Abstract
The double epidemic of substance abuse and HIV infection is a multifaceted problem To investigate mechanistic clues to the effects of substance abuse on infected individuals we preformed quantitative proteomic profiling of plasma in a methamphetamine treated nonhuman primate model for AIDS. A nontargeted quantitative approach identified extracellular superoxide dismutase to be significantly upregulated by SIV and methamphetamine treatment, and targeted studies revealed an increase in expression in the antixoidant glutathione S-transferase, thus pointing to a compensatory response to increased oxidative stress in methamphetamine-treated animals.
Methamphetamine (METH) abuse is a significant cofactor in human immunodeficiency virus (HIV) infection throughout the world.1 Both HIV and METH affect the central nervous system (CNS) and induce neuronal dysfunction and CNS pathology in the subcortical region of the brain.2,3 Many of the mechanisms and sites of damage resulting from METH converge with those in HIV-induced CNS damage; however, the nature of this interaction is not clear. A critical gap in the field of HIV/METH comorbidity is the identification of reliable molecular markers that will provide valuable insights into mechanisms of pathogenesis.
Given the difficulty in sampling the brain, most studies aimed at identifying biomarkers focus on using biofluids such as cerebrospinal fluid (CSF), plasma, and urine.4 In the current study, we hypothesized that the effects of METH could be exhibited throughout the body, and that proteomic profiling of plasma would yield clues to the effects of METH on HIV infection. To assess this in a controlled fashion, we utilized the simian immune deficiency virus (SIV)/rhesus monkey (Macaca mulatta) model of HIV pathogenesis and METH administration. All animal experiments were performed under IACUC approval and following NIH/USDA guidelines.
Our subjects consisted of rhesus monkeys that were infected with an in vivo serial passage derivative of SIVmac2515,6 and developed a stable viremia, as determined by bDNA testing of EDTA-anticoagulated plasma. At 19 weeks postinfection (pi) six monkeys had equivalent set point viral loads (average ± standard deviation, 5.33 ± 0.11 log10 viral genome equivalents per ml plasma). One group of three then received control vehicle (phosphate-buffered saline, PBS) injections whereas the other received METH (Sigma-Aldrich, St. Louis, MO), which was administered intramuscularly via a dose-escalation protocol to enable chronic administration.7 The escalating dose regimen of METH continued for 5 weeks, reaching a final dose of 25 mg/kg a week, which was maintained for an additional 18 weeks. This level mimics that used by human METH abusers.8 At 42 weeks pi (23 weeks of METH administration) the plasma viral load did not differ between the groups (5.60 ± 0.26 and 5.66 ± 1.07 for PBS and METH, respectively), and animals sacrificed and specimens taken for studies of the effects of METH on SIV infection, including plasma for the proteomic studies here. At this stage of infection, animals were still relatively healthy, but with alterations in immune cells, astrocyte activation, and brain viral load noted as presented elsewhere.9
Immunodepletion of relatively abundant proteins was performed to allow the identification of less plentiful proteins. High and medium abundant serum proteins were removed by immunodepletion using the IgY/SuperMix liquid chromatography columns (Genway Bio, San Diego, CA) connected linearly using an Akta Purifier FPLC (GE Health Care, Uppsala, Sweden). The flowthrough fraction was concentrated using Amicon Ultra-15 centrifugal filters (Millipore, Billerica, MA), the protein concentration measured using BCA (Pierce, Rockford, IL), and bovine serum albumin was used as a standard.
