al. 2006). Enhanced excitation of the reflex response in the shortened muscle would reinforce the actions of this voluntary drive. Importantly, it also would prevent the shortened muscle from becoming slack (Angel and Lewitt 1978), an event that would reduce the capacity to respond rapidly and accurately during precision tasks. Finally, the transient excitatory responses in the shortened muscle could serve to reduce the net torque about the elbow, resulting in a decreased reflex contribution to joint stiffness. This decrease in reflex mediated stiffness, coupled with the increased intrinsic stiffness known to occur with increased muscle activity, may serve to reduce the influence of co-contraction on the net joint stiffness. The reported experiments do not allow us to distinguish between these possibilities. Additional studies of shortening reflexes during co-contraction in a functionally relevant context may better elucidate the functional consequences of these responses.
Acknowledgments
The authors would like to thank TiVany Ceja for her technical assistance and Krista Settle for her contributions to data collection. The project was supported by NIH grant R01 NS053813 to EJP and a fellowship from the Brinson Foundation awarded to GNL.
Contributor Information
Gwyn N. Lewis, Health and Rehabilitation Research Institute, AUT University, Private Bag 92006, Auckland 1142, New Zealand
Colum D. MacKinnon, Department of Physical Therapy and Human Movement Sciences, Northwestern University, Evanston, IL, USA Department of Neurology, Northwestern University, Evanston, IL, USA.
Randy Trumbower, Sensory Motor Performance Program, Rehabilitation Institute of Chicago, Chicago, IL, USA; Division of Physical Therapy, Emory University, Druid Hills, GA, USA.
Eric J. Perreault, Sensory Motor Performance Program, Rehabilitation Institute of Chicago, Chicago, IL, USA Department of Physical Medicine and Rehabilitation, Northwestern University, Evanston, IL, USA; Department of Biomedical Engineering, Northwestern University, Evanston, IL, USA.
References
- Akazawa K, Milner TE, Stein RB. Modulation of reflex EMG and stiffness in response to stretch of human Wnger muscle. J Neurophysiol. 1983;49:16–27. doi: 10.1152/jn.1983.49.1.16. [DOI] [PubMed] [Google Scholar]
- Angel RW, Lewitt PA. Unloading and shortening reactions in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1978;41:919–923. doi: 10.1136/jnnp.41.10.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bawa P, Sinkjaer T. Reduced short and long latency reflexes during voluntary tracking movement of the human wrist joint. Acta Physiol Scand. 1999;167:241–246. doi: 10.1046/j.1365-201x.1999.00608.x. [DOI] [PubMed] [Google Scholar]
- Berardelli A, Hallett M. The shortening reaction of human tibialis anterior. Neurology. 1984;34:242–246. doi: 10.1212/wnl.34.2.242. [DOI] [PubMed] [Google Scholar]
- Carter RR, Crago PE, Gorman PH. Nonlinear stretch reflex interaction during cocontraction. J Neurophysiol. 1993;69:943–952. doi: 10.1152/jn.1993.69.3.943. [DOI] [PubMed] [Google Scholar]
- Colebatch JG, Gandevia SC, McCloskey DI, Potter EK. Subject instruction and long-latency reflex responses to muscle stretch. J Physiol. 1979;292:527–534. doi: 10.1113/jphysiol.1979.sp012869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crago PE, Houk JC, Hasan Z. Regulatory actions of human stretch reflex. J Neurophysiol. 1976;39:925–935. doi: 10.1152/jn.1976.39.5.925. [DOI] [PubMed] [Google Scholar]
- Crone C, Johnsen LL, Biering-Sorensen F, Nielsen JB. Appearance of reciprocal facilitation of ankle extensors from ankle flexors in patients with stroke or spinal cord injury. Brain. 2003;126:495–507. doi: 10.1093/brain/awg036. [DOI] [PubMed] [Google Scholar]
- Day SJ, Hulliger M. Experimental simulation of cat electromyogram: evidence for algebraic summation of motor-unit action-potential trains. J Neurophysiol. 2001;86:2144–2158. doi: 10.1152/jn.2001.86.5.2144. [DOI] [PubMed] [Google Scholar]
- Dietz V, Bischer M, Faist M, Trippel M. Amplitude modulation of the human quadriceps tendon jerk reflex during gait. Exp Brain Res. 1990;82:211–213. doi: 10.1007/BF00230854. [DOI] [PubMed] [Google Scholar]
- Dietz V, Discher M, Trippel M. Task-dependent modulation of short- and long-latency electromyographic responses in upper limb muscles. Electroencephalogr Clin Neurophysiol. 1994;93:49–56. doi: 10.1016/0168-5597(94)90091-4. [DOI] [PubMed] [Google Scholar]
