Abstract
Alcohol abuse and dependence is considered a developmental disorder with etiological onset during late childhood and adolescence, and understanding age-related differences in ethanol sensitivity is important. Low to moderate ethanol doses (0.5 and 2.0 g/kg, i.g.) induce single-trial, appetitive second-order place conditioning (SOC) in adolescent, but not adult, rats. Recent studies have demonstrated that adolescents may be less sensitive than adults to the aversive properties of ethanol, reflected by conditioned taste aversion. The present study assessed the aversive motivational effects of high-dose ethanol (3.0 and 3.25 g/kg, i.g., for adolescent and adults, respectively) using SOC. These doses were derived from Experiment 1, which found similar blood and brain ethanol levels in adolescent and adult rats given 3.0 and 3.25 g/kg ethanol, respectively. In Experiment 2, animals received ethanol or vehicle paired with intraoral pulses of sucrose (conditioned stimulus 1 [CS1]). After one, two, or three conditioning trials, rats were presented with the CS1 while in a distinctive chamber (CS2). When tested for CS2 preference, ethanol-treated animals exhibited reduced preference for the CS2 compared with controls. This result, indicative of ethanol-mediated aversive place conditioning, was similar for adolescents and adults, for females and males, and after one, two, or three training trials. One finding, however, suggested that adolescents were less sensitive than adults to ethanol’s aversive effects at the intermediate level of training. In conjunction with previous results, the present study showed that in adolescent rats subjected to SOC, ethanol’s hedonic effects vary from appetitive to aversive as the ethanol dose increases. Adolescent and adult animals appear to perceive the post-ingestive effects of high-dose ethanol as similarly aversive when assessed by SOC.
Keywords: adolescence, adulthood, ethanol, reinforcement, second-order conditioning
1. Introduction
Early initiation of alcohol consumption is associated with a greater likelihood of later alcohol abuse and dependence (“early debut effect,” Pedersen and Skrondal, 1998). This relationship is not linear, nor is it necessarily causal. Alcohol initiation at certain developmental stages is critically important to determine the pattern of alcohol consumption at adulthood. Specifically, the risk of alcohol abuse and dependence is greater when the onset of alcohol intake occurs during early adolescence (13-14 years old, Anthony and Petronis, 1995). Moreover, Grant and Dawson (1997) found that subjects who started drinking before age 15 were four-times more likely to develop alcohol dependence than those who started after age 21. These findings have strong public health implications, particularly when viewed in conjunction with the fact that alcohol intake usually begins during adolescence, with 28% of underage drinkers in the U.S. having started at age 13 (Johnston and O’Malley, 2007).
The use of animal models has identified factors that could help explain the avidity for alcohol during adolescence as well as the enduring consequences of such consumption. Ethanol intake in adolescent rats (animals 21-60 days of age, postnatal day [PD] 21-60, Spear, 2000) surpasses that observed in older animals (Doremus et al., 2005). Adolescents are also more sensitive than adults to the facilitating effects of low-dose ethanol on social behavior but are less sensitive to the disruptive effects that higher ethanol doses have on social behavior (Varlinskaya and Spear, 2002). Intriguingly, adolescents are remarkably resistant to several acute effects of ethanol (e.g., sedation, motor coordination, hypothermia, narcosis; Spear, 2004; White et al., 2002) that normally should serve to preclude further engagement in alcohol intake.
The motivational effects of ethanol are critical in the modulation of drug-seeking and self-administration (Cunningham et al., 2000). Adult rats readily detect an aversive component derived from alcohol intoxication. For example, they reject a taste that has been previously paired with ethanol’s effects (conditioned taste avoidance [CTA]; Davies and Parker, 1990). In contrast, evidence of the expression of ethanol-mediated conditioned preferences in adult rats has proven problematic. Unlike mice, rats tend to avoid locations or textures that signal the drug (conditioned place aversion; Cunningham et al., 1993). Some intriguing data suggest that adolescent rats may exhibit differential sensitivity to ethanol’s motivational effects compared with their more mature counterparts. Philpot et al. (2003) found ethanol-induced conditioned place preference (CPP) at PD25 (0.2 g/kg) and late in adolescence (PD45, 0.5 and 1 g/kg, intraperitoneally [i.p.]), whereas a trend toward conditioned aversion was found in young adults (PD60). A later study (Philpot and Kirstein, 2004) determined that dopaminergic tone correlated with repeated ethanol administration in adolescent and adult rats. Dopamine is a neurotransmitter hypothesized to mediate, at least partially, ethanol’s motivational effects (Samson and Chappel, 2003). Late-adolescent rats (PD45) had higher basal dopamine levels than younger or more mature subjects (Philpot and Kirstein, 2004).
A variation of the CPP procedure has provided another venue for the analysis of ethanol-mediated motivational learning. In this preparation, a gustatory stimulus (e.g., water or sucrose, conditioned stimulus 1 [CS1]) is paired with ethanol’s pharmacological effects. Animals are then stimulated with the CS1 while placed in a visually and tactually distinctive chamber (CS2). Preference or aversion toward the CS2 is then assessed in a choice procedure (CS2 vs. CS novel). The procedure has been described as second-order conditioning (SOC; Molina et al., 2006, 2007) because ethanol’s motivational effects are assessed not through direct responsiveness to the taste CS1 but rather by assessing whether the ethanol-paired taste can transfer motivational information to the CS2. The SOC procedure detected both appetitive and aversive effects of ethanol in 14-day-old, preweanling rats. Specifically, intraoral CSs paired in a single trial with either early or late effects of intragastric (i.g.) administration of a low dose of ethanol (0.5 g/kg) or with the early effects of a moderate dose (2 g/kg) became positive second-order reinforcers. Conversely, aversions emerged when the CS1 was paired with 2.0 g/kg 30-45 min post-administration (Molina et al., 2006, 2007).
One advantage of SOC is that it provides a relatively simple preparation for assessing ethanol reinforcement that can be employed with minimal modification across ontogeny. Indeed, we have recently assessed ethanol-mediated, one-trial SOC in adolescent and adult rats (PD32 and PD70, respectively; Pautassi et al., 2008b). The CS1 (a sucrose taste) was delivered through a surgically implanted catheter 5-15 min or 30-45 min following ethanol administration (0.5 or 2.0 g/kg, i.g.). The CS1 then acted as an appetitive second-order reinforcer in the adolescents, mediating the expression of CPP, which was particularly strong when the CS1 was originally paired with 2.0 g/kg ethanol. The adult rats did not exhibit changes in tactile preferences indicative of ethanol-mediated learning. These results suggest greater sensitivity to ethanol’s appetitive effects in adolescent rats than in adult rats assessed by SOC (Pautassi et al., 2008b).
