Abstract
Background
Ethanol consumption is considerably elevated during adolescence. Attractiveness of alcohol for humans during the adolescent developmental period is based, in part, on its ability to induce social facilitation—a facilitation of social interactions not only evident in human adolescents but also in adolescent rats. Endogenous opioid systems are among the multiple neural systems implicated in the behavioral and reinforcing effects of ethanol and may play a substantial role in modulating stimulatory effects of low doses of ethanol on social behavior during adolescence. This possibility was explored in the present study through the use of an animal model of peer-directed social behavior.
Methods
Sprague–Dawley rats were challenged early in adolescence with saline or ethanol intraperitoneally (i.p.), placed into an individual holding cage for 30 minutes, and then tested in a familiar situation with a nonmanipulated partner of the same age and sex. In Experiment 1, each test subject was injected subcutaneously with one of the three doses of a nonselective opioid antagonist naloxone (0, 0.05, and 0.1 mg/kg), 5 minutes prior to the social interaction test and 25 minutes following challenge with saline or ethanol (0.5 g/kg), whereas in Experiment 2 animals were challenged with one of the six doses of ethanol (0, 0.25, 0.5, 0.75, 1.0, and 1.25 g/kg) prior to injection of either saline or naloxone (0.05 mg/kg). In Experiment 3, animals were pretreated i.p. with the selective μ-opioid antagonist CTOP (0, 0.01, 0.025, 0.05, and 0.1 mg/kg) 30 minutes prior to challenge with saline or ethanol (0.5 g/kg).
Results
Low doses of ethanol (0.5 and 0.75 g/kg) produced social facilitation, as indexed by significant increases in play fighting and social investigation. Both doses of naloxone and the three highest doses of CTOP blocked the stimulatory effects of ethanol on play fighting but not on social investigation. These effects were not associated with alterations in ethanol pharmacokinetic properties or with shifts in the biphasic ethanol dose–response curve.
Conclusions
Ethanol-induced facilitation of social play behavior seen in adolescent animals is mediated in part through ethanol-induced release of endogenous ligands for the μ-opioid receptor or an ethanol-associated enhancement of sensitivity of these receptors for their endogenous ligands.
Keywords: Adolescence, Ethanol, Rat, Social Behavior, Opioid Receptors
Ethanol consumption is considerably elevated during adolescence. According to the 2007 Monitoring the Future National Survey, 8% of eighth graders, 19% of tenth graders, and 30% of twelfth graders were reported being drunk within the past 30 days, with approximately 11% of eighth graders, 22% of tenth graders, and 25% of high school seniors reported to show binge pattern of drinking (i.e., consumption of five or more drinks per occasion) in the last 2 weeks (Johnston et al., 2007). Given the importance of interactions with peers during adolescence (see Spear, 2000 for references and review), it is not surprising that human adolescents often drink with peers, with heavy drinkers, and problem drinkers expecting alcohol to make them more sociable and relaxed (Brown et al., 1987). Therefore, attractiveness of ethanol for human adolescents seems to be based, in part, on its ability to facilitate social interactions (Beck and Treiman, 1996; Beck et al.,1993).
Adolescence is a developmental transition during which an immature and dependent youth is gradually transformed into a mature and relatively independent adult. A similar developmental transition from immaturity toward maturity can be identified across different mammalian species. In humans, adolescence is commonly defined as the second decade of life (Petersen et al., 1996), with females generally maturing more rapidly than males (e.g., Buchanan et al., 1992). A conservative interval during which rats of both sexes and most breeding stock exhibit adolescent-typical neurobehavioral features is the range between postnatal day (P) 28 and P42 (Spear, 2000), with this age range being sometimes subdivided into three developmental phases, namely, early (around P28), mid (around P35), and late (around P42) adolescence (Adriani et al., 2002). Human adolescents and adolescents of other mammalian species demonstrate substantial commonalities in developmental history, age-typical behavioral predispositions, neural characteristics, and changing hormonal milieu (see Spear, 2007, for review). These across-species similarities provide sufficient face and construct validity to support elaboration of animal models of adolescence.
The use of a model of adolescence in the rat has demonstrated that the propensity for elevated ethanol intake and ethanol-induced social facilitation is not restricted to human adolescents. Adolescent rats likewise demonstrated greater voluntary ethanol intake than their adult counterparts under various circumstances (Brunell and Spear, 2005; Doremus et al., 2005; Lancaster et al., 1996; Vetter et al., 2007; although see also Yoshimoto et al., 2002). Moreover, acute exposure to relatively low experimental doses of ethanol (0.5 to 0.75 g/kg) administered intraperioneally (i.p.) has been shown to facilitate social interactions in adolescent rats tested under familiar, nonanxiogenic circumstances (Varlinskaya and Spear, 2002, 2006). These doses produce blood ethanol concentrations (BECs) from approximately 40 to 80 mg/dl—BECs that are within the moderate to heavy consumption range in humans (see Eckardt et al., 1998, for references and review). Ethanol-induced social facilitation is seen in both male and female adolescent rats and is predominantly characterized by an increase in play fighting—an adolescent-characteristic form of social interactions in rats (Vanderschuren et al., 1997). This social facilitation is most pronounced early in adolescence and declines gradually across the adolescent period to be no longer evident in late adolescents and adults (Varlinskaya and Spear, 2002, 2006). Adolescents develop tolerance to the social consequences of ethanol following repeated exposure, with ethanol-induced social facilitation emerging at higher ethanol doses in these tolerant animals (Varlinskaya and Spear, 2007).
Although sex differences in ethanol consumption, pharmacokinetics, and some ethanol-induced effects have been reported for adult rats (Blanchard and Glick, 1995; Blanchard et al., 1993; Brasser and Spear, 2002; Cailhol and Mormède, 2001; Doremus et al, 2005; Lancaster et al., 1996; Silveri and Spear, 1999; Varlinskaya and Spear, 2004; Webb et al., 2002), in sufficiently powered experiments, we have not observed significant sex differences in responsiveness to ethanol-induced social facilitation among adolescent animals (Varlinskaya and Spear, 2002, 2006). Likewise, although sex differences in social interactions with same sex peers have been reported in adult rats, as well as in some experiments with adolescent rats (Johnston and File, 1991; Pellis and Pellis, 1990; Pellis et al., 1997; Thor and Holloway, 1986), sex differences in baseline social activity have not emerged in group-housed adolescents in our studies (Varlinskaya and Spear, 2008). Variations across studies in the incidence of sex differences in social behavior may, in part, be strain-dependent. For instance, sex differences in play fighting have been observed in adolescent Long–Evans hooded but not Sprague–Dawley rats (Pellis et al., 1997). Given the reliable ethanol-induced social facilitation seen under our testing circumstances in adolescent animals regardless of sex (Varlinskaya and Spear, 2002, 2006), both male and female adolescent rats were used in the present study to explore the role of endogenous activity at opioid receptors in ethanol-induced social facilitation.
