Abstract
Systematic advancements in the field of musculoskeletal tissue engineering require clear communication about the mechanical environments that promote functional tissue growth. To support the rapid discovery of effective mechanostimulation protocols, this study developed and validated a mechanoactive transduction and evaluation bioreactor (MATE). The MATE provides independent and consistent mechanical loading of six specimens with minimal hardware. The six individual chambers accurately applied static and dynamic loads (1 and 10 Hz) in unconfined compression from 0.1 to 10 N. The material properties of poly(ethylene glycol) diacrylate hydrogels and bovine cartilage were measured by the bioreactor, and these values were within 10% of the values obtained from a standard single-chamber material testing system. The bioreactor was able to detect a 1-day 12% reduction (2 kPa) in equilibrium modulus after collagenase was added to six collagenase sensitive poly(ethylene glycol) diacrylate hydrogels (p = 0.03). By integrating dynamic stimulation and mechanical evaluation into a single batch-testing research platform, the MATE can efficiently map the biomechanical development of tissue-engineered constructs during long-term culture.
Introduction
A goal in tissue engineering (TE) is to create mechanically viable constructs in vitro that can functionally replace damaged or diseased tissue in vivo. Presently, the clinical application of TE technology in load-bearing tissue has been limited by the biomechanical inferiority of TE constructs.1–3 This lack of mechanical integrity is partly caused by the improper production and assembly of matrical components during culture.4 A promising strategy to improve the composition and ultrastructural distribution of the extracellular matrix, and thus the structural integrity of TE constructs, is to apply mechanical stimuli during culture with bioreactors.1,2,5 To allow the systematic discovery of optimal culture conditions, bioreactors must provide investigators a fast and reliable means to assess the functional outcome of specific mechanical stimulation regimes.
An ongoing challenge in applying accurate and reproducible mechanical stimulations is that TE constructs are normally cultured in batches. In cartilage engineering, this batch testing methodology has resulted in the common practice of compressing multiple constructs with a single actuator.6–10 Although this protocol permits physiological loading11 and high testing volume, it assumes that all specimens are being equally strained. However, construct thickness can vary by at least 10%11,12; therefore, a group of uniformly displaced constructs will be nonuniformly strained. The resulting divergences in strain application can impact construct development.13 Further, few studies that compress constructs account for alterations in specimen thickness during culture.14 These oversights cast uncertainty on the specific mechanical stimulations applied during culture, and confound efforts to reproduce experiments.
The evaluation of mechanical function in TE studies is equally insufficient. Of 205 cartilage engineering articles we examined that applied mechanical stimulation, only 29% quantified material properties. The evident deficiency in material property evaluation is likely related to time and expertise. Material testing is time consuming, as material testing systems that are accurate and economical often test only one specimen at a time.1,15 The expertise to properly quantify the viscoelastic material behavior of musculoskeletal tissue is also uncommon in many dedicated biology labs. The unfortunate consequence is that little is currently known about how specific culture regimes stimulate functional cartilage growth.16 Additionally, information is lacking on the transient physical maturation of TE constructs. Mapping the material properties of TE constructs could guide the development of effective culture protocols.17 This is particularly important in the design of biodegradable scaffolds, in which the rate of scaffold degradation should coincide with the rate of matrix deposition.18
A bioreactor is therefore needed that can provide researchers a platform to efficiently and accurately apply mechanical stimulations and assess material properties. The technology to achieve this goal may be feasible by using multiple electromagnetic voice-coil actuators. Single electromagnetic actuators have previously been implemented in TE bioreactors,11,19,20 and the coordination of multiple electromagnetic actuators could enable a practical and accurate methodology to control the mechanical environment of multiple specimens. The objective of this study was to implement and validate this technology in a mechanoactive transduction and evaluation bioreactor (MATE). Validation was performed by quantifying the MATE's accuracy to (1) deliver mechanical stimulations, (2) measure mechanical properties, and (3) detect alterations in mechanical properties during incubation.
Materials and Methods
Bioreactor design
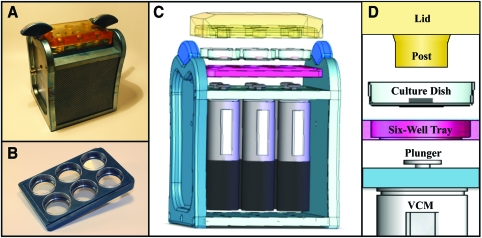
The MATE (Fig. 1A) was designed to accommodate the requirements and constraints of a tissue-culture laboratory environment. The culture tray that holds the constructs was built to imitate a six-well plate (Fig. 1B). This tray facilitates the use of standard 35 × 10 mm cell culture dishes. A translucent polysulfone lid21 interfaces with the culture tray and two butterfly fasteners secure these components to the MATE frame (Fig. 1C). The lid includes 15 mm diameter impermeable loading posts that are centered 6 mm over each culture well. When the culture tray and lid are joined, they create a contained environment that provides for air-flow and enables sterile transfer of constructs for medium exchange. To further minimize the potential for contamination, all electronic instrumentation is housed beneath the culture wells in an enclosed environment (Fig. 1C). The MATE weighs 7 kg, and its overall dimensions were kept sufficiently small (15 × 16 × 22 cm) for housing in a standard CO2 incubator.