Age-specific predisposition in terms of sensitivity to ethanol’s motivational effects may render adolescents at risk for ethanol-related problems. Adolescents may be more sensitive to ethanol’s appetitive effects (Pautassi et al., 2008b) but less sensitive to the aversive consequences of ethanol. The latter effects are easily observed in both adult and infant rats, particularly at doses ≥ 2.0 g/kg. Adolescent rats are less susceptible to CTA induced by psychoactive drugs (e.g., cocaine, amphetamine, and nicotine) and also by lithium chloride, an emetic, non-addictive substance (Schramm-Sapyta et al., 2006). Less is known, however, about ethanol’s ability to induce aversive learning in adolescent rats. A recent series of studies (Vetter-O’Hagen, Varlinskaya & Spear, submitted); Varlinskaya and Spear, 2008; Anderson et al., 2008) assessed age- and sex-related differences in terms of ethanol-mediated CTA in adult and adolescent rats (PD32 and PD74, respectively). Ethanol-induced CTA was evident in the adolescents, but at higher doses than in adults. The older animals showed CTA at i.p. doses of 1.0 and 1.5 g/kg, while CTA in adolescents was evident only at 2.0 g/kg. Moreover, in male adolescents (but not in adults or in female adolescents), the presence of a sober conspecific during intoxication inhibited the expression of ethanol-mediated CTA. These results suggest that, when assessed by CTA, adolescents may be less sensitive than their older counterparts to the aversive properties of ethanol. The results also underscore the role of social context in attenuating the aversive effects of ethanol (Vetter-O’Hagen, Varlinskaya & Spear, submitted; Varlinskaya and Spear, 2008).
To date, responsiveness to the aversive properties of ethanol as a function of age and sex has been studied by the CTA paradigm (Vetter-O’Hagen, Varlinskaya & Spear, submitted; Varlinskaya and Spear, 2008; Anderson et al., 2008) and, to a lesser extent, by CPP (Philpot et al., 2003). The study of sex-related differences during adolescence is important because epidemiological and preclinical data indicate substantial sex differences in alcohol consumption (Greenfield, 2002; Chester et al., 2006; Doremus et al., 2005). Little is known, however, about sex differences in terms of motivational learning promoted by ethanol during adolescence (but see Varlinskaya and Spear, 2008). The present study further explored these age- and sex-related differences using alternative learning preparations. Second-order conditioning has already proven sensitive to the detection of the motivational effects of ethanol in adolescent rats (Pautassi et al., 2008b). Therefore, the aim of the present study was to assess the expression of ethanol-mediated aversive learning during adolescence and adulthood using a SOC procedure. Specifically, we explored whether adolescents and adults differ in SOC when trained with a high ethanol dose (3.0 or 3.25 g/kg i.g.; see Experiment 1) in either one-trial training (Experiment 2a) or after more prolonged training (two or three daily conditioning sessions, Experiments 2a and 2b, respectively). The possibility of SOC differing across male and females was also fully assessed.
Pharmacokinetic differences could be major determinants of age-related differences in behavior (Walker and Ehlers, 2008). We recently observed significantly higher blood ethanol concentrations (BECs) in adolescent rats than adult rats 30 min after administration of 2.0 g/kg ethanol i.g. (Pautassi et al., 2008b. Therefore, equating the level of intoxication at both ages (Experiment 1) prior to proceeding with the analysis of potential age-related differences in SOC was important. Our underlying hypothesis was that high-dose ethanol would result in conditioned aversion and that adolescents may be less likely to show such an aversion, particularly when multiple conditioning trials are given. These expectations were based on previous data gathered with CTA (Varlinskaya and Spear, 2008) as well as on the possibility that daily training with ethanol intubations would differentially facilitate the development of tolerance to the aversive effects of ethanol in adolescent rats.
The assessment of ontogenetic differences in the development of tolerance to the hypnotic and sedative effects of ethanol has produced inconsistent results. Tolerance to the hypothermic effects of ethanol, as delivered via vapor inhalation, developed faster in adult than in adolescent rats (Ristuccia & Spear, 2005). A different profile was observed in a study that administered a high-dose ethanol i.g. twice a day for 7 days (4 g/kg; Swartzwelder et al., 1998) and found that tolerance to ethanol-induced sedation and hypothermia was greater in adolescent than in adult rats. In a studywhere adolescent and adult rats were equated for their initial level of ethanol-induced motor impairment, equivalent levels of tolerance following chronic ethanol were found across age (Silveri & Spear, 2001). Less is known about the development of tolerance to ethanol’s hedonic effects. It seems that the existence of ontogenetic differences in the development of ethanol tolerance is uncertain and more work is needed to clarify the conditions in which adults and adolescent differ in the expression of this phenomenon.
2. General Methods
2.1. Subjects
A total of 228 Sprague-Dawley rats were used. These animals were derived from 55 litters born and reared at the Center for Development and Behavioral Neuroscience (Binghamton University, Binghamton, NY, USA). Births were examined daily, and the day of parturition was considered PD0. Pups were housed with the dam in standard maternity cages with free access to water and food. The colony was maintained at 22-24°C with a 12 h light-dark cycle. Weaning was performed at PD21. On that day, eight animals from each litter (four males and four females) were assigned to these studies and transferred to clean tubs lined with pine shavings. On PD28, males and females from a given litter were transferred to clean tubs in same-sex groups of four. Animals that were tested during adulthood were further separated into pairs at PD60.
One hundred nine rats were tested during adolescence (Experiment 1, 12 subjects; Experiment 2a, 61 subjects; Experiment 2b, 36 subjects), and 118 rats were tested at PD67-71 (i.e., adults: Experiment 1, 30 animals; Experiment 2a, 58 animals; Experiment 2b, 30 subjects). Both males and females were tested. The litter representation was the following: Experiment 1, 9 litters; Experiment 2a, 30 litters; Experiment 2b, 16 litters. To eliminate confounds between litter and treatment effects, no more than one subject per litter was assigned to the same condition (Holson and Pearce, 1992). Experimental procedures complied with the Guide for the Care and Use of Laboratory Animals (National Research Council, 1996) and were approved by the Institutional Animal Care and Use Committee within a facility accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care.
2.2. Surgery and Cannulation Procedures
Animals were implanted with polyethylene tubing cannulae on P30 (adolescents) or P68 (adults). This device allowed control over the amount and timing of the intraoral stimulation provided by sucrose infusion (CS1). The surgical procedures were similar to those described in Pautassi et al. (2008b; also see Kiefer, 1995; Kiefer et al., 2005). Briefly, animals were anesthetized with isoflurane (via vapor 2.5%, oxygen carrier, 55 psi). After appropriate antiseptic procedures, an incision was made in the cheek using a thin-walled 14-gauge disposable needle (Harvard Instruments, Columbus, OH, USA). PE10 polyethylene tubing (10 cm section, Clay-Adams, Parsippany, NJ, USA) was run through the needle, which was then removed. Heat was applied to one end of the tubing, thus creating a small flange. The tubing was pulled through the medial internal surface of the cheek such that the flanged end of the cannula rested over the oral mucosae while the remainder exited from the mouth. Another needle was subsequently inserted at the back of the neck and was guided subcutaneously to exit close to the tubing site. The tubing was then run through the needle, thus exiting on the top of the neck where it was secured with a fast-acting adhesive (SuperGlue, Santa Anita, CA, USA). Completion of the procedure took approximately 10 min per animal. Animals remained isolated thereafter to avoid damage to the tubing.