Although multiple neurochemical brain systems have been implicated in the behavioral and reinforcing effects of ethanol (see Eckardt et al., 1998, for references and review), the current project focused on the role of endogenous opioid systems, as activation of μ-opioid receptors contributes to the positive reinforcing and stimulatory effects of ethanol. Ethanol has been shown to induce the release of endogenous ligands (including β-endorphin) for these receptors in the hypothalamus, nucleus accumbens, and ventral tegmental area (Boyadjieva and Sarkar, 1997; De Waele and Gianoulakis, 1993; De Waele et al., 1992; Olive et al., 2001; Rasmussen et al., 1998). The consequent interaction of these endogenous ligands with μ-opioid receptors located within the mesolimbic reward system is viewed as a central event underlying the euphoric, positively reinforcing effects of ethanol (Froehlich and Li, 1994; Gianoulakis, 1996; Herz, 1997; Oswald and Wand, 2004). Likewise, μ-opioid agonists (Beatty and Costello, 1982; Niesink and Van Ree, 1989; Vanderschuren et al., 1995) join ethanol, alpha-2 adrenoreceptor antagonists (Siviy and Baliko, 2000; Siviy et al., 1994), indirect cannabinoid agonists (Trezza and Vanderschuren, 2008a,b), and N-methyl-D-aspartate (NMDA) antagonists (Siviy et al., 1995) in being among the few pharmacological manipulations effective in precipitating play fighting in young adolescent animals. Together, such data support the hypothesis that the facilitation of play fighting in adolescent animals may be mediated, in part, via ethanol-induced release of endogenous ligands for the μ-opioid receptor or ethanol-associated enhancement of sensitivity of μ-opioid receptors to their endogenous ligands.
Therefore, the present study used an animal model of peer-directed social behavior to investigate the possible roles of endogenous opioid systems in the facilitation of social interactions by low doses of ethanol during early adolescence. Specifically, Experiment 1 assessed the effects of a nonselective opioid antagonist naloxone on ethanol-induced social facilitation in rats challenged with 0.5 g/kg ethanol, whereas Experiment 2 examined whether naloxone-induced blockade of the stimulatory effects of ethanol on social behavior reflected an overall shift in the dose–response curve for ethanol or pharmacokinetic factors. Finally, Experiment 3 investigated whether selective blockade of μ-opioid receptors was effective in attenuating ethanol-induced social facilitation during early adolescence.
General Methods
Subjects
Sprague–Dawley rats bred and reared in our colony at Binghamton University were used in these experiments. All animals were housed in a temperature-controlled (22°C) vivarium maintained on a 14-hour/10-hour light/dark cycle (lights on at 7:00 am) with ad libitum access to food (Purina Rat Chow, Lowell, MA) and water. Litters were culled to 10 pups (five males and five females) within 24 hours after birth and were housed until weaning with their mothers in standard opaque maternity cages with pine shavings as bedding material. On P21, rats were weaned and housed with same sex littermates. In all respects, maintenance and treatment of the animals were in accord with guidelines for animal care established by the National Institutes of Health, using protocols approved by the Binghamton University Institutional Animal Care and Use Committee.
Animals were tested on P28. To avoid the possible confounding of litter with treatment effects (Holson and Pearce, 1992), animals were assigned semirandomly to the experimental groups, with the constraint that no more than one subject per sex from a given litter was assigned to a particular treatment group. The order of testing was counterbalanced for all treatment groups. Equal numbers of males and females were included into each group to allow for maximal utilization of generated litters, although assessment of sex differences was not a focus of these studies, given prior evidence that sex is not a significant variable in the facilitation of social behavior by ethanol under these test circumstances (e.g., Varlinskaya and Spear, 2002, 2006).
Ethanol Exposure and Drug Challenge
As in our earlier studies that have demonstrated reliable ethanol-induced social facilitation in adolescent animals after i.p. administration (Varlinskaya and Spear, 2002, 2006), ethanol was administered i.p. as a 12.6% (v/v) solution in physiological saline, a relatively low-concentration that induces little (if any) tissue irritation at the site of injection. Dose of ethanol was varied by altering volume rather than concentration to avoid concentration-induced differences in ethanol absorption rate (Linakis and Cunningham, 1979). Control animals were injected i.p. with isotonic saline in a volume equal to the volume of the highest dose of ethanol. Solutions were administered at room temperature. The i.p. route of ethanol administration was employed in this study, as well as in our earlier studies, because it produces little variability in blood ethanol levels and has been the most commonly used route of administration in neuropharmacological studies of acute ethanol effects.
A nonselective opioid antagonist, naloxone (Sigma, Atlanta, GA), was dissolved in physiological saline and injected subcutaneously (s.c.) in a volume of 2 ml/kg. This competitive opioid antagonist was administered five minutes prior to the social interaction test and 25 minutes after ethanol challenge, given its extremely fast onset and short duration of action, as well as its rapid penetration and removal from the brain (Brown et al., 1983; Cohen and Coffman, 1980; Ngai et al., 1976). A cyclic octapeptide analogue of somatostatin (CTOP, Sigma), a highly potent and selective antagonist for μ-opioid receptors (Pelton et al., 1986), was dissolved in saline and injected i.p. in a volume of 2 ml/kg. Given the long duration of CTOP action and some findings suggesting a noncompetitive nature of its antagonist action (Kramer et al., 1989; Walker, 2006), this compound was administered 60 minutes prior to the social interaction test and 30 minutes prior to ethanol challenge. All solutions were administered at room temperature.
Testing Procedure
One day before testing (P27), each experimental animal was placed individually into the testing apparatus for 30 minutes to familiarize it with the experimental situation, as ethanol-induced social facilitation was seen under familiar, nonanxiogenic test circumstances (Varlinskaya and Spear, 2002). Each test apparatus (30 × 20 × 20 cm) composed of Plexiglas (Binghamton Plate Glass, Binghamton, NY) and contained clean pine shavings. The apparatus was divided into two equally sized compartments by a clear Plexiglas partition that contained an aperture (7 × 5 cm) to allow movement of the animals between compartments (Varlinskaya et al., 1999, 2001).
On test day (P28), experimental animals were injected with ethanol or saline, marked by a vertical line on the back, and placed individually into a holding cage for 30 minutes before testing. This pretest social deprivation in a novel environment was a standard procedure used to increase baseline levels of social behavior (see File, 1993) from which both stimulatory and inhibitory effects of ethanol on social interactions may be readily detected (Varlinskaya and Spear, 2002, 2006). At the onset of testing, each experimental animal was placed into the testing apparatus and immediately exposed to a non-drug-treated peer of the same sex and age. Social behavior (Pellis and McKenna, 1992, 1995; Varlinskaya et al., 1999) and social motivation (Varlinskaya et al., 1999) can be dramatically modified by social activity of the partner, with high socially active partners often precipitating high social activity in the animals with which they are paired. Consequently, low socially active partners were used in this as well as in our previous studies to increase sensitivity to ethanol-induced social facilitation. Given that both familiarity and prior social isolation enhanced social behavior at this age (Varlinskaya and Spear, 2008), low levels of social activity were fostered in the test partners by avoiding any social isolation prior to testing and by not familiarizing these partners to the test apparatus or the experimental animals with which they were paired for testing. Weight differences between test subjects and their partners were limited to at most a 5 g difference, with test subjects always being heavier than their partners. During the 10-minute test session, the behavior of the animals was recorded by a video camera (Panasonic model AF-X8, Secaucus, NJ), with real time being directly recorded onto the videotape for later scoring (Easy Reader II Recorder; Telcom Research TCG 550, Burlington, Ontario, Canada). After each test, the apparatus was wiped with 3% peroxide hydrochloride and the shavings were replaced with fresh ones.
Behavioral Measures
The frequency of a number of social activities of each test subject was analyzed from the video recordings (Meaney and Stewart, 1981; Thor and Holloway, 1984; Vanderschuren et al., 1997; Varlinskaya and Spear, 2002, 2006; Varlinskaya et al., 1999, 2001). Social investigation was defined as the sniffing of any part of the body of the partner. Play fighting was analyzed by scoring the frequencies of the following behavioral acts and postures: pouncing or playful nape attack (the experimental subject lunges at the partner with its fore-paws extended outward), following and chasing (the experimental animal rapidly pursues the partner), and pinning (the experimental subject stands over the exposed ventral area of the partner, pressing it against the floor). In addition to the assessment of social behaviors, total number of crossovers (movements between compartments) demonstrated by each experimental subject was determined and used as an index of general locomotor activity in this social context (Varlinskaya and Spear, 2002).