FIG. 1.
Images of the mechanoactive transduction and evaluation bioreactor (MATE). (A) The MATE is compact and readily fits into standard incubators. All machined surfaces consist of noncorrosive anodized aluminum. The operator controls the MATE from a dedicated PC with custom LabView software. (B) Specimens are loaded onto culture dishes that sit on a six-well tray. (C) The lid fastens the specimen-laden tray to the MATE frame. Electromagnetic voice coil motors (VCM) are dedicated to each chamber and are housed beneath the specimens. (D) The VCM raise the plunger and culture dish, thereby compressing specimens onto impermeable posts. Color images available online at www.liebertonline.com/ten.
Force and displacement were controlled and measured, respectively, by six electromagnetic actuators that are internally instrumented with optical encoders (resolution ± 1 μm). The electromagnetic actuators load each TE construct in unconfined compression by raising the specimens into contact with the loading posts (Fig. 1D). These actuators have a stroke of 15 mm, oscillate at velocities up to 1.5 m/s and apply peak and continuous forces of 18 and 10 N, respectively. The operator controls the MATE with custom LabView software (NI, 622X, Austin, TX) installed on a dedicated PC. The software generates a waveform that is converted into a continuous voltage signal by a data acquisition card (NI, PCI-6221; ± 10 V, ± 5 mA; 20 MHz). This signal is amplified by six linear current amplifiers housed in the PC case. Two cables transmit the actuator and encoder signals between the MATE and PC.
System calibration
To minimize hardware and system complexity, the current flowing through the electromagnetic actuators was used to predict the actual forces applied to the constructs. Calibration was performed in a custom casing equipped with a load cell (Honeywell, Morristown, NJ; resolution 0.005 N). The voltages necessary to deliver a 3, 6, and 9 N static load were recorded for all six actuators and the amplifiers were tuned for a synchronized calibration. Energy loss from kinetic friction was measured for each actuator during oscillation, and calibration offsets were adjusted accordingly for dynamic loading.
Mechanical stimulation
The calibration accuracy was quantified during variable static and dynamic loading. Static loads were applied at low magnitudes (0.1–1.0 N) and high magnitudes (1.0–10.0 N) to represent the forces commonly used to compress soft hydrogels and mature cartilage, respectively.17,22 For dynamic testing, a linear spring (Lee Spring, Brooklyn, NY; 10 N/mm) was loaded with variable sinusoidal amplitudes (0.1–10.0 N) and frequencies (1, 10 Hz). The spring was preloaded to 0.2 N before applying oscillations. The percent error of the system was calculated as the ratio of the measured force over the target force.
Material property evaluation
To verify that the six chambers of the MATE accurately measure material properties for a wide range of constructs, hydrogels and bovine cartilage specimens were evaluated in both the MATE and an Instron (DynaMight 8840; Instron, Norwood MA; force accuracy = 0.01 N, displacement accuracy = 1 μm). Six hydrogel scaffolds were synthesized by mixing 10% (w/v) poly(ethylene glycol) diacrylate (PEGDA, 6 kDa) with 0.06% (w/v) of the UV-sensitive photoinitiator Irgacure™ 2959. The monomer solution was cast into a stainless steel mold to form disk-shaped hydrogels (8 mm diameter) after 6 min of exposure to UV light (Spectroline: 6 mW/cm2, 365 nm). Hydrogels were incubated in phosphate-buffered saline (PBS) at 37°C for at least 48 h before mechanical testing. Six osteochondral plugs were extracted from a fresh-frozen bovine patella with a cylindrical punch (4 mm diameter). After removal of the deep zone,11 the cartilage plugs were equilibrated in PBS for at least 1-h before mechanical testing.