2.3. Conditioning and Testing Procedures
Experiment 2 used the SOC protocol developed by Molina et al. (2006, 2007) and later adapted for use in adolescent rats by Pautassi et al. (2008b). In this preparation, animals are given pairings of ethanol and intraoral infusion of sucrose (CS1, first-order conditioning phase), followed by pairings of sucrose and a chamber lined with sandpaper (CS2, second-order conditioning). Preference or aversion for the CS2 is then assessed in a two-way location preference paradigm (Fig. 1). A more detailed account of conditioning procedures follows.
Figure 1.
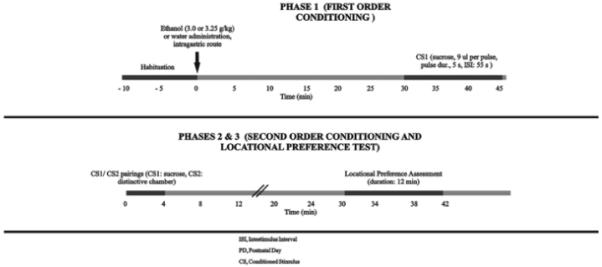
Methods for the analysis of the motivational properties of high-dose ethanol in adolescent and adult rats. Phase 1, first-order conditioning: Animals underwent an initial, nonreinforced habituation phase (10 min duration). After habituation, they were administered ethanol (3.0 or 3.25 g/kg, i.g., for adolescent and adult rats, respectively) or its vehicle (tap water) and then were given a conditioned stimulus (CS1) consisting of intraoral pulses of sucrose. CS1 delivery occurred 30-45 min after the intragastric administration. Phase 2, second-order conditioning: Animals were briefly stimulated with 10% sucrose (4 min duration) while placed in a visually and tactilely distinctive chamber (CS2). Phase 3, locational preference test, P33 or P71: The time spent in the CS2 chamber was recorded in a 12 min place preference test.
Phase 1 (first-order conditioning)
This phase occurred on PD32, PD33, and PD34 for adolescent subjects given three conditioning trials (Experiment 2b). Rats that received only one or two trials (Experiment 2a) were conditioned on PD32 or on PDs 32-33, respectively. Adult animals were given one (PD70), two (PD70-71), or three (PD70-72) conditioning trials. During each daily session, animals were individually placed in a square-shaped chamber (sides and height: 23 cm, lined with cotton). They remained in the chambers for 10 min (habituation phase). Animals were then weighed to the nearest 0.01 g (Sartorius, Gottingen, Germany) and were given ethanol i.g. at a dose of 3.0 or 3.25 g/kg for adults and adolescents, respectively, or its vehicle (tap water; i.e., 0.0 g/kg ethanol). These doses were achieved by administering 0.015 ml/kg of a 25.2, 27.3, or 0.0% ethanol solution, respectively (190-proof ethanol, Pharmaco, Brookfield). The rats were then returned to their individual holding chambers until 30 min post-administration. Exposure to the CS1 occurred in the square-shaped chambers. Specifically, animals received intraoral pulses of sucrose (CS1; 10% v/v; 9 μl per pulse; pulse duration, 5 s; interpulse interval, 55 s) 30-45 min post-administration. This post-administration time was selected on the basis of a previous study conducted in infant rats (Molina et al., 2007). Delivery of sucrose was conducted by slipping the free end of the cannula inside a second polyethylene tube (PE10), which in turn was connected to a Gilmont syringe (Barnant Co., Barrington, IL, USA) mounted in a rotary microsyringe infusion pump. Sucrose (Sigma-Aldrich, St. Louis, MO, USA) was prepared daily using distilled water as a vehicle. The experiment was conducted under bright room illumination (four 25 W fluorescent lamps located in the wall opposite the chambers).
Phase 2 (second-order conditioning)
Twenty-four hours after termination of the last conditioning session, animals were confined, by means of an acrylic barrier, to one section of the box utilized for evaluation of CPP (see Phase 3 below). This section (CS2) was made distinctive by visual and tactile cues. Specifically, the compartment (28 × 20 × 21 cm) had alternating vertical black and white stripes and was lined with a rough sandpaper sheet (Gatorgrit, 60 grit; Ali Industries, Fairborn, OH, USA). While in this compartment, animals were briefly stimulated with 10% sucrose (four 9 μl pulses; interstimulus interval, 55 s; trial duration, 4 min).
Phase 3 (location preference test)
Thirty minutes following Phase 2, animals were tested in a 12 min location preference test. The apparatus consisted of three interconnected chambers (28 × 20 × 21 cm each) with a central area made of white Plexiglas and lined with Formica and two end compartments. The animals had already experienced one of these compartments which contained a vertical striped pattern on the walls with a sandpaper floor (CS2), during Phase 2. The other compartment had a horizontal striped diagram on the walls with a smooth floor (the reverse side of a sandpaper sheet). Sandpaper sheets were replaced for each new test. During this phase, barriers separating the compartments were absent; therefore, animals could freely explore the three-chamber test box. The position of the test box during testing was the same as in Phase 2 of conditioning to keep potential distal spatial cues constant that could signal CS1 delivery (Cunningham et al., 2006). Preference assessment began by placing the animal in the central portion of the neutral compartment. The time spent over each end compartment of the apparatus was recorded. A subject was considered to be in a particular compartment when two paws and the head were over that section. Time spent in the middle section of the apparatus (i.e., start box) was not considered in the calculation of percentage time. The apparatus was cleaned with distilled water after each animal was tested.
2.4. Data Analysis
The main dependent variable in Experiment 2 was absolute time spent in the sandpaper-lined compartment (CS2) during the location preference test. Additionally, percent time spent in the compartment was analyzed. The latter variable measured time in CS2 compared with time spent in the smooth-floor compartment. The time spent in the central compartment was not taken into account. Percent time was calculated as the following: (total time spent over sandpaper × 100) / (total time spent over sandpaper + total time spent over smooth).
These variables were analyzed by separate three-way (Experiment 2b) or four-way (Experiment 2a) mixed-factor analyses of variance (ANOVAs). The between-group factors were age (adolescence or adulthood), sex (male or female), and drug treatment during conditioning (ethanol or its vehicle, tap water). In Experiment 2a, the ANOVA also included the factor length of training (i.e., number of conditioning trials conducted during the first-order conditioning phase: one or two conditioning trials). The loci of significant main effects or interactions were further examined using follow-up ANOVAs and post hoc comparisons (Fisher LSD tests). Values of p < 0.05 were considered statistically significant.
BECs and brain ethanol concentrations (BrECs) were analyzed separately using two-way ANOVAs (see Experiment 1 for details).
3. Experiment 1
When assessing ontogenetic differences in ethanol’s behavioral effects, verifying that similar alcohol levels are achieved across age following drug administration is critical (Walker and Ehlers, 2008). The objective of this first experiment was to find equivalence of ethanol levels across ages. To achieve this aim, adolescent rats were given 3.0 g/kg ethanol, and adults were administered 3.0, 3.25, or 3.5 g/kg ethanol. BECs and BrECs were measured at a post-administration time (32.5 min) that represented a time point close to the onset of the conditioning trial that was employed in the SOC procedure. Specifically, during phase 1 of conditioning, sucrose exposure occurred 30-45 min. following ethanol intubation. This experiment was a necessary step for the subsequent study aimed at analyzing the expression of ethanol-mediated SOC.