Blood Ethanol Determination
For analysis of BEC, trunk blood samples were collected immediately after behavioral testing using heparinized tubes (Experiments 2 and 3). Blood samples were then rapidly frozen and maintained at −80°C. Samples were assessed for BECs via headspace gas chromatography using a Hewlett Packard (HP) 5890 series II Gas Chromatograph (Wilmington, DE). At the time of assay, blood samples were thawed and 25 μl aliquots were placed in airtight vials. Vials were placed in a HP 7694E Auto-Sampler which heated each individual vial for 8 minutes and then extracted and injected a 1.0 ml sample of the gas headspace into the chromatograph. Ethanol concentrations in each sample were determined using HP Chemstation software which compares the peak area under the curve in each sample with those of standard curves derived from reference standard solutions.
Data Analysis
Behavioral data were scored from the videotape records by two observers without knowledge of the drug treatment condition of any animal. Agreement between observers scoring the same videotape was in excess of 90% for each measure of social behavior.
Frequencies of social investigation and play fighting, overall number of crossovers, and BECs were examined by using separate between group ANOVAs. Tukey/Kramer tests were used to explore significant main effects and interactions. A significance level of p < 0.05 was used for all analyses and comparisons. Given that these studies were not designed to focus on assessments of sex differences and hence were not powered for these assessments, sex was excluded as a factor in the ANOVAs of the data.
Experiment 1: Effects of a Nonselective Opioid Antagonist Naloxone on Ethanol-Induced Facilitation of Play Fighting and Social Investigation of Adolescent Rats
Experiment 1 was designed to assess whether endogenous opioid systems play a role in mediating adolescent-characteristic social facilitation induced by ethanol. This social facilitation was evident under familiar test circumstances following i.p. administration of low doses of ethanol, with both early (P28) and mid (P35) adolescents responding to 0.5 g/kg ethanol with increases in social investigation and play fighting (Varlinskaya and Spear, 2002, 2006). To the extent that this social facilitation is related to the activation of the endogenous opioid system by ethanol, a nonselective opioid antagonist should attenuate the stimulatory effects of ethanol on social behavior of adolescent animals. This possibility was tested by assessing the effectiveness of low doses of the nonselective opioid antagonist, naloxone, for blocking ethanol-induced social facilitation.
Methods
A total of 60 animals served as experimental subjects and 60 served as partners in this experiment. Twenty four hours after preexposure to the testing apparatus (see General Methods), P28 experimental subjects were challenged either with 0 (saline) or 0.5 g/kg of ethanol and placed into individual holding cages. Five minutes prior to the social interaction test and 25 minutes after ethanol challenge, each animal was injected s.c. with one of the three doses of naloxone (0, 0.05, and 0.1 mg/kg). Therefore, the design of this experiment was a 2 (ethanol challenge dose) × 3 (naloxone dose) factorial, with 10 animals placed into each of the six experimental conditions.
Results
Similarly to our previous findings, ethanol-induced social facilitation was evident in adolescent rats tested in a familiar environment. As seen in Fig. 1 (left and center), the 0.5 g/kg dose of ethanol enhanced social investigation [main effect of ethanol dose, F(1,54) = 20.11, p < 0.0001] and play fighting [ethanol challenge dose × naloxone dose interaction, F(2,54) = 4.27, p < 0.05]. Ethanol-induced increases in social interactions did not reflect nonspecific activating effects of ethanol, given that overall locomotor activity in the social context indexed by the total number of crossovers between compartments, was not affected by ethanol (Fig. 1, right).
Fig. 1.
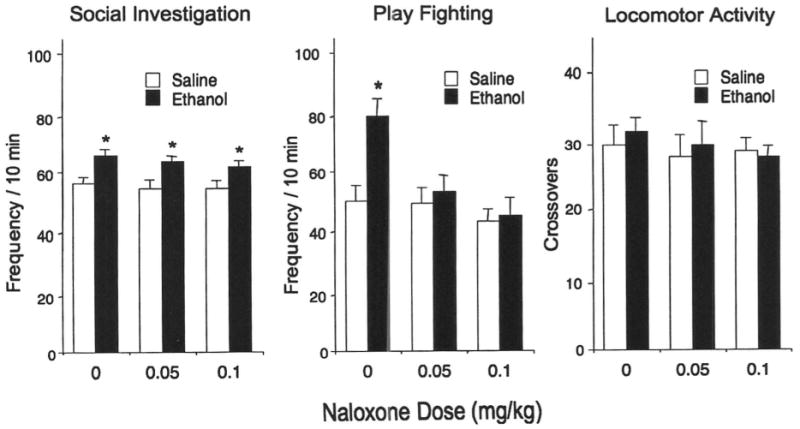
Social investigation (left), play fighting (middle), and overall locomotor activity (right) for adolescent rats challenged either with saline or 0.5 g/kg ethanol and injected with one of the three doses of naloxone in Experiment 1. Significant changes induced by ethanol challenge are marked by asterisks, p < 0.05.
Both doses of naloxone blocked the stimulatory effect of ethanol on play fighting, whereas pretest administration of naloxone had no effects on ethanol-induced facilitation of social investigation. Social behavior and locomotor activity of saline-challenged controls were not affected by naloxone.
The results of Experiment 1 confirmed previous observations (Varlinskaya and Spear, 2006) that social behavior was facilitated by a low dose of ethanol among early adolescents tested in a familiar context. This ethanol-induced facilitation of play fighting (an adolescent-characteristic form of social behavior) was attenuated by naloxone. However, ethanol-related increases in social investigation (a more adult-like form of social interactions) were not affected by this nonselective opioid antagonist.
Experiment 2: Effects of Naloxone on Social Behavior of Adolescent Rats Challenged With Different Doses of Ethanol
Effects of ethanol on social behavior of early adolescent rats were dose-dependent, with low doses (0.5 to 0.75 g/kg) producing social facilitation of play fighting, moderate doses (1.0 g/kg) having no effects, and higher doses (1.25 g/kg and higher) eliciting social inhibition (Varlinskaya and Spear, 2006). Therefore, the lack of ethanol-induced facilitation of play fighting following naloxone administration in Experiment 1 may be related, at least partly, to a naloxone-induced shift of the dose-response curve for ethanol. Experiment 2 was designed to investigate whether naloxone indeed blocked the stimulatory effects of ethanol on play fighting by assessing its effects on social behavior of P28 adolescent rats challenged with ethanol, across a range of doses that captures both stimulatory and inhibitory effects of ethanol on social interactions. An additional aim of this experiment was to assess possible effects of naloxone on BECs.
Methods
A total of 120 animals served as experimental subjects, and 120 served as test partners in Experiment 2. Following preexposure to the testing apparatus on P27, P28 animals were challenged with one of the six doses of ethanol (0, 0.25, 0.5, 0.75, 1.0, and 1.25 g/kg) and placed into individual holding cages. Five minutes prior to the social interaction test and 25 minutes after ethanol challenge, each animal was injected s.c. with either saline or 0.05 mg/kg of naloxone. Trunk blood samples were collected immediately after behavioral testing for BEC analyses. Therefore, the design of Experiment 2 was a 6 (ethanol challenge dose) × 2 (naloxone exposure) factorial, with 10 animals placed into each of the 12 experimental conditions.