The mechanical testing protocols were selected to be consistent with the literature1,23,24 and minimally invasive.28 Specimens were placed in cell culture dishes with 2 mL of PBS. Specimen area (ao) was determined using the average of three diameter measurements with a digital caliper (Mitutoyo, San Jose, CA; accuracy ± 0.02 mm), and specimen thickness (lo) was measured by the testing system after applying a 0.1 N preload. For PEGDA hydrogels, specimens were compressed to 0.20 N at 0.05 N/s, allowed 60 s to creep, and sinusoidally loaded for 10 cycles (frequency = 1 Hz, amplitude = 0.15 N). For bovine cartilage, specimens were compressed to 0.70 N at 0.05 N/s, allowed 100 s to creep, and sinusoidally loaded for 10 cycles (frequency = 1 Hz, amplitude = 0.50 N). Each specimen was mechanically tested three times in both the MATE and Instron, alternating between systems for successive tests. Since the Instron is a single-stage testing system, all twelve specimens had to be individually tested three times (36 total tests), whereas sets of six specimens were batch-tested in the MATE (6 total tests).
Material properties were automatically calculated from the MATE and Instron tests using custom LabVIEW software. Stress–strain curves were generated using the first Piola-Kirchhoff stress (f/ao, where f is the current force and ao is the reference area) and engineering strain ([l − lo]/lo, where l is the current thickness and lo is the reference thickness).24 Since the MATE is not instrumented with a force sensor, LabView software used the prescribed force to calculate stress for the MATE tests. Equilibrium modulus was calculated as the ratio of stress and strain at the end of the creep period. Dynamic modulus was calculated by fitting the last four cycles of oscillation data (stress and strain) to a four-parameter sine wave function and finding the ratio of stress amplitude to strain amplitude.24,25
Mechanical evaluation during incubation
The MATE monitored the effect of collagenase in two groups of six cell-free hydrogels: a nondegradable group (10% w/v PEGDA) and degradable group (10% w/v of a collagenase sensitive PEGDA). Degradable hydrogels were synthesized by covalently embedding a collagenase sensitive peptide,26 and exposing for 2 min to visible light and an initiator system of 0.01 mM eosin Y, 0.1% triethanolamine (TEA), and 37 mM 1-vinyl-2-pyrrolidinone (NVP). Each hydrogel was placed in culture dishes with 2 mL of PBS that contained 0.005% collagenase type II (Worthington Biochemical Corporation, Lakewood, NJ).
Daily mechanical tests were performed by the MATE to measure material properties during 10 days of culture under standard incubation conditions (37°C, 5% CO2). Before each mechanical test, specimen diameter was determined from the average of three measurements, and thickness was measured by the MATE after applying a preload of 0.10 N. Specimens were quasi-statically compressed to 0.20 N at 0.05 N/s, allowed 1 min to creep, and sinusoidally loaded for 10 cycles (frequency = 1 Hz, amplitude = 0.1 N). Equilibrium and dynamic modulus were automatically calculated using the previously described LabVIEW software.
Statistics
Paired t-tests determined the effect of the test system (MATE vs. Instron) on measured material properties. Time-dependent effects of incubation were determined using repeated measures ANOVA. If an overall significance was detected (Greenhouse-Geisser), then pairwise comparisons between consecutive days were made using a Least Significance Difference test. Confidence intervals were used for equivalence testing.27 Significance was set to p < 0.05 and all results are reported as mean ± standard deviation.
Results
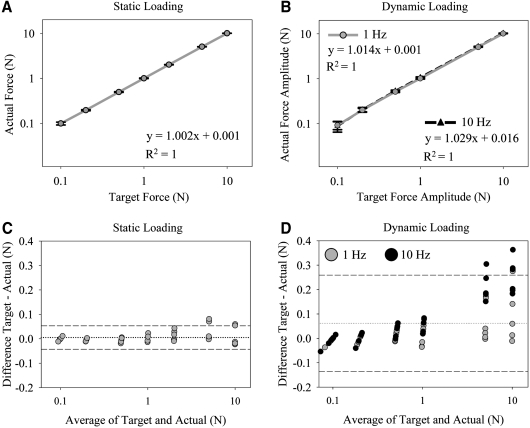
The average forces delivered by the MATE strongly correlated with the target forces prescribed by the user during static (Fig. 2A) and dynamic loading (Fig. 2B). For static loading, there was small variability between the six MATE chambers when 0.1 N was prescribed (0.10 ± 0.01 N, error =0% ± 10%) and when 10 N was prescribed (10.01 ± 0.04 N, error = 0.1% ± 0.4%). The variability between the six MATE chambers increased when loaded dynamically (Fig. 2C, D), with the largest error when a 0.1 N amplitude waveform was prescribed (0.09 ± 0.02 N, error = 9% ± 18%). However, each MATE chamber exhibited good accuracy with waveform amplitudes from 0.2 N (0.20 ± 0.02 N, error = 0% ± 8%) to 10 N (10.2 ± 0.12 N, error = 2% ± 1%). At higher waveform amplitudes, the MATE tended to over apply force (Fig. 2C, D), and the force output at 10 Hz was on average 2.1% greater than the force output at 1 Hz (p < 0.001).
FIG. 2.