The procedure of isobaric tag for relative and absolute quantitation (iTRAQ) labeling followed by mass spectrometry (MS) was employed to quantitatively analyze immunodepleted plasma. A 7-plex approach was employed (using the 8-plex iTRAQ kit, Applied Biosystems, Foster City, CA) wherein the non-METH samples were labeled with 113, 114, 115, METH-treated samples were labeled with 116, 117, and 118, and a reference pool containing all the six samples was labeled with 121. Samples were then combined and subjected to individual off-line cleanup and fractionation using a strong cation-exchange cartridge followed by reverse-phase liquid chromatography electrospray ionization tandem mass spectrometry analysis on an LTQ linear ion trap (Thermo Scientific, Waltham, MA) run in a pulsed-Q-dissociation/collision-induced dissociation hybrid mode. Data were searched against the UniProtKB/Swiss-Prot Release 57.13 of January 19, 2010 using the primates taxonomy with the Mascot 2.2 search engine (Matrix Science Limited, UK). Settings for the MS and database search were previously described.10
We identified a total of 110 proteins, of which 83 proteins had iTRAQ ratios for at least one animal (Supplementary Table 1; Supplementary Data are available online at www.liebertonline.com/aid) and 65 proteins with ratios for all six animals. For initial identification of potential candidate proteins statistical analysis was performed on the proteins quantified in all six animals to identify differentially expressed proteins between the two groups. We found four proteins to be altered by SIV/METH (compared to SIV/PBS) treatment, all of them increased: extracellular superoxide dismutase (EC-SOD), complement factor I (CFI), IgG lambda chain, and mannan binding lectin serine protease 2 (MASP2) (Table 1). Given its importance as an antioxidant enzyme against reactive oxygen species (ROS) and particularly superoxide anion radicals, we focused on EC-SOD.
Table 1.
Differential Expression of Plasma Proteins After METH Administrationa
| Protein identified | iTRAQ ratio SIV + PBS | iTRAQ ratio SIV + METH | Average fold change | p-value |
|---|---|---|---|---|
| IgG lambda chain | 0.722 ± 0.126 | 2.119 ± 0.120 | +2.93 | 0.001 |
| Extracellular superoxide dismutase | 0.707 ± 0.022 | 1.729 ± 0.281 | +2.45 | 0.022 |
| Complement factor I | 0.543 ± 0.095 | 1.243 ± 0.197 | +2.29 | 0.033 |
| Mannan-binding lectin serine protease 2 | 0.930 ± 0.069 | 1.610 ± 0.129 | +1.73 | 0.010 |
The average iTRAQ ratios (± standard error of mean) from the control and METH-administered monkeys were used to compute the fold changes and significance (as determined by an unpaired Student's t-test).
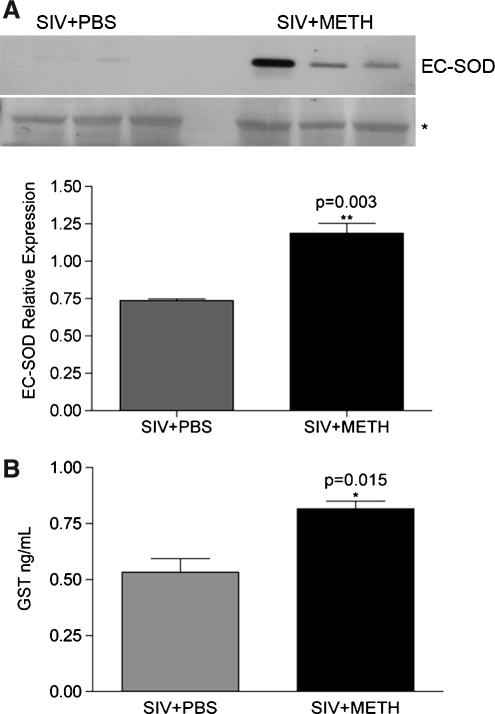
To validate the proteomic data, we next performed Western blot analysis as previously described,10 using anti-EC-SOD (1:500 dilution Santa Cruz), followed by secondary antibody (1:10,000 dilution of HRP-conjugated antigoat IgG; GE Healthcare, Little Chalfont, UK), and development with a 1:1 solution of Super Signal West Pico Chemiluminescent Substrate and Luminol/Enhancer (Thermo Fisher Scientific, Rockford, IL). Western blot analysis indeed confirmed the upregulation of EC-SOD in these samples (Fig. 1A).
FIG. 1.
Antioxidant proteins are increased in plasma in METH-treated animals. (A) Western blot analysis using anti-EC-SOD antibody on immunodepleted plasmas from the six monkeys used in the study (top). Loading was confirmed by Ponceau S staining of membranes and data were normalized to a band expressed equally in all samples (below, indicated by an asterisk). The averages of the normalized values of EC-SOD expression from the six monkeys from the two groups before and after METH treatment are shown as bar graphs (bottom). (B) Immunoassay revealed a significant increase in GST in METH-treated animals. Data are represented as mean ± SEM. An unpaired t-test was used to assess the data for statistical significance.