Results
As seen in Fig. 2 (left), the facilitation of social investigation by 0.5 g/kg of ethanol was unaffected by naloxone [main effect of ethanol challenge dose, F(5,108) = 15.77, p < 0.0001]. The stimulatory effects of 0.5 and 0.75 g/kg of ethanol on play fighting evident in saline-treated animals were blocked by naloxone (see Fig. 2, right), whereas this nonselective opioid antagonist did not alter the inhibition of this adolescent characteristic form of social interactions seen following a higher (1.25 g/kg) dose of ethanol [ethanol challenge dose × naloxone exposure interaction. F(5,108) = 3.07, p < 0.05]. There was no effect of naloxone exposure on overall locomotor activity in the social context (Fig. 3, left), with activity in this context significantly suppressed by 1.25 g/kg of ethanol [main effect of ethanol dose, F(5,108) = 2.55, p < 0.05]. BECs increased in a dose-dependent fashion [main effect of ethanol challenge dose, F(4,90) = 662.52, p < 0.0001] and did not differ as a function of naloxone treatment (see Fig. 3, right).
Fig. 2.
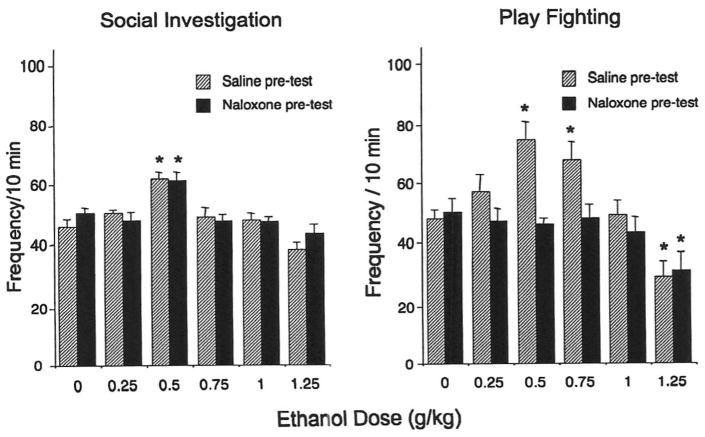
Social investigation (left) and play fighting (right) for adolescent rats challenged with one of the five doses of ethanol or saline and injected either with saline or naloxone pretest in Experiment 2. Significant changes induced by ethanol challenge are marked by asterisks, p < 0.05.
Fig. 3.
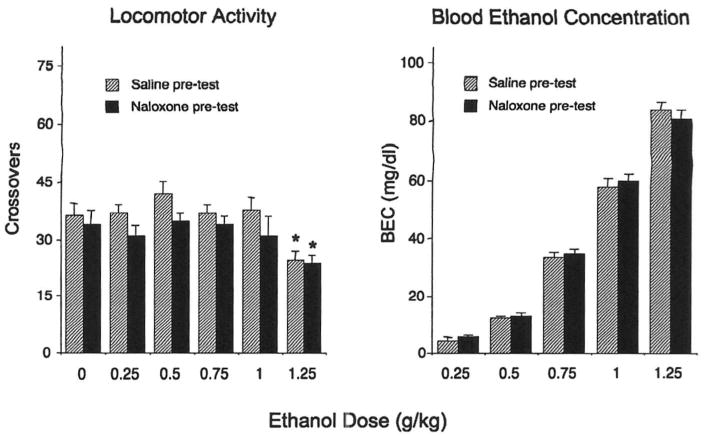
Locomotor activity (left) for adolescent rats challenged with one of the five doses of ethanol or saline and injected either with saline or naloxone pretest in Experiment 2. Significant changes induced by ethanol challenge are marked by asterisks, p < 0.05. Blood ethanol concentrations (right) are presented for animals challenged with ethanol.
As in Experiment 1, the stimulatory effects of ethanol on play fighting but not on social investigation were attenuated by naloxone. Social and locomotor inhibition evident at the highest dose of ethanol was not affected by naloxone exposure. These results demonstrated that the naloxone-related blockade of the stimulatory effects of ethanol on play fighting was not associated with a shift in the overall biphasic dose–response curve for ethanol or with alterations in ethanol's pharmacokinetic properties.
Experiment 3: Effects of the Selective μ-Opioid Antagonist CTOP on Ethanol-induced Facilitation of Play Fighting and Social Investigation in Adolescent Rats
The attenuation of ethanol-induced facilitation of play fighting by naloxone seen in Experiments 1 and 2 suggested that endogenous activity at opioid receptors might play a role in mediation of the stimulatory effects of ethanol on this adolescent characteristic form of social interactions. Experiment 3 was designed to investigate whether the stimulatory effect of ethanol on play fighting was related to ethanol-induced activation of the endogenous μ-opioid system by assessing the effectiveness of the selective μ-opioid antagonist CTOP in blocking ethanol-induced social facilitation.
Methods
A total of 100 animals served as experimental subjects and 100 served as partners for Experiment 3. On P28, experimental animals were pretreated i.p. with one of the five doses of the selective μ antagonist CTOP (0, 0.01, 0.025, 0.05, and 0.1 mg/kg) and returned to their home cage for 30 minutes (Kim et al., 2000; Pastor and Aragon, 2006). Then each animal was injected i.p. with either saline (0 g/kg ethanol) or 0.5 g/kg of ethanol and placed individually into a holding cage for a 30-minute period prior to behavioral testing. Therefore, the design of this experiment was a 2 (ethanol challenge dose) × 5 (CTOP dose) factorial, with 10 animals placed into each of the 10 experimental conditions. Trunk blood samples were collected immediately after behavioral testing for BEC analyses.
Results
Reminiscent of Experiments 1 and 2, ethanol-induced facilitation of social investigation [main effect of ethanol challenge dose, F(l,90) = 115.88, p < 0.0001] was not affected by CTOP pretreatment (see Fig. 4, left). In contrast, stimulatory effects of ethanol on play fighting, evident in adolescents exposed to saline prior to ethanol challenge, were attenuated by the selective μ-opioid antagonist CTOP [ethanol challenge dose × CTOP dose interaction, F(4,90) = 4.37, p < 0.01—see Fig. 4, right]. Overall locomotor activity in the social context, indexed in terms of crossovers between compartments, was not affected either by ethanol, F(1,90) = 0.02, p > 0.05, or CTOP, F(4,90) = 0.27, p > 0.05 (see Fig. 5, left). Similarly, BECs did not differ as a function of CTOP dose, F(4,45) = 0.06, p > 0.05 (Fig. 5, right).
Fig. 4.
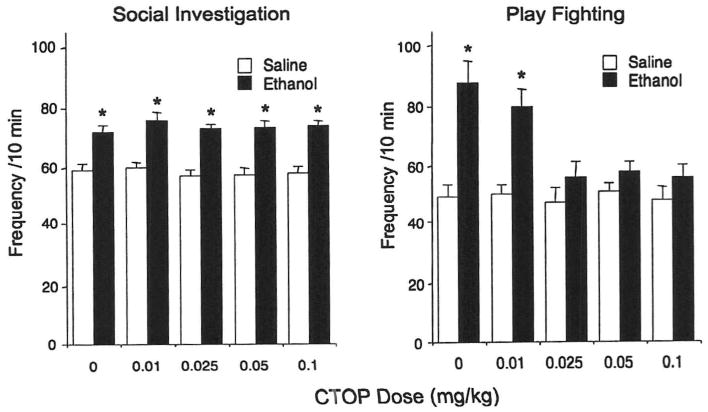
Social investigation (left) and play fighting (right) for adolescent rats challenged with either ethanol or saline and pretreated with one of the five doses of CTOP in Experiment 3. Significant changes induced by ethanol challenge are marked by asterisks, p < 0.05.