Plots showing agreement between the forces prescribed by the operator (target) and the forces experimentally measured on the MATE by a force sensor (actual). Linear regression plots demonstrate a strong correlation between target and actual forces (averaged from all six MATE chambers) for (A) static loading (p < 0.001) and (B) dynamic loading at frequencies of 1 Hz (p < 0.001) and 10 Hz (p < 0.001). Bars represent standard deviation (SD). Bland-Altman plots display variability between individual MATE chambers during (C) static loading and (D) dynamic loading. The dashed lines indicate mean ± 2SD; dotted line indicates mean.
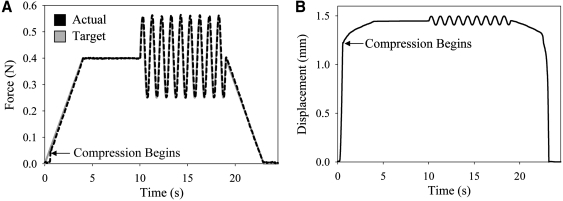
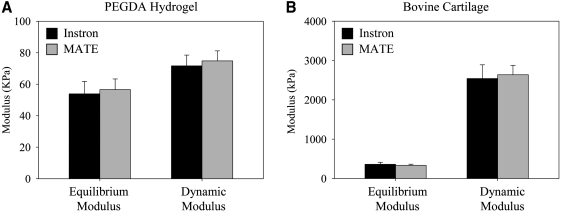
The MATE's force–displacement data (Fig. 3) yielded material properties that were not significantly different from the material properties acquired by the Instron (Fig. 4). On average, the equilibrium and dynamic modulus determined from the MATE's six chambers were within 5% of Instron results for soft hydrogels (p = 0.3, p = 0.4, respectively; Fig. 4A), and within 8% for mature cartilage (p = 0.2, p = 0.3, respectively; Fig. 4B). There was no difference in intra-specimen standard deviation between the test systems (p = 0.15). The material testing protocols applied maximum strains under 20% for all tested specimens,11 and there was an average decrease of 0.8% ± 0.4% in strain between the start and end of oscillations for all specimens. No time dependence existed in the repeated testing of hydrogel and cartilage specimens (p = 0.42, p = 0.13, respectively).
FIG. 3.
Force and displacement data acquired by a MATE chamber during mechanical testing of a hydrogel. (A) The force delivered by the MATE (actual) matches the force prescribed by the user (target). (B) Displacement of the platen during testing.
FIG. 4.
Material test results of the MATE and Instron. (A) For poly(ethylene glycol) diacrylate (PEGDA) hydrogels (n = 6), there was no difference between the systems in determining equilibrium modulus (p = 0.3) and dynamic modulus (p = 0.4). (B) For bovine patellar cartilage (n = 6), there was no difference between the systems in determining equilibrium modulus (p = 0.2) and dynamic modulus (p = 0.3). Bars represent standard deviation.
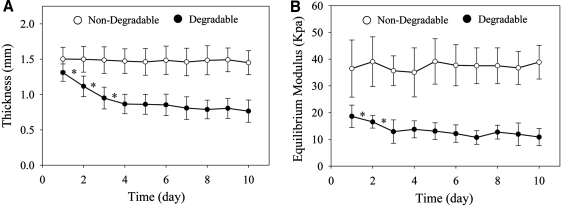
The MATE system was able to detect minor changes in the material properties of degradable and nondegradable hydrogels over a 10-day time period (Fig. 5). Material characteristics of degradable hydrogels were altered during incubation (41% change in thickness, p < 0.001; 42% change in equilibrium modulus, p = 0.02; 20% change in dynamic modulus, p = 0.005), but were unaltered in the nondegradable group (1% change in thickness, p = 0.19; 3% change in equilibrium modulus, p = 0.47; 1% change in dynamic modulus, p = 0.27). In the degradable group, a 24 h reduction was evident in thickness (15%, p < 0.001) and equilibrium modulus (12%, p = 0.03). In the nondegradable group, any overall changes to thickness, equilibrium modulus, and dynamic modulus were < 16%, 16%, and 19%, respectively (95% confidence interval). Maximum strains during mechanical testing were 17% ± 5%.
FIG. 5.
Time-dependent material behavior of hydrogels during collagenase digestion. (A) The thickness of the degradable group was reduced by 41% during incubation (p < 0.001), whereas the nondegradable group was unaltered (p = 0.2). (B) The equilibrium modulus of the degradable group was reduced by 42% during incubation (p < 0.001), whereas the nondegradable group was unaltered (p = 0.5). Most alterations in the degradable group occurred in the first 2 days of collagenase digestion. Bars represent standard deviation (*p < 0.05).