3.1. Experimental Design and Procedures
The design consisted of four independent groups defined by the age of the animals and the ethanol dose administered. Adolescents (PD32) were given 3.0 g/kg ethanol, whereas adults (PD70) were administered 3.0, 3.25, or 3.50 g/kg ethanol. Both males and females were used. Groups were composed of 8-12 subjects each. Animals were individually placed in pine-shaving-lined containers, with brain and blood ethanol samples taken 32.5 min after ethanol administration. The procedure was similar to that described in Pautassi et al. (2008b). Briefly, blood samples obtained through decapitation were centrifuged at high speed (3000 rpm, 15 min; Micro-Haematocrit Centrifuge, Hawksley & Sons, Sussex, England) and then processed using an AM1 Alcohol Analyzer (Analox Instruments, Lunenburg, MA, USA). Whole brains were collected, sonicated in a water solution (Silveri and Spear, 2000), and analyzed for ethanol using a head-space gas-chromatograph (Hewlett Packard 5890 series II, Wilmington, DE, USA). BECs and BrECs were expressed as milligrams of ethanol per deciliter of body fluid (mg/dl = mg%).
3.2. Results
Figure 2 depicts BECs and BrECs in adolescent and adult animals. BECs and BrECs were analyzed using separate two-way ANOVAs [(sex male or female) × group assignment (PD32/3.0, PD70/3.0, PD70/3.25, or PD70/3.50, in which the decimal numbers indicate the ethanol doses received by the subjects)]. The BEC and BrEC data yielded very similar results (see Fig. 2). A significant main effect of group assignment was found (BEC: F3,32 = 6.42, p < 0.005; BrEC: F3,32 = 6.36; p < 0.005). Subsequent post hoc tests revealed that adults given 3.0 g/kg ethanol had lower BECs and BrECs than adolescents administered 3.0 g/kg ethanol. In contrast, BECs and BrECs assessed in the latter group (PD32/3.0) were equivalent (i.e., not statistically different) to those found in adult rats administered 3.25 g/kg ethanol. Post hoc analysis also indicated that the PD70/3.50 group had higher BECs and BrECs than any other group. The analyses showed no significant main effects of sex or significant interactions with this factor.
Figure 2.
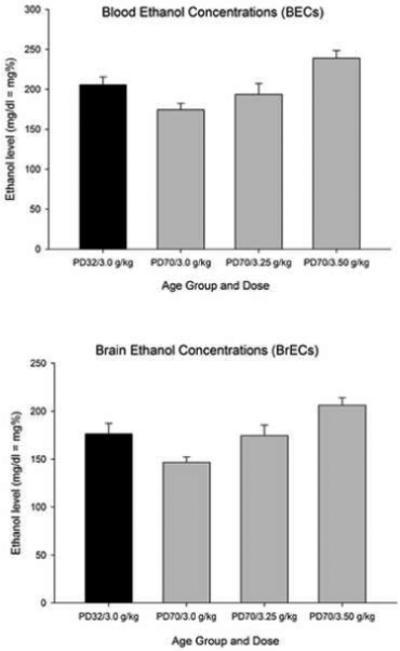
Blood and brain ethanol levels (BECs and BrECs, top and bottom panels, respectively; mg/dl = mg%) in adolescent rats given 3.0 g/kg ethanol (Group PD32/3.0) and adult rats given 3.0, 3.25, or 3.50 g/kg ethanol (Groups PD70/3.0, PD70/3.25 and PD70/3.5; respectively). Blood and brain samples were collected 32.5 min post-administration of ethanol (intragastric). Data were collapsed across sex (male or female). The sex factor did not exert a significant main effect or significantly interact with the remaining variables. Vertical bars indicate the standard error of the mean.
Consistent with other reports(Walker and Ehlers, 2008; Pautassi et al., 2008b), these results suggest faster ethanol metabolism following i.g. ethanol in mature than in juvenile rats. Considering these results, subsequent experiments tested motivational consequences associated with i.g. administration of 3.0 and 3.25 g/kg in adolescent and adult rats, respectively.
4. Experiment 2
The present experiment assessed the motivational effects of high-dose ethanol (3.0 and 3.25 g/kg for adolescent and adult rats, respectively) in adolescent and adult rats using a similar SOC procedure. The preparation was similar to that previously used to detect the appetitive effects of low- and moderate-dose ethanol in adolescent and infant rats (Molina et al., 2006, 2007; Pautassi et al., 2008b). Animals were given one, two (Experiment 2a), or three (Experiment 2b) pairings of an intraoral tastant (sucrose, CS1) with ethanol’s effects. Ethanol doses were derived from Experiment 1, which found similar BECs and BrECs in adolescent and adult rats given 3.0 and 3.25 g/kg, respectively.
4.1. Experiment 2a
4.1.1 Experimental design
The design was a 2 × 2 × 2 × 2 factorial. Experimental subjects were divided into 16 groups defined by age (adolescence or adulthood), sex (male or female), drug treatment (ethanol or its vehicle, tap water), and length of training (one or two conditioning trials). Each condition consisted of 6-9 subjects.
4.1.2. Conditioning and testing procedures
A full account of the second-order procedure has been provided in the General Methods section (Fig. 1). Briefly, rats were given one or two pairings of water or ethanol (3.0 and 3.25 g/kg, for adolescent and adult rats, respectively) and a novel tastant (sucrose, CS1). Sucrose was delivered 30-45 min post-administration in a pulsate pattern by means of an infusion pump connected to an intraoral cannula. Twenty-four hours after the last conditioning session, animals were briefly re-exposed to the taste CS1 while confined to a distinctive chamber (CS2). Thirty min thereafter, the animals were tested for their preference for CS2 in a three-way preference test. The preference/avoidance observed toward the section of the test cage originally paired with the ethanol-related CS1 was considered an index of the hedonic properties of ethanol.
4.1.3. Results
As depicted in Fig. 3 (top panel), animals treated with ethanol during conditioning spent less absolute time on the sandpaper-lined compartment (CS2) of the testing cage than animals that had been given vehicle. The corresponding ANOVA indicated that this pattern, indicative of ethanol-mediated aversive place learning, was similar across age (adolescence or adulthood), sex (female or male), and length of training (one or two trials). Specifically, the ANOVA revealed a significant main effect of drug treatment (F1,103 = 6.56; p < 0.05) that failed to significantly interact with the remaining variables. Additionally, the analysis indicated a borderline main effect of gender, with females displaying a slightly greater preference for the CS2 (180.35 +/− 10.51) at test than males (158.24 +/− 7.81) (F1,103 = 3.80, p = 0.053).
Figure 3.