Fig. 5.
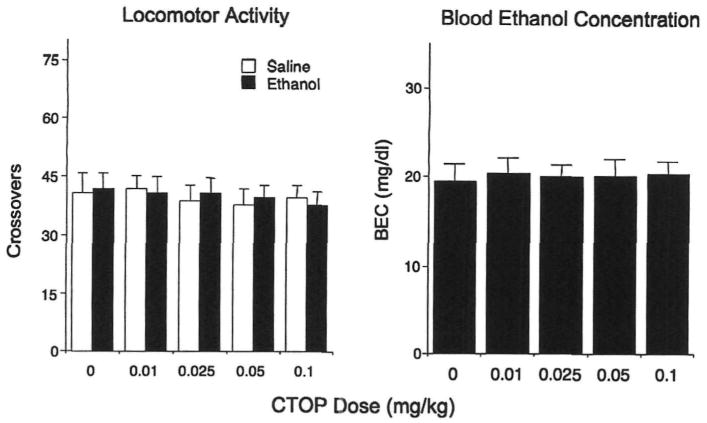
Locomotor activity (left) for adolescent rats challenged with either ethanol or saline and pretreated with one of the five doses of CTOP in Experiment 3. Blood ethanol concentrations (right) are presented for animals challenged with ethanol.
The results of Experiment 3 suggest that ethanol-induced facilitation of play fighting seen in adolescent animals was related to the activation of the endogenous μ-opioid system, as the selective μ antagonist, CTOP, reliably attenuated this social facilitation while producing no effects on social investigation, overall locomotor activity, or BECs.
Discussion
The results of the present experimental series were in agreement with our previous observations. In a familiar, nonstressful environment, low doses of ethanol enhanced social investigation and play fighting in early adolescent animals tested on P28. This low dose stimulation was specific to social behavior, given that ethanol did not affect locomotor activity, as indexed by overall number of crossovers between compartments. Similar to our earlier findings (Varlinskaya and Spear, 2004, 2006), play fighting was more sensitive to the stimulatory effects of ethanol than social investigation, with play fighting being notably enhanced by 0.5 and 0.75 g/kg of ethanol, whereas social investigation was slightly but significantly increased only after the dose of 0.5 g/kg.
The facilitation of play fighting by low doses of ethanol in early adolescent rats was attenuated by the nonselective opioid antagonist naloxone (Experiments 1 and 2) as well as by the selective μ antagonist CTOP (Experiment 3). The attenuation of ethanol-induced facilitation of social behavior was specific to play fighting and was not evident in terms of social investigation. This pattern of results suggested that endogenous activity at μ-opioid receptors was necessary for mediation of the stimulatory effects of ethanol on an adolescent-associated form of social behavior, namely, play fighting. The results of the present study were added to the list of the stimulatory and reinforcing effects of ethanol that were mediated, in part, via opioid receptor systems.
There were some occasional reports that opioid antagonists could affect ethanol absorption and modify BECs, although there was inconsistency in the directionality of these effects. For instance, Linseman and Lê (1997) reported naltrexone-induced decreases in BECs following intragastric ethanol administration, whereas Benitez and others (1987) found that brain and blood ethanol levels were elevated following naloxone and the same route of ethanol administration. Assessment of BECs in the present study, however, revealed no effects of naloxone or CTOP administration, confirming that under the circumstances of these tests, both antagonists blocked the socially activating effects of ethanol without altering its pharmacokinetic properties. The lack of effects of opioid antagonists on ethanol pharmacokinetics in the present study is likely to be related to the relatively rapid absorption of ethanol following its i.p. administration.
Whereas a number of studies had reported that nonselective opioid antagonists reliably decreased play fighting in adolescent animals (e.g., Beatty and Costello, 1982; Niesink and Van Ree, 1989; Panksepp et al., 1980; Siegel and Jensen, 1986), we did not observe naloxone-induced attenuation of play fighting in adolescents challenged with saline. Similarly, β-FNA, a selective μ antagonist has been reported to significantly decrease play fighting in P21 animals (Vanderschuren et al., 1995), whereas the selective μ antagonist, CTOP, produced no effects on the levels of play fighting of adolescents challenged with saline in Experiment 3. The discrepancy between the present study and those earlier studies may be related to procedural differences, including housing conditions, strain of rats as well as doses of the antagonists. For instance, in the studies reporting declines in play fighting following naloxone or naltrexone administration, play behavior of the experimental animals was enhanced by individual housing (Beatty and Costello, 1982; Niesink and Van Ree, 1989; Panksepp et al., 1980; Siegel and Jensen, 1986) or pair-housed animals were tested repeatedly following administration of different naloxone doses (Panksepp et al, 1985; Siegel et al., 1985), with the dose ranges being much higher in these earlier studies than that used in the present study (1.0 to 10.0 mg/kg vs. 0.05 to 0.1 mg/kg, respectively). Therefore, it is likely that opioid antagonists are effective in decreasing play fighting in adolescent animals when levels of play fighting are elevated by either pretest manipulations or pharmacologically, with high antagonist doses perhaps producing general behavioral suppression as well. The suggestion that opioid antagonists are effective in decreasing play fighting only when previously enhanced by pharmacological and/or behavioral manipulations seems reasonable, given that endogenous opioid systems demonstrates little or no intrinsic activity under normal circumstances, but rather are activated by a number of biologically relevant stimuli (Herz, 1997).
Although ethanol-induced facilitation of play fighting in adolescent rats is antagonized by μ-opioid receptor blockers, the exact mechanisms underlying opioid-related socially activating effects of ethanol remain to be investigated. These socially facilitating effects may be mediated through ethanol-induced release of endogenous ligands for the μ-opioid receptors as well as ethanol-related enhancement of sensitivity of these receptors to their endogenous ligands. Indeed, ethanol has been demonstrated to stimulate the release of endogenous ligands for μ- and δ-opioid receptors (β-endorphin, enkephalins) in distinct brain regions associated with reward and reinforcement (Boyadjieva and Sarkar, 1997; De Waele and Gianoulakis, 1993; De Waele et al., 1992; Olive et al., 2001; Rasmussen et al., 1998), release that is transient and is rapidly followed by a return to basal levels (De Waele and Gianoulakis, 1993; Keith et al., 1986). In vitro studies had shown dose-dependent effects of ethanol on β-endorphin release from the pituitary or hypothalamus (De Waele et al., 1992; Gianoulakis, 1990; Keith et al., 1986), with lower concentrations inducing more pronounced increase in β-endorphin release than higher ethanol concentrations (i.e., an inverted U-shaped dose–response curve). Such ethanol-induced release of β-endorphin in the hypothalamus, nucleus accumbens, and ventral tegmental area (Boyadjieva and Sarkar, 1997; De Waele and Gianoulakis, 1993; De Waele et al., 1992; Marinelli et al., 2003; Olive et al., 2001; Rasmussen et al., 1998) and consequent interaction of this endogenous ligand with μ-opioid receptors located within the mesolimbic reward system may be viewed as one of the possible mechanisms underlying ethanol-induced social facilitation. There are other possibilities as well. For instance, indirect cannabinoid agonists (Trezza and Vanderschuren, 2008a,b) and NMDA antagonists (Siviy et al., 1995) like ethanol, facilitated social behavior in adolescence, with these systems being implicated in ethanol intake and reinforcement (Vengeliene et al., 2008). Consequently, these systems are likely candidates with respect to contributing to ethanol-induced social facilitation in adolescents.