Discussion
A bioreactor (MATE) was developed and validated to support the potential discovery of culture conditions that promote mechanically viable engineered tissue. The MATE provides independent and continuous mechanical loading of six specimens with minimized complexity and hardware. These design attributes enable two key innovations for the TE community. First, mechanical stimulation can be applied in a consistent and repeatable manner to all specimens throughout batch culture. Second, mechanical properties can be periodically evaluated to map functional development. Further, by leveraging system redundancies, the MATE has a portable and practical design. This design includes a compact frame for insertion into standard incubators and a culture tray that replicates a six-well plate.
A clear purpose of the MATE was to provide accurate and repeatable mechanical conditioning for a wide range of materials. For compliant constructs, a 0.1 N static force and 0.2 N dynamic force (≤10 Hz) can be applied with an error <10%. For relatively stiff constructs, a 10 N static force and 10 N dynamic force (≤10 Hz) can be applied with an error <3%. For standard specimen diameters of 4–8 mm, these loads correspond to stresses of 2–500 kPa, and cover the spectrum of stimulation magnitudes and frequencies normally applied in cartilage TE studies.17,22
This level of loading accuracy for a batch-testing bioreactor is uncommon, and differentiates the MATE from contemporary bioreactors that load multiple TE constructs. In an independent review of 205 peer-reviewed articles that applied compressive loads to TE constructs, 84% displaced multiple specimens with a single mechanoelectric actuator, and 95% did not report accounting for alterations in specimen thickness during culture. This strategy is vulnerable to nonuniform and inconsistent specimen deformation, and confounds efforts to reproduce experiments. In the MATE, each specimen is independently deformed under force-control loading. Therefore, the user-specified load will be accurately and continuously transmitted to multiple specimens throughout culture. This loading strategy does, however, limit the number of specimens that can be simultaneously stimulated. Since TE studies normally require additional samples for terminal testing (e.g., gene expression and histology), the six-specimen capacity of the MATE is more restrictive than bioreactors that concurrently stimulate 20 or more samples.6,9 A direct method to increase throughput is to operate several MATE systems at one time (eight MATE bioreactors can readily fit in a standard 79 × 86 × 64 cm incubator interior). A second method is for a single MATE to load multiple six-well culture trays by manually exchanging trays during the same experimental timeframe.14 The number of culture trays that can be stimulated with this strategy will depend on the stimulation duty cycle. For example, if mechanical stimulations are being applied for <2 h per day, four culture trays could be stimulated during an 8-h period (24 constructs total). This method is facilitated by the MATE's tray and lid assembly (Fig. 1C), which preserves sterility during handling.
The ability of the MATE to apply specimen-specific deformation also qualifies the MATE to evaluate material properties during culture. Force and displacement data acquired from each chamber of the MATE were used to determine equilibrium and dynamic modulus of PEGDA hydrogels and bovine patellar cartilage. These modulus values are an indicator of interstitial fluid pressurization, which is fundamental to normal cartilage function.11,28 The modulus values obtained by the MATE were within 10% of values obtained using a standard Instron, and the intra-specimen variability in measuring material properties was similar between the systems. These mechanical tests lasted <3 min, which was sufficient to compare the mechanical testing systems and to give modulus values comparable to previous studies.29,30 More accurate measurements of modulus values, at a given stress level, would require longer test protocols with extended creep periods.31 However, since material tests will naturally stimulate the constructs, the load and duration of material tests should be carefully selected to minimize cellular mechanotransduction and therefore permit an unequivocal evaluation of the prescribed stimulation regimes.
The material analysis of multiple specimens during long-term culture is a feature with considerable potential to the TE community. Material properties represent a clear parameter to assess functional outcome, yet the majority of cartilage engineering studies do not report mechanical behavior.6–10 By integrating batch mechanical stimulation and evaluation into one device, investigators can now efficiently observe the time-dependent influence of specific culture conditions on material properties. This may facilitate the rapid dissemination of techniques that promote functional development, and lead to stimulation regimens that adjust during maturation to optimize growth.16
The MATE's ability to map transient alterations in material properties was tested by monitoring degradable and nondegradable PEGDA hydrogels during incubation in a collagenase medium. In the degradable hydrogels, the MATE detected significant physical alterations, most occurring in the first 2 days. A change in bulk mechanical properties infers that collagenase diffused into the gel and cleaved interior crosslinks. This bulk degradation process has been described by an experimentally validated kinetic model of hydrophilic hydrogels.32,33 In the nondegradable hydrogels, the MATE determined, with 95% confidence, that modulus values changed by <20%. Since the MATE tests six specimens at a time, each daily evaluation lasted <5 min. Therefore, the MATE can assist TE researchers by quantifying the functional effect of specific culture conditions with minimal time and materials.