- Doemges F, Rack PM. Task-dependent changes in the response of human wrist joints to mechanical disturbance. J Physiol. 1992;447:575–585. doi: 10.1113/jphysiol.1992.sp019019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoka RM. Neural strategies in the control of muscle force. Muscle Nerve. 1997;S5:66–69. [PubMed] [Google Scholar]
- Enriquez-Denton M, Nielsen JB, Perreault MC, Morita H, Petersen N, Hultborn H. Presynaptic control of transmission along the pathway mediating disynaptic reciprocal inhibition in the cat. J Physiol. 2000;526:623–637. doi: 10.1111/j.1469-7793.2000.t01-1-00623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb GL, Myklebust BM, Penn RD, Agarwal GC. Reciprocal excitation of muscle antagonists by the primary aVerent pathway. Exp Brain Res. 1982;46:454–456. doi: 10.1007/BF00238640. [DOI] [PubMed] [Google Scholar]
- Hammond PH. The influence of prior instruction to the subject on an apparently involuntary neuro-muscular response. Society. 1956:17P–18P. [PubMed] [Google Scholar]
- Hultborn H, Jankowska E, Lindstrom S. Recurrent inhibition from motor axon collaterals of transmission in the Ia inhibitory pathway to motoneurons. J Physiol. 1971;215:591–612. doi: 10.1113/jphysiol.1971.sp009487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultborn H, Illert M, Santini M. Convergence on interneurones mediating the reciprocal Ia inhibition of motoneurones. II. Effects from segmental flexor reflex pathways. Acta Physiol Scand. 1976;96:351–367. doi: 10.1111/j.1748-1716.1976.tb10205.x. [DOI] [PubMed] [Google Scholar]
- Humphrey DR. Separate cell systems in the motor cortex of the monkey for the control of joint movement and of joint stiffness. Electroencephalogr Clin Neurophysiol. 1982;36:393–408. [PubMed] [Google Scholar]
- Humphrey DR, Reed DJ. Separate cortical systems for control of joint movement and joint stiffness: reciprocal activation and co-activation of antagonist muscles. Adv Neurol. 1983;39:347–372. [PubMed] [Google Scholar]
- Jankowska E, Padel Y, Tanaka R. Disynaptic inhibition of spinal motoneurones from the motor cortex in the monkey. J Physiol. 1976;258:467–487. doi: 10.1113/jphysiol.1976.sp011431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E, Johannisson T, Lipski J. Common interneurones in reflex pathways from group 1a and 1b afferents of ankle extensors in the cat. J Physiol. 1981;310:381–402. doi: 10.1113/jphysiol.1981.sp013556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannsen P, Christensen LOD, Sinkjaer T, Nielsen JB. Cerebral functional anatomy of voluntary contractions of ankle muscles in man. J Physiol. 2001;535:397–406. doi: 10.1111/j.1469-7793.2001.00397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz R, Penicaud A, Rossi A. Reciprocal Ia inhibition between elbow flexors and extensors in the human. J Physiol. 1991;437:269–286. doi: 10.1113/jphysiol.1991.sp018595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornecki S. Mechanism of muscular stabilization process in joints. J Biomech. 1992;25:235245. doi: 10.1016/0021-9290(92)90023-t. [DOI] [PubMed] [Google Scholar]
- Krutky MA, Ravichandran VJ, Trumbower RD, Perreault EJ. Interactions between limb and environmental mechanics influence stretch reflex sensitivity in the human arm. J Neurophysiol. 2010;103:429–440. doi: 10.1152/jn.00679.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacquaniti F, Borghese NA, Carrozzo M. Transient reversal of the stretch reflex in human arm muscles. J Neurophysiol. 1991;66:939–954. doi: 10.1152/jn.1991.66.3.939. [DOI] [PubMed] [Google Scholar]
- Lance JW, Degail P. Spread of phasic muscle reflexes in normal and spastic subjects. J Neurol Neurosurg Psychiatry. 1965;28:328–334. doi: 10.1136/jnnp.28.4.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laporte Y, Lloyd DPC. Nature and significance of the reflex connections established by large afferent fibers of muscular origin. Am J Physiol. 1952;169:609–621. doi: 10.1152/ajplegacy.1952.169.3.609. [DOI] [PubMed] [Google Scholar]
- Lewis GN, MacKinnon CD, Perreault EJ. The effect of task instruction on the excitability of spinal and supraspinal reflex pathways projecting to the biceps muscle. Exp Brain Res. 2006;174:413–425. doi: 10.1007/s00221-006-0475-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews PBC. Observations on the automatic compensation of reflex gain on varying the pre-existing level of motor discharge in man. J Physiol. 1986;374:73–90. doi: 10.1113/jphysiol.1986.sp016066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner TE, Cloutier C. Compensation for mechanically unstable loading in voluntary wrist movement. Exp Brain Res. 1993;94:522–532. doi: 10.1007/BF00230210. [DOI] [PubMed] [Google Scholar]