Given that ethanol-induced social facilitation is seen under normal circumstances in adolescent but not adult rats, it is likely that ethanol activates the endogenous β-endorphin system of adolescents and adults differentially, with this activation being more pronounced in younger than older animals. However, age-related differences in ethanol-induced social facilitation may also be associated with an ontogenetic decline in sensitivity to positive, stimulatory effects of endogenous ligands for μ-opioid receptors on social behavior, in general, and play fighting, in particular, with early adolescent animals being most sensitive and adults being insensitive to these stimulatory effects. Despite the lack of direct age comparisons, there are some hints that adolescents may be more sensitive than adults to the social stimulatory effects of μ-opioid agonists. Effectiveness of μ-opioid agonists in facilitation of play fighting has been demonstrated in preadolescent and early adolescent rats by a number of investigators (Niesink and Van Ree, 1989; Vanderschuren et al., 1995), whereas data regarding the effects of opioid manipulations on social behavior of adult rats are neither plentiful nor consistent. For instance, decreases in social investigation and contact behavior (Meyerson, 1981) as well as increases in contact behavior but not social investigation (Van Ree and Niesink, 1983) had both been reported in adult male rats following β-endorphin administration.
In summary, age-related differences in responsiveness to the stimulatory effects of ethanol on social behavior, with adolescents showing notable ethanol-induced social facilitation that is not typically seen in adults, may stem from enhanced sensitivity to ethanol-induced activation of the endogenous μ-opioid system during adolescence. However, precise opioid mechanisms underlying this pronounced age-associated difference in sensitivity to ethanol-induced social facilitation still remain to be elucidated.
Acknowledgments
The research presented in this paper was supported by NIH grants R01 AA012453 to Elena I. Varlinskaya and R37 AA012525, R01 AA016887 to Linda P. Spear.
Footnotes
No claim to original U.S. government works
References
- Adriani W, Macrí S, Pacific R, Laviola G. Peculiar vulnerability to nicotine oral self-administration in mice during early adolescence. Neuropsychopharmacol. 2002;27:212–224. doi: 10.1016/S0893-133X(02)00295-6. [DOI] [PubMed] [Google Scholar]
- Beatty WW, Costello KB. Naloxone and play fighting in juvenile rats. Pharmacol Biochem Behav. 1982;17:905–907. doi: 10.1016/0091-3057(82)90470-1. [DOI] [PubMed] [Google Scholar]
- Beck KH, Thombs DL, Summons TG. The social context of drinking scales: construct validation and relationship to indicants of abuse in an adolescent population. Addict Behav. 1993;18:159–169. doi: 10.1016/0306-4603(93)90046-c. [DOI] [PubMed] [Google Scholar]
- Beck KH, Treiman KA. The relationship of social context of drinking, perceived social norms, and parental influence to various drinking patterns of adolescents. Addict Behav. 1996;21:633–644. doi: 10.1016/0306-4603(95)00087-9. [DOI] [PubMed] [Google Scholar]
- Benitez M, Boada J, Díaz E, Feria M, Prunell M. Naloxone-induced increase in blood and brain ethanol concentrations in rats. Pharmacol Res Commun. 1987;19:723–729. doi: 10.1016/0031-6989(87)90102-0. [DOI] [PubMed] [Google Scholar]
- Blanchard BA, Glick SD. Sex differences in mesolimbic dopamine responses to ethanol and relationship to ethanol intake in rats. Recent Dev Alcohol. 1995;12:231–241. doi: 10.1007/0-306-47138-8_15. [DOI] [PubMed] [Google Scholar]
- Blanchard BA, Steindorf S, Wang S, Glick SD. Sex differences in ethanol-induced dopamine release in nucleus accumbens and in ethanol consumption in rats. Alcohol Clin Exp Res. 1993;17:968–973. doi: 10.1111/j.1530-0277.1993.tb05650.x. [DOI] [PubMed] [Google Scholar]
- Boyadjieva NI, Sarkar DK. Effects of ethanol on basal and prostaglandin E1-induced increases in beta-endorphin release and intracellular cAMP levels in hypothalamic cells. Alcohol Clin Exp Res. 1997;21:1005–1009. [PubMed] [Google Scholar]
- Brasser SM, Spear NE. Physiological and behavioral effects of acute ethanol hangover in juvenile, adolescent, and adult rats. Behav Neurosci. 2002;116:305–320. doi: 10.1037//0735-7044.116.2.305. [DOI] [PubMed] [Google Scholar]
- Brown SA, Christiansen BA, Goldman MS. The Alcohol Expectancy Questionnaire: an instrument for the assessment of adolescent and adult alcohol expectancies. J Stud Alcohol. 1987;48:483–491. doi: 10.15288/jsa.1987.48.483. [DOI] [PubMed] [Google Scholar]
- Brown DR, Robertson MJ, Goldberg LI. Reversal of morphine-induced catalepsy in the rat by narcotic antagonists and their quaternary derivates. Neuropharmacology. 1983;22:317–321. doi: 10.1016/0028-3908(83)90246-0. [DOI] [PubMed] [Google Scholar]
- Brunell SC, Spear LP. Effect of stress on the voluntary intake of a sweetened ethanol solution in pair housed adolescent and adult rats. Alcohol Clin Exp Res. 2005;29:1641–1653. doi: 10.1097/01.alc.0000179382.64752.13. [DOI] [PubMed] [Google Scholar]
- Buchanan CM, Ecdes JS, Becker JB. Are adolescents the victims of raging hormones: evidence for activational effects of hormones on moods and behavior at adolescence. Psychol Bull. 1992;111:62–107. doi: 10.1037/0033-2909.111.1.62. [DOI] [PubMed] [Google Scholar]
- Cailhol S, Mormède P. Sex and strain differences in ethanol drinking: effects of gonadectomy. Alcohol Clin Exp Res. 2001;25:594–599. [PubMed] [Google Scholar]
- Cohen RA, Coffman JD. Naloxone reversal of morphine-induced peripheral vasodilatation. Clin Pharmacol Ther. 1980;28:541–544. doi: 10.1038/clpt.1980.200. [DOI] [PubMed] [Google Scholar]
- De Waele JP, Gianoulakis C. Effects of single and repeated exposures to ethanol on hypothalamic beta-endorphin and CRH release by the C57BL/6 and DBA/2 strains of mice. Neuroendocrinology. 1993;57:700–709. doi: 10.1159/000126428. [DOI] [PubMed] [Google Scholar]
- De Waele JP, Papachristou DN, Gianoulakis C. The alcohol-preferring C57BL/6 mice present an enhanced sensitivity of the hypothalamic beta-endorphin system to ethanol than the alcohol-avoiding DBA/2 mice. J Pharmacol Exp Ther. 1992;26:788–794. [PubMed] [Google Scholar]
- Doremus TL, Brunell SC, Rajendran P, Spear LP. Factors influencing elevated ethanol consumption in adolescent relative to adult rats. Alcohol Clin Exp Res. 2005;29:1796–1808. doi: 10.1097/01.alc.0000183007.65998.aa. [DOI] [PubMed] [Google Scholar]