The absence of bioreactors with the MATE's functionality is likely related to inherent limitations in standard bioreactor instrumentation. Most bioreactors capable of material evaluation control specimen displacement with a mechanoelectric actuator and measure force with a load cell.9,13,34 Expanding this standard design to multiple chambers becomes cost-prohibitive and complex for TE applications. For example, there are no design redundancies, as each chamber requires unique instrumentation to acquire material properties. Further, when operating in displacement-control, loading protocols must be tailored to avoid separation between the platen and specimen.2 Although an electromechanical actuator can avoid platen lift-off by operating in force-control, this strategy becomes impractical as it necessitates a closed-loop control mechanism (e.g., proportional–integral–derivative (PID) controller).35 Closed-loop controls require material-specific input parameters, which can take considerable time and expertise to optimize.
A novel design has enabled the MATE to overcome these technical challenges. The MATE uses multiple electromagnetic actuators, which directly apply force proportional to the user-defined driving current, thereby eliminating the need for a closed-loop system. To maintain the stability of the current-to-force calibration, linear re-circulating bearings were selected with a rating life of 325 years,36 and a magnet was selected that retains stability under 300°C.37 After an estimated 2 million loading cycles, this electromagnetic actuator maintained its current-to-force calibration constant to within 0.2% (data not shown).
It is important to outline limitations of this study. Although this study demonstrates that the MATE can reliably apply mechanical stimulations and evaluate TE constructs in an established culture environment,11 this study did not stimulate cultured cells. At present, the MATE is only capable of controlling all six chambers with the same user-defined loading parameters, and the resulting force waveforms always load the specimens in unconfined compression during mechanical stimulations and mechanical tests. The MATE offers a wide range of loading magnitudes, but the lowest preload that can be reliably applied to measure thickness is 0.05 N. This preload is not uncommon in cartilage engineering studies,38 but will prestrain soft TE constructs. Since the MATE operates in force control, the strain level that material properties are evaluated can only be indirectly controlled. Finally, material testing protocols need to be determined on a tissue-specific basis to ensure tests are repeatable24,39 and have negligible influence on biosynthesis.
In summary, the creation of functional TE constructs demands not only a concerted effort by the TE community, but also clear and reliable communication with regard to the functional outcome of specific TE designs under specific culture conditions. The MATE offers a reliable tool to facilitate this communication and expedite the potential transfer of TE technology to clinical use.40
Acknowledgments
Financial support for this research was provided by the Medical Research Foundation of Oregon and by NIH Grant 1R41AR059433.
Disclosure Statement
Dr. Bottlang and Dr. Madey have a patent pending on the MATE. Dr. Lujan, Kyle Wirtz, Dr. Chelsea Bahney, and Dr. Johnstone have no conflicts of interests.
References
- 1.Hung C.T. Mauck R.L. Wang C.C. Lima E.G. Ateshian G.A. A paradigm for functional tissue engineering of articular cartilage via applied physiologic deformational loading. Ann Biomed Eng. 2004;32:35. doi: 10.1023/b:abme.0000007789.99565.42. [DOI] [PubMed] [Google Scholar]
- 2.Mauck R.L. Soltz M.A. Wang C.C. Wong D.D. Chao P.H. Valhmu W.B., et al. Functional tissue engineering of articular cartilage through dynamic loading of chondrocyte-seeded agarose gels. J Biomech Eng. 2000;122:252. doi: 10.1115/1.429656. [DOI] [PubMed] [Google Scholar]
- 3.Butler D.L. Goldstein S.A. Guilak F. Functional tissue engineering: the role of biomechanics. J Biomech Eng. 2000;122:570. doi: 10.1115/1.1318906. [DOI] [PubMed] [Google Scholar]
- 4.van Osch G.J. Mandl E.W. Marijnissen W.J. van der Veen S.W. Verwoerd-Verhoef H.L. Verhaar J.A. Growth factors in cartilage tissue engineering. Biorheology. 2002;39:215. [PubMed] [Google Scholar]