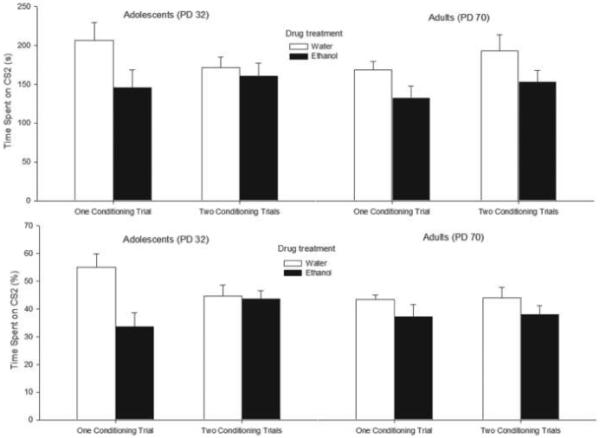
Total time (s) spent in the sandpaper lined chamber (CS2, top panel) and the corresponding percent preference for the chamber (bottom panel) during the 12 min test session as a function of drug treatment during conditioning (ethanol or water), length of training (one or two first-order conditioning trials), and age of the subjects (adolescence or adulthood). Data were collapsed across sex (male or female). Vertical bars indicate the standard error of the mean.
Percent preference for the CS2 is shown in Fig. 3 (bottom panel). Visual inspection of Fig. 3 appears to indicate that length of training had a differential effect on conditioned aversion in adolescent vs. adult rats. This impression received some support from the inferential analyses. The ANOVA for percent preference yielded a complex pattern of results. Significant main effects of gender and drug treatment were observed (F1,103 = 5.10 and F1,103 = 7.63, p < 0.05 and p < 0.001, respectively). The following interactions also achieved significance: length of training × drug treatment (F1,103 = 5.02, p < 0.05) and length of training × drug treatment × age (F1,103 = 5.66, p < 0.05). The four-way interaction (gender × length of training × drug treatment × age) yielded a trend toward significance (F1,103 = 3.10, p = 0.081). To better understand these interactions, follow-up ANOVAs (gender × drug treatment × length of training) were performed for each age group. With adult animals, only significant main effects of drug treatment and gender were observed (F1,50 = 11.91 and F1,50 = 4.37, p < 0.005 and p < 0.05, respectively). Females spent more percent time in CS2 (46.13 +/− 2.26) than males (40.18 +/− 1.92). More importantly, ethanol treatment in adults resulted in significantly less time on CS2 (sandpaper), indicating ethanol-induced second-order aversion for the texture.
When considering adolescent rats, the ANOVA revealed a significant drug treatment × length of training interaction (F1,53= 8.22, p < 0.01). Subsequent post hoc analysis revealed that percent time spent in the sandpaper-lined chamber (CS2) was lower in ethanol-treated adolescents given one, but not two, conditioning trials compared with their appropriate vehicle-treated controls. In animals given two conditioning trials, ethanol-treated adolescents apparently spent as much time in CS2 as their counterparts given vehicle (i.e., water). Also importantly, post hoc analysis indicated that ethanol-treated adolescents given one conditioning trial exhibited a significantly lower predilection for CS2 than their counterparts conditioned with ethanol in two conditioning trials.
Visual inspection of Fig. 3 may suggest that differences occurred between control groups in time spent in CS2 as a function of age and length of the first-order training. This supposition, however, was not corroborated by the inferential analysis. Specifically, relevant two-way ANOVAs conducted on animals that received vehicle indicated a lack of significant main effects or significant interactions. This is an important result indicating that, at this level of training, no age-related differences were observed in terms of basal level of responsiveness toward the target CS or, looking at it from the converse perspective, for the novel portion of the apparatus.
4.2. Experiment 2b
The main result of Experiment 2a was that after one or two pairings of high-dose ethanol and a distinctive taste (CS1), the ethanol-paired tastant endowed a paired tactile/spatial cue (CS2) with aversive, second-order reinforcing capabilities. Results of the ANOVA for percentage or absolute time spent on CS2 indicated that age did not affect selection of CS2. In other words, conditioned aversion was not statistically different for adolescents and adults. However, within-age analyses conducted on the percent preference data revealed a potential ontogenetic difference as a function of length of training, with less aversion in adolescents, but not in adults, rats after two conditioning trials than after only one. This suggested that still more conditioning trials might reveal significant age-related differences in ethanol-mediated conditioned aversion. Therefore, in Experiment 2b, adolescents and adults were given three conditioning trials, i.e., three pairings of an intraoral tastant and the post-absorptive effects of ethanol. Animals were then briefly exposed to the CS1-CS2 pairing followed shortly thereafter by assessment of CS2 preference. By replicating a substantial part of the procedures of Experiment 2a, we also sought to assess the stability and reliability of the SOC preparation for detecting learning mediated by the hedonic component of ethanol.
4.2.1. Experimental design and procedures
A 2 (age: adolescence or adulthood) × 2 (sex: male or female) × 2 (drug treatment: ethanol or water) design was employed. Each of the eight groups consisted of 7-10 subjects each.
4.2.2. Procedures
Conditioning and testing procedures followed those described in Experiment 2a, with the exception that the first-order conditioning phase had one more training episode: adolescents were given daily pairings of ethanol and intraoral sucrose on PD32, PD33, and PD34, whereas adults received similar training on PD70, PD71, and PD72. The second-order conditioning phase (i.e., CS1-CS2 pairings) and the location preference assessment were conducted 24 h after the last conditioning session.
4.2.3. Results
Fig. 4 illustrates ethanol-induced conditioned place aversion, with the magnitude of this avoidance response similar across age groups. These observations were supported by the corresponding statistical analysis. The ANOVAs for absolute and relative time spent in the sandpaper-lined chamber yielded very similar results. Age (absolute time: F1,58 = 8.51, p < 0.01; percent time: F1,58 = 5.06, p < 0.05) and Dose (absolute time: F1,58 = 13.03, p < 0.001; percent time: F1,58 = 8.98, p < 0.005) exerted independent significant main effects. Sex achieved a borderline effect with regard to absolute time, with females having greater scores (231.97 +/− 23.49) than males (197.97 +/− 18.90) (F1,58 = 3.65, p = 0.061). Adult animals spent significantly more time in CS2 than their younger counterparts, regardless of condition. Furthermore, absolute and percent time in CS2 was significantly lower in ethanol-treated subjects than in controls given CS1-water pairings, thus revealing aversive conditioning supported by high-dose ethanol.
Figure 4.
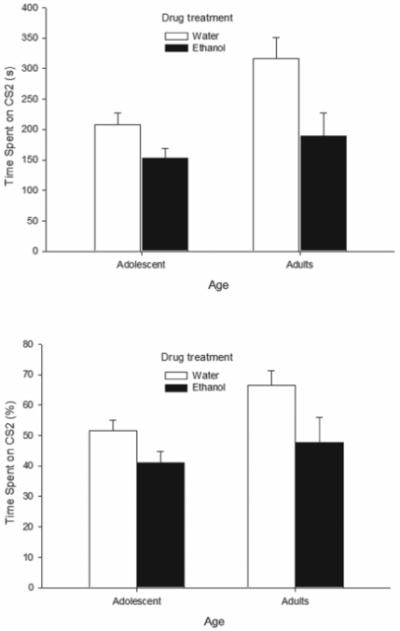
Total time (s) spent in the sandpaper lined chamber (CS2, top panel) and the corresponding percent preference for the chamber (bottom panel) during the 12 min test session after three first-order conditioning sessions. Data are plotted as a function of drug treatment during conditioning (ethanol or water) and age of the subjects (adolescence or adulthood). Data were collapsed across sex (male or female). The sex factor did not exert a significant main effect or significantly interact with the remaining variables. Vertical bars indicate the standard error of the mean.