This interesting observation led us to perform a targeted multiplexed bead-based assay that comprised a number of damaging and protective factors (HumanMAP, Rules Based Medicine Inc., Austin, TX). Profiling of the plasma samples identified glutathione S-transferase (GST), an enzyme that effectively scavenges free radicals during oxidative stress, to be significantly upregulated in the METH-treated (compared to the PBS-treated) SIV-infected monkeys (Fig. 1B).
These data point to activation of defense mechanisms to oxidative stress. In a study on a different drug of abuse (morphine) on SIV-infected monkeys, oxidative stress and defenses were also examined.11,12 Although the experimental protocol differed (animals were administered morphine before inoculation with a mixture of viral strains that rapidly depletes CD4+ T cells), interesting comparisons can be made in chronic infection at a similar pi time point (48 weeks). The morphine-dependent animals had increased plasma malondialdehyde, a marker of lipid peroxidation, and decreased catalase, an antioxidant enzyme that breaks down hydrogen peroxide. Plasma superoxide dismutase (which is predominantly EC-SOD), although not different between the groups at this time point, manifested a rise due to morphine given before infection, which then dropped with SIV infection. Additional studies at earlier time points revealed similar changes in other markers for oxidative stress (an increase in 8-isoprostane) and protective mechanisms (a decrease in glutathione) in the presence of morphine. Intriguingly in these studies, an increased rate of rapid progression to simian AIDS was found in the morphine-treated animals. Together, these data suggest that administration of morphine during infection results in loss of the protective antioxidant activity, whereas it is augmented by METH.
Our studies identified the circulating antioxidant EC-SOD to be significantly upregulated after METH administration in the context of SIV infection. Excessive production of ROS associated with oxidative stress can overwhelm antioxidant systems and lead to cellular damage. Earlier studies have shown that METH can cause oxidative stress by increasing ROS production.13–15 A potent role for oxidative mechanisms in METH toxicity is confirmed by reports that antioxidants such as vitamin C, N-acetyl-l-cysteine, selenium, and melatonin protect against METH-induced destruction of dopamingeric terminals.16–19 Furthermore, CuZn-SOD transgenic mice that have higher CuZn-SOD activity are less prone to METH-induced damage on dopaminergic terminals.15,20,21 Although not specific to METH, in HIV-infected intravenous drug abusers an increase in nitrosative stress was found on CSF proteins.22
Another key antioxidant we identified to be upregulated after SIV/METH treatment is GST, which aids in metabolizing byproducts of oxidative stress.23 Together with the upregulation of EC-SOD, this suggests that in the SIV/METH-treated animals there is an upregulation of the antioxidant system, reflecting compensatory responses to increased oxidative stress. Therefore, the observed increase in the antioxidants is likely protective in nature.
Our study also identified increases in MASP-224,25 and CFI,26 which are proteases associated with the lectin and alternative pathways of the complement system, respectively. MASP-2 alone is sufficient to trigger complement activation through the lectin pathway, whereas CFI is a key negative regulator of the alternative pathway. No significant changes in other complement proteins were observed in our untargeted (where no change was found in complement components C1r, C1s, C2, C5, C8α, C8γ, or C9) or targeted (C3) proteomics. Taken together, the effects on the complement system may be balanced to yield no net effect on complement activation.
Although this study focused on plasma, the combined actions of SIV/METH likely affect other compartments including the brain. Whereas we find an increase in the defense systems to oxidative stress in the plasma, in other organ systems or perhaps with increased time such defense mechanisms may be overwhelmed. The contrast between morphine, where there is a reduction in such defense mechanisms, and METH, where they are increased, is intriguing, but the experimental conditions were quite distinct between these experiments in terms of timing of drug administration and viral inoculum. Additional studies to understand how extrinsic factors such as drugs of abuse increase or decrease host defenses are important in the now prolonged time course of HIV infection.