- Milner TE, Cloutier C. Damping of the wrist joint during voluntary movement. Exp Brain Res. 1998;122:309–317. doi: 10.1007/s002210050519. [DOI] [PubMed] [Google Scholar]
- Miscio G, Pisano F, Del Conte C, Pianca D, Colombo R, Schieppati M. The shortening reaction of forearm muscles: the influence of central set. Clin Neurophysiol. 2001;112:884–894. doi: 10.1016/s1388-2457(01)00468-0. [DOI] [PubMed] [Google Scholar]
- Myklebust BM, Gottlieb GL, Penn RD, Agarwal GC. Reciprocal excitation of antagonistic muscles as a differentiating feature of spasticity. Ann Neurol. 1982;12:367–374. doi: 10.1002/ana.410120409. [DOI] [PubMed] [Google Scholar]
- Nicolas G, Marchand-Pauvert V, Burke D, Pierrot-Deseilligny E. Corticospinal excitation of presumed cervical propriospinal neurones and its reversal to inhibition in humans. J Physiol. 2001;533:903–919. doi: 10.1111/j.1469-7793.2001.t01-1-00903.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen J, Kagamihara Y. The regulation of presynaptic inhibition during co-contraction of antagonist muscles in man. J Physiol. 1993;464:575–593. doi: 10.1113/jphysiol.1993.sp019652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen JB, Pierrot-Deseilligny E. Evidence of facilitation of soleus-coupled Renshaw cells during voluntary co-contraction of antagonistic ankle muscles in man. J Physiol. 1996;493:603–611. doi: 10.1113/jphysiol.1996.sp021407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen J, Petersen N, Deuschl G, Ballegaard M. Task-related changes in the effect of magnetic brain stimulation on spinal neurones in man. J Physiol. 1993;471:223–243. doi: 10.1113/jphysiol.1993.sp019899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen J, Sinkjaer T, Toft E, Kagamihara Y. Segmental reflexes and ankle joint stiffness during co-contraction of antagonistic ankle muscles in man. Exp Brain Res. 1994;102:350–358. doi: 10.1007/BF00227521. [DOI] [PubMed] [Google Scholar]
- Osu R, Franklin DW, Kato H, Gomi H, Domen K, Yoshioka T, Kawato M. Short- and long-term changes in joint co-contraction associated with motor learning as revealed from surface EMG. J Neurophysiol. 2002;88:991–1004. doi: 10.1152/jn.2002.88.2.991. [DOI] [PubMed] [Google Scholar]
- Perreault EJ, Chen K, Trumbower RD, Lewis GN. Interactions with compliant loads alter stretch reflex gains but not intermuscular coordination. J Neurophysiol. 2008;99:2101–2113. doi: 10.1152/jn.01094.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierrot-Deseilligny E. Transmission of the cortical command for human voluntary movement through cervical propriospinal pre-motoneruons. Prog Neurobiol. 1996;48:489–517. doi: 10.1016/0301-0082(96)00002-0. [DOI] [PubMed] [Google Scholar]
- Pierrot-Deseilligny E, Bergego C, Katz R. Reversal in cutaneous control of Ib pathways during human voluntary contraction. Brain Res. 1982;233:400–403. doi: 10.1016/0006-8993(82)91213-6. [DOI] [PubMed] [Google Scholar]
- Pruszynski JA, Kurtzer I, Scott SH. Rapid motor responses are appropriately tuned to the metrics of a visuospatial task. J Neurophysiol. 2008;100:224–238. doi: 10.1152/jn.90262.2008. [DOI] [PubMed] [Google Scholar]
- Rothwell JC, Traub MM, Marsden CD. Influence of voluntary intent on the human long-latency stretch reflex. Nature. 1980;286:496–498. doi: 10.1038/286496a0. [DOI] [PubMed] [Google Scholar]
- Selen LPJ, Beek PJ, Van Dieen JH. Impedance is modulated to meet accuracy demands during goal-directed arm movements. Exp Brain Res. 2006;172:129–138. doi: 10.1007/s00221-005-0320-7. [DOI] [PubMed] [Google Scholar]
- Shemmell J, Je HA, Perreault EJ. The differential role of motor cortex in stretch reflex modulation induced by changes in environmental mechanics and verbal instruction. J Neurosci. 2009;29:13255–13263. doi: 10.1523/JNEUROSCI.0892-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AM. The coactivation of antagonist muscles. Can J Physiol Pharmacol. 1981;59:733–747. doi: 10.1139/y81-110. [DOI] [PubMed] [Google Scholar]
- Solomonow M, Baratta R, Bernardi M, Zhou B, Lu Y, Zhu M, Acierno S. Surface and wire EMG crosstalk in neighbouring muscles. J Electromyogr Kinesiol. 1994;4:131–142. doi: 10.1016/1050-6411(94)90014-0. [DOI] [PubMed] [Google Scholar]
- Xia R, Rymer WZ. Reflex reciprocal facilitation of antagonist muscles in spinal cord injury. Spinal Cord. 2005;43:14–21. doi: 10.1038/sj.sc.3101656. [DOI] [PubMed] [Google Scholar]