5. Discussion
The present study assessed the motivational effects of high-dose ethanol using SOC. Prior to comparing adolescent and adult subjects in ethanol-mediated learning, ethanol doses yielding equivalent BECs and BrECs across age were determined. Generally, ethanol elimination rates tend to increase across ontogeny when ethanol is infused intragastrically. For example, Kelly et al. (1987) found an increase from 7.5 mg/dl/h in neonates to 42.2 mg/dl/h by young adulthood following i.g. challenges with ethanol. Moreover, adult Wistar rats appear to metabolize ethanol faster than adolescents (Walker and Ehlers, 2008). Adolescent rats were recently shown to exhibit significantly higher BECs than adult rats 30 min after being administered 2.0 g/kg ethanol i.g. (Pautassi et al., 2008b). Consistent with these previous reports, the present study found greater BECs and BrECs in adolescent rats than in adult Sprague-Dawley rats 32.5 min after administration of 3.0 g/kg ethanol (BEC: 205 vs. 174 mg%; BrEC: 176 vs. 146 mg%; adolescents and adults, respectively; Fig. 1). In contrast, BECs and BrECs did not differ statistically across age when adolescent and adult rats were given 3.0 and 3.25 g/kg ethanol, respectively. The latter doses were employed in the subsequent behavioral experiment.
In Experiment 2, animals were trained in a SOC procedure, with high-dose ethanol as the unconditioned stimulus (US). Ethanol-treated animals exhibited reduced preference for the target CS at test compared with controls. This result, indicative of ethanol-mediated second-order aversive place learning, was substantially similar across age (adolescence or adulthood) and length of training (one, two, or three trials). Important information provided by the present study was that sex did not appear to affect the development of ethanol-induced aversions. Sex effects were limited to females having a slightly, yet significant, greater overall predilection than males for the CS2. This could reflect a greater preference for novelty in males. Some earlier findings have revealed that, under some test circumstances, adolescent males show a greater preference for novelty than adults or adolescent females (Douglas et al, 2003).
There was, however, a suggestion for the adolescents to be less sensitive to ethanol’s aversive effects. Relative (percent) preference for sandpaper was similar in adolescents given two training sessions, regardless of whether they were treated with ethanol or vehicle. Notably, however, the inferential analysis allowed such a statement only with regard to the relative preference for CS2, not in terms of absolute time spent on CS2. Furthermore, if repeated training with ethanol reduced the adolescent’s ability to learn the drug’s aversive effects in the SOC preparation, we should have observed little or no conditioned responding in Experiment 2b, which employed three conditioning trials. This, however, was not the case. In Experiment 2b, adolescents displayed clear ethanol-mediated place avoidance, similar in magnitude to that found in their more mature counterparts. These results indicate some lability in the expression of the hypothetical age-related difference and preclude a definitive statement about this phenomenon. Nonetheless, these results raise the possibility that, under the specific conditions defined by the intermediate level of training, adolescent animals may perhaps display a lessened sensitivity to the aversive effects derived from ethanol administration. Rapid tolerance to ethanol’s aversive effects could be the mechanism underlying this hypothetical phenomenon. Employing ethanol doses similar to those used here, Silveri and Spear (1999) found that the ability to develop rapid tolerance to ethanol-induced hypnosis emerged during adolescence.
Repeated exposure to ethanol prior to testing its reinforcement effects has yielded mixed results, with some studies reporting that it facilitates ethanol-mediated learning (Bienkowsky et al., 1996; Bozarth et al., 1990), others indicating that it weakens the drug’s ability to act as a US (Davies and Parker, 1991), and still others reporting no effect (Pautassi et al., 2005). Given the frequent association of dopamine and ethanol reinforcement, it is interesting that repeated ethanol exposure appears to elevate the availability of basal dopamine in both adolescent and adult rats (Philpot and Kirstein, 2004). The effects of repeated exposure to training with ethanol appear to be quite complex, and more work is needed to understand the conditions in which it affects ethanol reinforcement.
There are other possibilities for explaining the apparent age-related difference observed in Exp. 2a. For instance, it could have been that animals may have had some hangover effects 24 hrs following the last ethanol administration; that is, at the time they were given the CS1-CS2 pairings. If so, the conditioned aversion could have been favored because the CS2 would have been paired with hangover, and perhaps differentially across age. This possibility seems unlikely, though. It would be expected that the magnitude of the hangover, and hence its hypothetical effect upon conditioned aversion, would be greater as a function of repeated treatment. Yet, the magnitude of the aversive learning was quite similar across number of trials at both ages. Another possibility is that secondary aversive conditioning was affected by differences in novelty preference. Under this framework, adolescents may have spent less time in the CS2 chamber than adults just because they were more prone to explore the novel, alternative chamber. However, in Experiment 2a there were no differences in basal sandpaper preference across age in the basic control conditions (i.e., water-treated animals). Furthermore, in general terms preference for novelty seems to be greater in male adolescent than in adult or female adolescents (see Douglas et al., 2003). If novelty affects secondary aversion, some sex difference in the expression of the conditioned aversion would be expected. That was not the case in the present set of experiments.
It is useful to consider the present findings within the framework of Varlinskaya and Spear’s study (2008; also see Vetter-O’Hagen, Varlinskaya & Spear, submitted; Anderson et al., 2008). These authors observed age-related differences in ethanol-mediated CTA. Adult, but not adolescent, rats expressed CTA after 1.0 and 1.5 g/kg ethanol i.p., whereas adolescents showed CTA only at a higher doses (2.0 g/kg). The fact that the present SOC experiment does not report age-related differences, but rather similar aversive conditioning at both ages, might be explained by procedural differences. One obvious difference is the higher dose employed in the present study. Age-related differences in SOC can be postulated to emerge at low or moderate, but not high, ethanol doses. Previous data support this possibility. As stated above, adolescent, but not adult, rats show appetitive SOC when using 0.5 or 2.0 g/kg ethanol (Pautassi et al., 2008b). Notably, however, at the same dose (2.0 g/kg) and age (adolescence; approximately PD32), ethanol exerted appetitive effects in SOC but aversive effects in CTA (Pautassi et al., 2008b; Varlinskaya and Spear, 2008; respectively). This provides further information toward understanding the apparent discrepancy between Varlinskaya & Spear’s (2008) CTA study and the present results. CTA is widely thought to depend on some degree of the animal’s biological “preparedness” for processing the particular confirmation of stimuli comprising the aversive conditioning (Gemberling and Domjan, 1982) and might be better suited for detecting age-related differences in aversive learning than SOC. In particular, expression of a conditioned aversion to visceral or pharmacological distress is generally clearer with a gustatory CS (Varlinskaya & Spear, 2008) than a tactile CS such as CS2 in the present study. Another possibility is that adolescents and adults may differ in their ability to acquire or express higher-order associative learning (e.g., SOC in the present study). This possibility cannot be completely excluded.