- Eckardt MJ, File SE, Gessa GL, Grant KA, Guerri C, Hoffman PL, Kalant H, Koob GF, Li TK, Tabakoff B. Effects of moderate alcohol consumption on the central nervous system. Alcohol Clin Exp Res. 1998;22:998–1040. doi: 10.1111/j.1530-0277.1998.tb03695.x. [DOI] [PubMed] [Google Scholar]
- File S. The social interaction test of anxiety. Neurosci Protocol. 1993;10:1–7. [Google Scholar]
- Froehlich JC, Li TK. Opioid involvement in alcohol drinking. Ann NY Acad Sci. 1994;739:156–167. doi: 10.1111/j.1749-6632.1994.tb19817.x. [DOI] [PubMed] [Google Scholar]
- Gianoulakis C. Characterization of the effects of acute ethanol administration on the release of beta-endorphin peptides by the rat hypothalamus. Europ J Pharmacol. 1990;180:21–29. doi: 10.1016/0014-2999(90)90588-w. [DOI] [PubMed] [Google Scholar]
- Gianoulakis C. Implications of endogenous opioids and dopamine in alcoholism: human and basic science studies. Alcohol Alcohol. 1996;31(Suppl 1):33–42. [PubMed] [Google Scholar]
- Herz A. Endogenous opioid systems and alcohol addiction. Psychopharmacology. 1997;129:99–111. doi: 10.1007/s002130050169. [DOI] [PubMed] [Google Scholar]
- Holson RR, Pearce B. Principles and pitfalls in the analysis of prenatal treatment effects in multiparous species. Neurotoxicol Teratol. 1992;14:221–228. doi: 10.1016/0892-0362(92)90020-b. [DOI] [PubMed] [Google Scholar]
- Johnston AL, File SE. Sex differences in animal tests of anxiety. Physiol Behav. 1991;49:245–250. doi: 10.1016/0031-9384(91)90039-q. [DOI] [PubMed] [Google Scholar]
- Johnston LD, O'Malley PM, Bachman JG, Schulenberg JE. Monitoring the Future National Survey Results on Drug Use, 1975–2006, Volume 1, Secondary School Students (NIH Publication No 07-6205) National Institute on Drug Abuse; Bethesda, MD: 2007. [Google Scholar]
- Keith LD, Crabbe JC, Robertson LM, Kendall JW. Ethanol-stimulated endorphin and corticotropin secretion in vitro. Brain Res. 1986;367:222–229. doi: 10.1016/0006-8993(86)91595-7. [DOI] [PubMed] [Google Scholar]
- Kim SG, Stromberg MF, Kim MJ, Volpicelli JR, Park JM. The effect of antagonists selective for mu- and delta-opioid receptor subtypes on alcohol consumption in C57BL/6 mice. Alcohol. 2000;22:85–90. doi: 10.1016/s0741-8329(00)00109-9. [DOI] [PubMed] [Google Scholar]
- Kramer TH, Shook JE, Kazmierski W, Ayres EA, Wire WS, Hruby VJ, Burks TF. Novel peptidic mu opioid antagonists: pharmacologic characterization in vitro and in vivo. J Pharmacol Exp Ther. 1989;249:544–551. [PubMed] [Google Scholar]
- Lancaster FE, Brown TD, Coker KL, Elliott JA, Wren SB. Sex differences in alcohol preference and drinking patterns emerge during the early postpubertal period. Alcohol Clin Exp Res. 1996;20:1043–1049. doi: 10.1111/j.1530-0277.1996.tb01945.x. [DOI] [PubMed] [Google Scholar]
- Linakis JG, Cunningham CL. Effects of concentration of ethanol injected intraperitoneally on taste aversion, body temperature, and activity. Psychopharmacology. 1979;64:61–65. doi: 10.1007/BF00427346. [DOI] [PubMed] [Google Scholar]
- Linseman MA, Lê AD. Effects of opioids on the absorption of alcohol. Pharmacol Biochem Behav. 1997;58:79–84. doi: 10.1016/s0091-3057(97)00002-6. [DOI] [PubMed] [Google Scholar]
- Marinelli PW, Quirion Rm, Gianoulakis C. A microdialysis profile of beta-endorphin and catecholamines in the rat nucleus accumbens following alcohol administration. Psychopharmacology. 2003;169:60–67. doi: 10.1007/s00213-003-1490-2. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Stewart J. A descriptive study of social development in the rat (Rattus norvegicus) Anim Behav. 1981;29:34–45. [Google Scholar]
- Meyerson BJ. Comparison of the effects of beta-endorphin and morphine on exploratory and socio-sexual behaviour in the male rat. Europ J Pharmacol. 1981;69:453–463. doi: 10.1016/0014-2999(81)90449-0. [DOI] [PubMed] [Google Scholar]
- Ngai SH, Berkowitz BA, Yang JC, Hempstead J, Spector S. Pharmacokinetics of naloxone in rats and men: basis for its potency and short duration of action. Anesthesiology. 1976;44:398–401. doi: 10.1097/00000542-197605000-00008. [DOI] [PubMed] [Google Scholar]
- Niesink RJ, Van Ree JM. Involvement of opioid and dopaminergic systems in isolation-induced pinning and social grooming of young rats. Neuropharmacology. 1989;28:411–418. doi: 10.1016/0028-3908(89)90038-5. [DOI] [PubMed] [Google Scholar]
- Olive MF, Koenig HN, Nannini MA, Hodge CW. Stimulation of endorphin neurotransmission in the nucleus accumbens by ethanol, cocaine, and amphetamine. J Neurosci. 2001;21:RC184–RC184. doi: 10.1523/JNEUROSCI.21-23-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oswald LM, Wand GS. Opioids and alcoholism. Physiol Behav. 2004;81(2):339–358. doi: 10.1016/j.physbeh.2004.02.008. [DOI] [PubMed] [Google Scholar]
- Panksepp J, Herman BH, Vilberg T, Bishop P, DeEskinazi FG. Endogenous opioids and social behavior. Neurosci Biobeh Rev. 1980;4:473–487. doi: 10.1016/0149-7634(80)90036-6. [DOI] [PubMed] [Google Scholar]
- Panksepp J, Jalowiec J, DeEskinazi FG, Bishop P. Opiates and play dominance in juvenile rats. Behav Neurosci. 1985;99:441–453. doi: 10.1037//0735-7044.99.3.441. [DOI] [PubMed] [Google Scholar]
- Pastor Rl, Aragon CMG. The role of opioid receptor subtypes in the development of behavioral sensitization to ethanol. Neuropsychopharmacology. 2006;31:1489–1499. doi: 10.1038/sj.npp.1300928. [DOI] [PubMed] [Google Scholar]
- Pellis SM, Field EF, Smith LK, Pellis VC. Multiple differences in the play fighting of male and female rats. Implications for the causes and functions of play. Neurosci Biobehav Rev. 1997;21:105–120. doi: 10.1016/0149-7634(95)00060-7. [DOI] [PubMed] [Google Scholar]
- Pellis SM, McKenna MM. Intrinsic and extrinsic influences on play fighting in rats: effects of dominance, partner's playfulness, temperament and neonatal exposure to testosterone propionate. Behav Brain Res. 1992;50:135–145. doi: 10.1016/s0166-4328(05)80295-5. [DOI] [PubMed] [Google Scholar]
- Pellis SM, McKenna MM. What do rats find rewarding in play fighting? An analysis using drug-induced non-playful partners. Behav Brain Res. 1995;68:65–73. doi: 10.1016/0166-4328(94)00161-8. [DOI] [PubMed] [Google Scholar]
- Pellis SM, Pellis VC. Differential rates of attack, defense, and counterattack during the developmental decrease in play fighting by male and female rats. Dev Psychobiol. 1990;23:215–231. doi: 10.1002/dev.420230303. [DOI] [PubMed] [Google Scholar]
- Pelton JT, Kazmierski W, Gulya K, Yamamura HI, Hruby VJ. Design and synthesis of conformationally constrained somatostatin analogues with high potency and specificity for mu opioid receptors. J Med Chem. 1986;29:2370–2375. doi: 10.1021/jm00161a037. [DOI] [PubMed] [Google Scholar]