- 5.Nugent G.E. Aneloski N.M. Schmidt T.A. Schumacher B.L. Voegtline M.S. Sah R.L. Dynamic shear stimulation of bovine cartilage biosynthesis of proteoglycan 4. Arthritis Rheum. 2006;54:1888. doi: 10.1002/art.21831. [DOI] [PubMed] [Google Scholar]
- 6.Cassino T.R. Anderson R. Love B.J. Huckle W.R. Seamans D.K. Forsten-Williams K. Design and application of an oscillatory compression device for cell constructs. Biotechnol Bioeng. 2007;98:211. doi: 10.1002/bit.21422. [DOI] [PubMed] [Google Scholar]
- 7.Chahine N.O. Ateshian G.A. Hung C.T. The effect of finite compressive strain on chondrocyte viability in statically loaded bovine articular cartilage. Biomech Model Mechanobiol. 2007;6:103. doi: 10.1007/s10237-006-0041-2. [DOI] [PubMed] [Google Scholar]
- 8.Mauck R.L. Nicoll S.B. Seyhan S.L. Ateshian G.A. Hung C.T. Synergistic action of growth factors and dynamic loading for articular cartilage tissue engineering. Tissue Eng. 2003;9:597. doi: 10.1089/107632703768247304. [DOI] [PubMed] [Google Scholar]
- 9.Aufderheide A.C. Athanasiou K.A. A direct compression stimulator for articular cartilage and meniscal explants. Ann Biomed Eng. 2006;34:1463. doi: 10.1007/s10439-006-9157-x. [DOI] [PubMed] [Google Scholar]
- 10.Lee D.A. Knight M.M. Mechanical loading of chondrocytes embedded in 3D constructs: in vitro methods for assessment of morphological and metabolic response to compressive strain. Methods Mol Med. 2004;100:307. doi: 10.1385/1-59259-810-2:307. [DOI] [PubMed] [Google Scholar]
- 11.Park S. Hung C.T. Ateshian G.A. Mechanical response of bovine articular cartilage under dynamic unconfined compression loading at physiological stress levels. Osteoarthritis Cartilage. 2004;12:65. doi: 10.1016/j.joca.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 12.Davisson T. Kunig S. Chen A. Sah R. Ratcliffe A. Static and dynamic compression modulate matrix metabolism in tissue engineered cartilage. J Orthop Res. 2002;20:842. doi: 10.1016/S0736-0266(01)00160-7. [DOI] [PubMed] [Google Scholar]
- 13.Waldman S.D. Spiteri C.G. Grynpas M.D. Pilliar R.M. Kandel R.A. Long-term intermittent compressive stimulation improves the composition and mechanical properties of tissue-engineered cartilage. Tissue Eng. 2004;10:1323. doi: 10.1089/ten.2004.10.1633. [DOI] [PubMed] [Google Scholar]
- 14.Butler D.L. Hunter S.A. Chokalingam K. Cordray M.J. Shearn J. Juncosa-Melvin N., et al. Using functional tissue engineering and bioreactors to mechanically stimulate tissue-engineered constructs. Tissue Eng Part A. 2009;15:741. doi: 10.1089/ten.tea.2008.0292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stolz M. Raiteri R. Daniels A.U. VanLandingham M.R. Baschong W. Aebi U. Dynamic elastic modulus of porcine articular cartilage determined at two different levels of tissue organization by indentation-type atomic force microscopy. Biophys J. 2004;86:3269. doi: 10.1016/S0006-3495(04)74375-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martin I. Wendt D. Heberer M. The role of bioreactors in tissue engineering. Trends Biotechnol. 2004;22:80. doi: 10.1016/j.tibtech.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 17.Preiss-Bloom O. Mizrahi J. Elisseeff J. Seliktar D. Real-time monitoring of force response measured in mechanically stimulated tissue-engineered cartilage. Artif Organs. 2009;33:318. doi: 10.1111/j.1525-1594.2009.00723.x. [DOI] [PubMed] [Google Scholar]
- 18.Bryant S.J. Anseth K.S. Hydrogel properties influence ECM production by chondrocytes photoencapsulated in poly(ethylene glycol) hydrogels. J Biomed Mater Res. 2002;59:63. doi: 10.1002/jbm.1217. [DOI] [PubMed] [Google Scholar]
- 19.Chokalingam K. Hunter S.A. Gooch C. Frede C. Florer J.L. Wenstrup R., et al. Three-dimensional in vitro effects of compression and time in culture on aggregate modulus and on gene expression and protein content of collagen type II in murine chondrocytes. Tissue Eng Part A. 2009;15:2807. doi: 10.1089/ten.tea.2008.0560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bottlang M. Simnacher M. Schmitt H. Brand R.A. Claes L. A cell strain system for small homogeneous strain applications. Biomed Tech (Berl) 1997;42:305. doi: 10.1515/bmte.1997.42.11.305. [DOI] [PubMed] [Google Scholar]
- 21.Li K.W. Williamson A.K. Wang A.S. Sah R.L. Growth responses of cartilage to static and dynamic compression. Clin Orthop Relat Res. 2001:S34. doi: 10.1097/00003086-200110001-00005. [DOI] [PubMed] [Google Scholar]