With regard to the generality of the SOC results, animals in this procedure are first given ethanol and later exposed to the CS1 (e.g., sucrose). In the present series of experiments, this pairing was conducted 30-45 min post-intubation. This CS-US design could be argued to involve backward conditioning (i.e., CS follows the US; Minnier et al., 2007) rather than simultaneous or delayed conditioning, depending on how one views the timing of the US effect in question. Thus, the sensitivity of SOC for detecting ethanol-induced learning may be related to possible age-related differences in backward conditioning. Although future work will scrutinize this phenomenon, a recent study in our laboratory found conditioned preferences by low-dose ethanol in infant rats through the use of simultaneous conditioning (e.g., Pautassi et al, 2008a). This result is quite consistent with the SOC preferences found by Molina et al. (2006, 2007).
Experiment 2b also indicated that adult animals spent significantly more time in CS2 than their younger counterparts, a result that was not affected by dose or sex. This result is consistent with our earlier work that revealed less preference for the sandpaper-lined floor in adolescents than adults (Pautassi et al., 2008b). We discussed in this paper that rats have “natural preference scales” (Rakover-Atar and Weller, 1997) for tactile and odor stimuli which may vary ontogenetically. It could also be the case that this reflected heightened novelty preference among adolescents relative to adults. Regardless of any differential level of CS2 preference, both age groups expressed clear and significant ethanol-mediated conditioned aversion.
It is notable that more extensive training (i.e., three first-order trials) in Experiment 2b relative to 2a resulted in higher levels of baseline preference for the sandpaper texture. This is an interesting phenomenon, particularly when considering that all groups were equivalent in terms of their experience with sandpaper, suggesting that the animals may have developed a preexposure effect toward the CS1 (i.e., an increase in preference for a given stimulus after repeated nonreinforced exposure; Chotro and Alonso, 1999). Subsequently, during the CS1-CS2 transfer phase, this increased preference may have transferred to the sandpaper texture (CS2). Again, however, this change in overall preference for the target CS did not affect the detection of significant ethanol-mediated aversion in adolescent and adult rats.
The main result of this report is that adolescent and adult rats appear to perceive the effects of high-dose ethanol as similarly aversive when assessed by SOC. Appetitive effects of ethanol in adolescent rats assessed by SOC have been associated with BECs in the range of 50-137 mg% (Pautassi et al., 2008b). This, together with the present study, suggests that, in adolescents assessed with SOC, ethanol’s hedonics may fluctuate from appetitive to aversive as the ethanol dose increases. This switch from appetitive to aversive in this learning context appears to occur when BECs reach approximately 150-200 mg%.
Acknowledgements
This work was supported by NIAAA grants AA011960, AA013098, and AA015992 to NES, NIMH grant MH035219 to NES, NIAAA grants AA012525, AA16887 and AA018036 to LPS, and the Agencia Nacional de Promocion Cientifica y Tecnologica (PICT 05-14024) to JCM. The authors wish to express their gratitude to Teri Tanenhaus, Carlos Martínez, and Judy Sharp.
6. References
- Anderson RI, Varlinskaya EI, Spear LP. Isolation Stress And Ethanol-Induced Conditioned Taste Aversion In Adolescent And Adult Male Rats; Paper presented at the 41st Annual Meeting of the International Society for Developmental Psychobiology; Washington DC. Nov. 12-15.2008. [Google Scholar]
- Anthony JC, Petronis KR. Early-onset drug use and risk of later drug problems. Drug Alcohol Depend. 1995;40:9–15. doi: 10.1016/0376-8716(95)01194-3. [DOI] [PubMed] [Google Scholar]
- Bienkowski P, Kuca P, Piasecki J, Kostowski W. Low dose of ethanol induces conditioned place preference in rats after repeated exposures to ethanol or saline injections. Alcohol Alcohol. 1996;31:547–553. doi: 10.1093/oxfordjournals.alcalc.a008190. [DOI] [PubMed] [Google Scholar]
- Bozarth MA. Evidence for the rewarding effects of ethanol using the conditioned place preference method. Pharmacol. Biochem. Behav. 1990;35:485–487. doi: 10.1016/0091-3057(90)90191-j. [DOI] [PubMed] [Google Scholar]
- Chester JA, de Paula Barrenha G, DeMaria A, Finegan A. Different effects of stress on alcohol drinking behaviour in male and female mice selectively bred for high alcohol preference. Alcohol Alcohol. 2006;41:44–53. doi: 10.1093/alcalc/agh242. [DOI] [PubMed] [Google Scholar]
- Chotro M, Alonso G. Effects of stimulus preexposure on the generalization of conditioned taste aversions in infant rats. Dev. Psychobiol. 1999;35:304–317. doi: 10.1002/(sici)1098-2302(199912)35:4<304::aid-dev5>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Fidler TL, Hill KG. Animal models of alcohol’s motivational effects. Alcohol Res. Health. 2000;24:85–92. [PMC free article] [PubMed] [Google Scholar]
- Cunningham CL, Niehus JS, Noble D. Species difference in sensitivity to ethanol’s hedonic effects. Alcohol. 1993;10:97–102. doi: 10.1016/0741-8329(93)90087-5. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Patel P, Milner L. Spatial location is critical for conditioning place preference with visual but not tactile stimuli. Behav. Neurosci. 2006;120:1115–1132. doi: 10.1037/0735-7044.120.5.1115. [DOI] [PubMed] [Google Scholar]
- Davies BT, Parker LL. Novel versus familiar ethanol: a comparison of aversive and rewarding properties. Alcohol. 1990;7:523–529. doi: 10.1016/0741-8329(90)90043-c. [DOI] [PubMed] [Google Scholar]
- Doremus TL, Brunell SC, Rajendran P, Spear LP. Factors influencing elevated ethanol consumption in adolescent relative to adult rats. Alcohol. Clin. Exp. Res. 2005;29:1796–1808. doi: 10.1097/01.alc.0000183007.65998.aa. [DOI] [PubMed] [Google Scholar]
- Douglas L, Varlinskaya E, Spear L. Novel-object place conditioning in adolescent and adult male and female rats: effects of social isolation. Physiology & Behavior. 2003;80:317–325. doi: 10.1016/j.physbeh.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Grant BF, Dawson DA. Age at onset of alcohol use and its association with DSM-IV alcohol abuse and dependence: results from the National Longitudinal Alcohol Epidemiologic Survey. J. Subst. Abuse. 1997;9:103–110. doi: 10.1016/s0899-3289(97)90009-2. [DOI] [PubMed] [Google Scholar]
- Gemberling GA, Domjan M. Selective associations in one-day-old rats: taste-toxicosis and texture-shock aversion learning. J. Comp. Physiol. Psychol. 1982;96:105–113. doi: 10.1037/h0077855. [DOI] [PubMed] [Google Scholar]