- Petersen AC, Silbereisen RK, Sörensen S. In: Adolescent development: a global perspective, in Social Problems and Social Contexts in Adolescence. Hurrelmann K, Hamilton SF, editors. Aldine de Gruyter; New York: 1996. pp. 3–37. [Google Scholar]
- Rasmussen DD, Bryant CA, Boldt BM, Colasurdo EA, Levin N, Wilkinson CW. Acute alcohol effects on opiomelanocortinergic regulation. Alcohol Clin Exp Res. 1988;22(4):789–801. [PubMed] [Google Scholar]
- Siegel MA, Jensen RA. The effects of naloxone and cage size on social play and activity in isolated young rats. Behav Neural Biol. 1986;45:155–168. doi: 10.1016/s0163-1047(86)90739-9. [DOI] [PubMed] [Google Scholar]
- Siegel MA, Jensen RA, Panksepp J. The prolonged effects of naloxone on play behavior and feeding in the rat. Behav Neural Biol. 1985;44:509–514. doi: 10.1016/s0163-1047(85)91024-6. [DOI] [PubMed] [Google Scholar]
- Silveri MM, Spear LP. Ontogeny of rapid tolerance to the hypnotic effects of ethanol. Alcohol Clin Exp Res. 1999;23:1180–1184. [PubMed] [Google Scholar]
- Siviy SM, Baliko CN. A further characterization of alpha-2 adrenoceptor involvement in the rough-and-tumble play of juvenile rats. Dev Psychobiol. 2000;37:25–34. doi: 10.1002/1098-2302(200007)37:1<25::aid-dev4>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Siviy SM, Fleischhauer AE, Kuhlman SJ, Atrens DM. Effects of alpha-2 adrenoceptor antagonists on rough-and-tumble play in juvenile rats: evidence for a site of action independent of non-adrenoceptor imidazoline binding sites. Psychopharmacology. 1994;113:493–499. doi: 10.1007/BF02245229. [DOI] [PubMed] [Google Scholar]
- Siviy SM, Line BS, Darcy EA. Effects of MK-801 on rough-and-tumble play in juvenile rats. Physiol Behav. 1995;57:843–847. doi: 10.1016/0031-9384(94)00361-8. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Spear LP. In: The developing brain and adolescent-typical behavior patterns: an evolutionary approach, in Adolescent Psychopathology and the Developing Brain: Integrating Brain and Prevention Science. Walker E, Romer D, editors. Oxford University Press; New York: 2007. pp. 9–30. [Google Scholar]
- Thor DH, Holloway WR., Jr Social play in juvenile rats: a decade of methodological and experimental research. Neurosci Biobehav Rev. 1984;8:455–464. doi: 10.1016/0149-7634(84)90004-6. [DOI] [PubMed] [Google Scholar]
- Thor DH, Holloway WR., Jr Social play soliciting by male and female juvenile rats: effects of neonatal androgenization and sex of cagemates. Behav Neurosci. 1986;100:275–279. doi: 10.1037//0735-7044.100.2.275. [DOI] [PubMed] [Google Scholar]
- Trezza V, Vanderschuren LJMJ. Bidirectional cannabinoid modulation of social behavior in adolescent rats. Psychopharmacology. 2008a;197:217–227. doi: 10.1007/s00213-007-1025-3. [DOI] [PubMed] [Google Scholar]
- Trezza V, Vanderschuren LJMJ. Cannabinoid and opioid modulation of social play behavior in adolescent rats: differential behavioral mechanisms. Eur Neuropsychopharmacol. 2008b;18:519–530. doi: 10.1016/j.euroneuro.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Ree JM, Niesink RJM. Low doses of beta-endorphin increase social contacts of rats tested in dyadic encounters. Life Sci. 1983;33(Suppl 1):611–614. doi: 10.1016/0024-3205(83)90577-5. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJ, Niesink RJ, Spruijt BM, Van Ree JM. Mu- and kappa-opioid receptor-mediated opioid effects on social play in juvenile rats. Europ J Pharmacol. 1995;276:257–266. doi: 10.1016/0014-2999(95)00040-r. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJ, Niesink RJ, van Ree JM. The neurobiology of social play behavior in rats. Neurosci Biobehav Rev. 1997;21:309–326. doi: 10.1016/s0149-7634(96)00020-6. [DOI] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Acute effects of ethanol on social behavior of adolescent and adult rats: role of familiarity of the test situation. Alcohol Clin Exp Res. 2002;26:1502–1511. doi: 10.1097/01.ALC.0000034033.95701.E3. [DOI] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Acute ethanol withdrawal (hangover) and social behavior in adolescent and adult male and female Sprague-Dawley rats. Alcohol Clin Exp Res. 2004;28(1):40–50. doi: 10.1097/01.ALC.0000108655.51087.DF. [DOI] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Differences in the social consequences of ethanol emerge during the course of adolescence in rats: social facilitation, social inhibition, and anxiolysis. Develop Psychobiol. 2006;48:146–161. doi: 10.1002/dev.20124. [DOI] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Chronic tolerance to the social consequences of ethanol in adolescent and adult Sprague–Dawley rats. Neurotoxicol Teratol. 2007;29:23–30. doi: 10.1016/j.ntt.2006.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Social interactions in adolescent and adult Sprague–Dawley rats: impact of social deprivation and test context familiarity. Behav Brain Res. 2008;188:398–405. doi: 10.1016/j.bbr.2007.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP, Spear NE. Social behavior and social motivation in adolescent rats: role of housing conditions and partner's activity. Physiol Behav. 1999;67:475–482. doi: 10.1016/s0031-9384(98)00285-6. [DOI] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP, Spear NE. Acute effects of ethanol on behavior of adolescent rats: role of social context. Alcohol Clin Exp Res. 2001;25:377–385. [PubMed] [Google Scholar]
- Vengeliene V, Bilbao A, Molander A, Spanagel R. Neuropharmacology of alcohol addiction. Br J Pharmacol. 2008;15:299–315. doi: 10.1038/bjp.2008.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetter CS, Doremus-Fitzwater TL, Spear LP. Time course of elevated ethanol intake in adolescent relative to adult rats under continuous, voluntary-access conditions. Alcohol Clin Exp Res. 2007;31:1159–1168. doi: 10.1111/j.1530-0277.2007.00417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker EA. In vivo pharmacological resultant analysis reveals noncompetitive interactions between opioid antagonists in the rat tail-withdrawal assay. Brit J Pharmacol. 2006;149:1071–1082. doi: 10.1038/sj.bjp.0706946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb B, Burnett PW, Walker DW. Sex differences in ethanol-induced hypnosis and hypothermia in young Long–Evans rats. Alcohol Clin Exp Res. 2002;26:695–704. [PubMed] [Google Scholar]
- Yoshimoto K, Hori M, Sorimachi Y, Watanabe T, Yano T, Yasuhara M. Increase of rat alcohol drinking behavior depends on the age of drinking onset. Alcohol Clin Exp Res. 2002;26(Suppl 8):63S–65S. doi: 10.1097/01.ALC.0000026977.19902.61. [DOI] [PubMed] [Google Scholar]