Supplementary Material
Acknowledgments
We thank James Buescher and Rufina Dominic Salvio for technical assistance. This is manuscript #07 from the UNMC CITN. This work was supported by NIH Grants P30 MH062261, R01 MH073490, and P01 DA026146.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Degenhardt L. Mathers B. Guarinieri M, et al. Methamphetamine use and associated HIV: Implications for global policy and public health. Int J Drug Policy. 2010;21(5):347–358. doi: 10.1016/j.drugpo.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 2.Cadet JL. Krasnova IN. Interactions of HIV and methamphetamine: Cellular and molecular mechanisms of toxicity potentiation. Neurotox Res. 2007;12(3):181–204. doi: 10.1007/BF03033915. [DOI] [PubMed] [Google Scholar]
- 3.Theodore S. Stolberg S. Cass WA. Maragos WF. Human immunodeficiency virus-1 protein tat and methamphetamine interactions. Ann NY Acad Sci. 2006;1074:178–190. doi: 10.1196/annals.1369.018. [DOI] [PubMed] [Google Scholar]
- 4.Shaw LM. Korecka M. Clark CM. Lee VM. Trojanowski JQ. Biomarkers of neurodegeneration for diagnosis and monitoring therapeutics. Nat Rev Drug Discov. 2007;6(4):295–303. doi: 10.1038/nrd2176. [DOI] [PubMed] [Google Scholar]
- 5.Burdo TH. Marcondes MC. Lanigan CM. Penedo MC. Fox HS. Susceptibility of Chinese rhesus monkeys to SIV infection. AIDS. 2005;19(15):1704–1706. doi: 10.1097/01.aids.0000186823.76230.33. [DOI] [PubMed] [Google Scholar]
- 6.Watry D. Lane TE. Streb M. Fox HS. Transfer of neuropathogenic simian immunodeficiency virus with naturally infected microglia. Am J Pathol. 1995;146(4):914–923. [PMC free article] [PubMed] [Google Scholar]
- 7.Madden LJ. Flynn CT. Zandonatti MA, et al. Modeling human methamphetamine exposure in nonhuman primates: Chronic dosing in the rhesus macaque leads to behavioral and physiological abnormalities. Neuropsychopharmacology. 2005;30(2):350–359. doi: 10.1038/sj.npp.1300575. [DOI] [PubMed] [Google Scholar]
- 8.Thompson PM. Hayashi KM. Simon SL, et al. Structural abnormalities in the brains of human subjects who use methamphetamine. J Neurosci. 2004;24(26):6028–6036. doi: 10.1523/JNEUROSCI.0713-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marcondes MC. Flynn C. Watry DD. Zandonatti M. Fox HS. Methamphetamine increases brain viral load and activates NK cells in SIV-infected monkeys. Am J Pathol. 2010;177:355–361. doi: 10.2353/ajpath.2010.090953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pendyala G. Trauger SA. Siuzdak G. Fox HS. Quantitative plasma proteomic profiling identifies the vitamin E binding protein afamin as a potential pathogenic factor in SIV induced CNS disease. J Proteome Res. 2010;9(1):352–358. doi: 10.1021/pr900685u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perez-Casanova A. Husain K. Noel RJ., Jr. Rivera-Amill V. Kumar A. Interaction of SIV/SHIV infection and morphine on plasma oxidant/antioxidant balance in macaque. Mol Cell Biochem. 2008;308(1–2):169–175. doi: 10.1007/s11010-007-9625-0. [DOI] [PubMed] [Google Scholar]
- 12.Perez-Casanova A. Noel RJ., Jr. Rivera-Amill V. Husain K. Kumar A. Morphine-mediated deterioration of oxidative stress leads to rapid disease progression in SIV/SHIV-infected macaques. AIDS Res Hum Retroviruses. 2007;23(8):1004–1007. doi: 10.1089/aid.2006.0286. [DOI] [PubMed] [Google Scholar]