- Greenfield SF. Women and alcohol use disorders. Harv. Rev. Psychiatry. 2002;10:76–85. doi: 10.1080/10673220216212. [DOI] [PubMed] [Google Scholar]
- Holson RR, Pearce B. Principles and pitfalls in the analysis of prenatal treatment effects in multiparous species. Neurotoxicol. Teratol. 1992;14:221–228. doi: 10.1016/0892-0362(92)90020-b. [DOI] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM. Monitoring the Future Study: National Survey Results on Drug Use, 1975-2006: Vol 1, Secondary School Students. National Institute on Drug Abuse; Bethedesa MD: 2007. NIH Pub. No. 07-6206. [Google Scholar]
- Kelly SJ, Bonthius DJ, West JR. Developmental changes in alcohol pharmacokinetics in rats. Alcohol. Clin. Exp. Res. 1987;11:281–286. doi: 10.1111/j.1530-0277.1987.tb01308.x. [DOI] [PubMed] [Google Scholar]
- Kiefer S. Alcohol, palatability, and taste reactivity. Neurosci. Biobehav. Rev. 1995;19:133–141. doi: 10.1016/0149-7634(94)00027-x. [DOI] [PubMed] [Google Scholar]
- Kiefer SW, Hill KG, Coonfield DL, Ferraro FM., 3rd Ethanol familiarity and naltrexone treatment affect ethanol responses in rats. Alcohol. 2005;37:167–172. doi: 10.1016/j.alcohol.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Minnier E, Misanin JR, Hinderliter CF. Age and interstimulus interval in forward and backward long-trace taste-aversion conditioning. Percept. Mot. Skills. 2007;105:1223–1226. doi: 10.2466/pms.105.4.1223-1226. [DOI] [PubMed] [Google Scholar]
- Molina JC, Ponce LF, Truxell E, Spear NE. Infantile sensitivity to ethanol’s motivational effects: ethanol reinforcement during the third postnatal week. Alcohol. Clin. Exp. Res. 2006;30:1506–1519. doi: 10.1111/j.1530-0277.2006.00182.x. [DOI] [PubMed] [Google Scholar]
- Molina JC, Pautassi RM, Truxell E, Spear N. Differential motivational properties of ethanol during early ontogeny as a function of dose and postadministration time. Alcohol. 2007;41:41–55. doi: 10.1016/j.alcohol.2007.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council . Guide for the Care and Use of Laboratory Animals. National Academy Press; Washington DC: 1996. [Google Scholar]
- Pautassi R, Ponce L, Molina J. Effects of early exposure to ethanol on subsequent learning mediated by the unconditional attributes of the drug. Revista Latinoamericana de Psicologia. 2005;37:149–166. [Google Scholar]
- Pautassi RM, Molina JC, Spear N. Infant rats exhibit aversive learning mediated by ethanol’s orosensory effects but are positively reinforced by ethanol’s post-ingestive effects. Pharmacol. Biochem. Behav. 2008a;88:393–402. doi: 10.1016/j.pbb.2007.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pautassi RM, Myers M, Spear LP, Molina JC, Spear NE. Adolescent but not adult rats exhibit ethanol-mediated appetitive second-order conditioning. Alcohol. Clin. Exp. Res. 2008b;32:2016–2027. doi: 10.1111/j.1530-0277.2008.00789.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen W, Skrondal A. Alcohol consumption debut: predictors and consequences. J. Stud. Alcohol. 1998;59:32–42. doi: 10.15288/jsa.1998.59.32. [DOI] [PubMed] [Google Scholar]
- Philpot R, Kirstein C. Developmental differences in the accumbal dopaminergic response to repeated ethanol exposure. Ann. N. Y. Acad. Sci. 2004;1021:422–426. doi: 10.1196/annals.1308.056. [DOI] [PubMed] [Google Scholar]
- Philpot RM, Badanich KA, Kirstein CL. Place conditioning: age-related changes in the rewarding and aversive effects of alcohol. Alcohol. Clin. Exp. Res. 2003;27:593–599. doi: 10.1097/01.ALC.0000060530.71596.D1. [DOI] [PubMed] [Google Scholar]
- Rakover-Atar S, Weller A. The influence of natural preference for tactile stimuli on appetitive learning in rat pups. Dev. Psychobiol. 1997;30:29–39. doi: 10.1002/(sici)1098-2302(199701)30:1<29::aid-dev3>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Ristuccia RC, Spear LP. Sensitivity and tolerance to autonomic effects of ethanol in adolescent and adult rats during repeated vapor inhalation sessions. Alcohol. Clin. Exp. Res. 2005;29:1809–1820. doi: 10.1097/01.alc.0000183010.72764.cd. [DOI] [PubMed] [Google Scholar]
- Samson HH, Chappell A. Dopaminergic involvement in medial prefrontal cortex and core of the nucleus accumbens in the regulation of ethanol self-administration: a dual-site microinjection study in the rat. Physiol. Behav. 2003;79:581–590. doi: 10.1016/s0031-9384(03)00126-4. [DOI] [PubMed] [Google Scholar]
- Schramm-Sapyta NL, Morris RW, Kuhn CM. Adolescent rats are protected from the conditioned aversive properties of cocaine and lithium chloride. Pharmacol. Biochem. Behav. 2006;84:344–352. doi: 10.1016/j.pbb.2006.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silveri MM, Spear LP. Ontogeny of rapid tolerance to the hypnotic effects of ethanol. Alcohol. Clin. Exp. Res. 1999;23:1180–1184. [PubMed] [Google Scholar]
- Silveri MM, Spear LP. Ontogeny of ethanol elimination and ethanol-induced hypothermia. Alcohol. 2000;20:45–53. doi: 10.1016/s0741-8329(99)00055-5. [DOI] [PubMed] [Google Scholar]
- Silveri MM, Spear LP. Acute, rapid, and chronic tolerance during ontogeny: Observations when equating ethanol perturbation across age. Alcohol. Clin. Exp. Res. 2001;25:1301–1308. [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci. Biobehav. Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Spear LP. Adolescence and the trajectory of alcohol use: introduction to part VI. Ann. N. Y. Acad. Sci. 2004;1021:202–205. doi: 10.1196/annals.1308.025. [DOI] [PubMed] [Google Scholar]
- Swartzwelder HS, Richardson RC, Markwiese-Foerch B, Wilson WA, Little PJ. Developmental differences in the acquisition of tolerance to ethanol. 1998;15:311–314. doi: 10.1016/s0741-8329(97)00135-3. [DOI] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Acute effects of ethanol on social behavior of adolescent and adult rats: role of familiarity of the test situation. Alcohol. Clin. Exp. Res. 2002;26:1502–1511. doi: 10.1097/01.ALC.0000034033.95701.E3. [DOI] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Attenuated aversive effects of ethanol among adolescent rats are diminished further in adolescent males by the presence of a social partner. Alcohol. Clin. Exp. Res. 2008;32(Suppl 1):94A. [Google Scholar]
- Walker BM, Ehlers CL. Age-related differences in the blood alcohol levels of Wistar rats. Pharmacol. Biochem. Behav. 2008;91:560–565. doi: 10.1016/j.pbb.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White AM, Truesdale MC, Bae JG, Ahmad S, Wilson WA, Best PJ, Swartzwelder HS. Differential effects of ethanol on motor coordination in adolescent and adult rats. Pharmacol. Biochem. Behav. 2002;73:673–677. doi: 10.1016/s0091-3057(02)00860-2. [DOI] [PubMed] [Google Scholar]