- 22.Kim Y.J. Sah R.L. Grodzinsky A.J. Plaas A.H. Sandy J.D. Mechanical regulation of cartilage biosynthetic behavior: physical stimuli. Arch Biochem Biophys. 1994;311:1. doi: 10.1006/abbi.1994.1201. [DOI] [PubMed] [Google Scholar]
- 23.Woodfield T.B. Malda J. de Wijn J. Peters F. Riesle J. van Blitterswijk C.A. Design of porous scaffolds for cartilage tissue engineering using a three-dimensional fiber-deposition technique. Biomaterials. 2004;25:4149. doi: 10.1016/j.biomaterials.2003.10.056. [DOI] [PubMed] [Google Scholar]
- 24.Lujan T.J. Underwood C.J. Jacobs N.T. Weiss J.A. Contribution of glycosaminoglycans to viscoelastic tensile behavior of human ligament. J Appl Physiol. 2009;106:423. doi: 10.1152/japplphysiol.90748.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bonifasi-Lista C. Lake S.P. Small M.S. Weiss J.A. Viscoelastic properties of the human medial collateral ligament under longitudinal, transverse and shear loading. J Orthop Res. 2005;23:67. doi: 10.1016/j.orthres.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 26.Mann B.K. Gobin A.S. Tsai A.T. Schmedlen R.H. West J.L. Smooth muscle cell growth in photopolymerized hydrogels with cell adhesive and proteolytically degradable domains: synthetic ECM analogs for tissue engineering. Biomaterials. 2001;22:3045. doi: 10.1016/s0142-9612(01)00051-5. [DOI] [PubMed] [Google Scholar]
- 27.Schuirmann D.J. A comparison of the two one-sided tests procedure and the power approach for assessing the equivalence of average bioavailability. J Pharmacokinet Biopharm. 1987;15:657. doi: 10.1007/BF01068419. [DOI] [PubMed] [Google Scholar]
- 28.Mow V.C. Kuei S.C. Lai W.M. Armstrong C.G. Biphasic creep and stress relaxation of articular cartilage in compression? Theory and experiments. J Biomech Eng. 1980;102:73. doi: 10.1115/1.3138202. [DOI] [PubMed] [Google Scholar]
- 29.Laasanen M.S. Toyras J. Korhonen R.K. Rieppo J. Saarakkala S. Nieminen M.T., et al. Biomechanical properties of knee articular cartilage. Biorheology. 2003;40:133. [PubMed] [Google Scholar]
- 30.Villanueva I. Hauschulz D.S. Mejic D. Bryant S.J. Static and dynamic compressive strains influence nitric oxide production and chondrocyte bioactivity when encapsulated in PEG hydrogels of different crosslinking densities. Osteoarthritis Cartilage. 2008;16:909. doi: 10.1016/j.joca.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bian L. Fong J.V. Lima E.G. Stoker A.M. Ateshian G.A. Cook J.L., et al. Dynamic mechanical loading enhances functional properties of tissue-engineered cartilage using mature canine chondrocytes. Tissue Eng Part A. 2010;16:1781. doi: 10.1089/ten.tea.2009.0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rice M.A. Sanchez-Adams J. Anseth K.S. Exogenously triggered, enzymatic degradation of photopolymerized hydrogels with polycaprolactone subunits: experimental observation and modeling of mass loss behavior. Biomacromolecules. 2006;7:1968. doi: 10.1021/bm060086+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Metters A.T. Bowman C.N. Anseth K.S. A Statistical kinetic model for the bulk degradation of PLA-b-PEG-b-PLA hydrogel networks. J Phys Chem. 2000;104:7043. [Google Scholar]
- 34.Demarteau O. Jakob M. Schafer D. Heberer M. Martin I. Development and validation of a bioreactor for physical stimulation of engineered cartilage. Biorheology. 2003;40:331. [PubMed] [Google Scholar]
- 35.Pouline E. Pomerleau A. Desbiens A. Hodouins D. Development and evaluation of an auto-tuning and adaptive PID controller. Automatica. 1995;32:71. [Google Scholar]
- 36.Nippon Thomposon Co. L. Special Selection IKO. 2001.
- 37.Magnetic Materials Producers. A Standard Specifications for Permanent Magnet Materials. 1964.
- 38.Bian L. Stoker A.M. Marberry K.M. Ateshian G.A. Cook J.L. Hung C.T. Effects of dexamethasone on the functional properties of cartilage explants during long-term culture. Am J Sports Med. 2010;38:78. doi: 10.1177/0363546509354197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lujan T.J. Underwood C.J. Henninger H.B. Thompson B.M. Weiss J.A. Effect of dermatan sulfate glycosaminoglycans on the quasi-static material properties of the human medial collateral ligament. J Orthop Res. 2007;25:894. doi: 10.1002/jor.20351. [DOI] [PubMed] [Google Scholar]
- 40.Martin I. Riboldi S.A. Jakob M. Wendt D. SnapShot: Bioreactors systems in tissue engineering (TE) & regenerative medicine (RM) Biomaterials. 2010;31:3114. doi: 10.1016/j.biomaterials.2010.01.046. [DOI] [PubMed] [Google Scholar]