- 13.Gluck MR. Moy LY. Jayatilleke E. Hogan KA. Manzino L. Sonsalla PK. Parallel increases in lipid and protein oxidative markers in several mouse brain regions after methamphetamine treatment. J Neurochem. 2001;79(1):152–160. doi: 10.1046/j.1471-4159.2001.00549.x. [DOI] [PubMed] [Google Scholar]
- 14.Harold C. Wallace T. Friedman R. Gudelsky G. Yamamoto B. Methamphetamine selectively alters brain glutathione. Eur J Pharmacol. 2000;400(1):99–102. doi: 10.1016/s0014-2999(00)00392-7. [DOI] [PubMed] [Google Scholar]
- 15.Jayanthi S. Ladenheim B. Cadet JL. Methamphetamine-induced changes in antioxidant enzymes and lipid peroxidation in copper/zinc-superoxide dismutase transgenic mice. Ann NY Acad Sci. 1998;844:92–102. [PubMed] [Google Scholar]
- 16.Ali SF. Martin JL. Black MD. Itzhak Y. Neuroprotective role of melatonin in methamphetamine- and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced dopaminergic neurotoxicity. Ann NY Acad Sci. 1999;890:119. doi: 10.1111/j.1749-6632.1999.tb07986.x. [DOI] [PubMed] [Google Scholar]
- 17.De Vito MJ. Wagner GC. Methamphetamine-induced neuronal damage: A possible role for free radicals. Neuropharmacology. 1989;28(10):1145–1150. doi: 10.1016/0028-3908(89)90130-5. [DOI] [PubMed] [Google Scholar]
- 18.Fukami G. Hashimoto K. Koike K. Okamura N. Shimizu E. Iyo M. Effect of antioxidant N-acetyl-L-cysteine on behavioral changes and neurotoxicity in rats after administration of methamphetamine. Brain Res. 2004;1016(1):90–95. doi: 10.1016/j.brainres.2004.04.072. [DOI] [PubMed] [Google Scholar]
- 19.Imam SZ. Ali SF. Selenium, an antioxidant, attenuates methamphetamine-induced dopaminergic toxicity and peroxynitrite generation. Brain Res. 2000;855(1):186–191. doi: 10.1016/s0006-8993(99)02249-0. [DOI] [PubMed] [Google Scholar]
- 20.Cadet JL. Ali S. Epstein C. Involvement of oxygen-based radicals in methamphetamine-induced neurotoxicity: Evidence from the use of CuZnSOD transgenic mice. Ann NY Acad Sci. 1994;738:388–391. doi: 10.1111/j.1749-6632.1994.tb21827.x. [DOI] [PubMed] [Google Scholar]
- 21.Hirata H. Ladenheim B. Carlson E. Epstein C. Cadet JL. Autoradiographic evidence for methamphetamine-induced striatal dopaminergic loss in mouse brain: Attenuation in CuZn-superoxide dismutase transgenic mice. Brain Res. 1996;714(1-2):95–103. doi: 10.1016/0006-8993(95)01502-7. [DOI] [PubMed] [Google Scholar]
- 22.Li W. Malpica-Llanos TM. Gundry R, et al. Nitrosative stress with HIV dementia causes decreased L-prostaglandin D synthase activity. Neurology. 2008;70(19 Pt 2):1753–1762. doi: 10.1212/01.wnl.0000282761.19578.35. [DOI] [PubMed] [Google Scholar]
- 23.Hayes JD. Flanagan JU. Jowsey IR. Glutathione transferases. Annu Rev Pharmacol Toxicol. 2005;45:51–88. doi: 10.1146/annurev.pharmtox.45.120403.095857. [DOI] [PubMed] [Google Scholar]
- 24.Matsushita M. Thiel S. Jensenius JC. Terai I. Fujita T. Proteolytic activities of two types of mannose-binding lectin-associated serine protease. J Immunol. 2000;165(5):2637–2642. doi: 10.4049/jimmunol.165.5.2637. [DOI] [PubMed] [Google Scholar]
- 25.Thiel S. Vorup-Jensen T. Stover CM, et al. A second serine protease associated with mannan-binding lectin that activates complement. Nature. 1997;386(6624):506–510. doi: 10.1038/386506a0. [DOI] [PubMed] [Google Scholar]
- 26.Nelson RA., Jr. Jensen J. Gigli I. Tamura N. Methods for the separation, purification and measurement of nine components of hemolytic complement in guinea-pig serum. Immunochemistry. 1966;3(2):111–135. doi: 10.1016/0019-2791(66)90292-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.