Abstract
Objective
This paper describes a high-quality, multisite telemammography system to enable “almost real-time” remote patient management while the patient remains in the clinic. One goal is to reduce the number of women who would physically need to return to the clinic for additional imaging procedures (termed “recall”) to supplement “routine” imaging of screening mammography.
Materials and Methods
Mammography films from current and prior (when available) examinations are digitized at three remote sites and transmitted along with other pertinent information across low-level communication systems to the central site. Images are automatically cropped, wavelet compressed, and encrypted prior to transmission to the central site. At the central site, radiologists review and rate examinations on a high-resolution workstation that displays the images, computer-assisted detection results, and the technologist's communication. Intersite communication is provided instantly via a messaging “chat” window.
Results
The technologists recommended additional procedures at 2.7 times the actual clinical recall rate for the same cases. Using the telemammography system during a series of “off-line” clinically simulated studies, radiologists recommended additional procedures at 1.3 times the actual clinical recall rate. Percent agreement and kappa between the study and actual clinical interpretations were 66.1% and 0.315, respectively. For every physical recall potentially avoided using the telemammography system, approximately one presumed “unnecessary” imaging procedure was recommended.
Conclusion
Remote patient management can reduce the number of women recalled by as much as 50% without performing an unreasonable number of presumed “unnecessary” procedures.
Key Words: Teleradiology, telemammography, mammography, breast cancer screening, remote decision making
Introduction
Periodic mass screening of asymptomatic women for early detection of breast cancer is an accepted and widely implemented practice with extensive public and scientific support,1,2 despite sporadic reports of limited benefits.3,4 The number of mammograms being acquired is increasing because of improved compliance, longer life expectancy, and an earlier recommended age for initial examination. Shortages of expert mammographers in many locations and efforts to improve radiologists' practice and performance combined with the desire to improve patient compliance resulted in a distributed acquisition-centralized expert review-type practices in a large number of institutions.5,6 Immediate or shortened time intervals between the recognized need for and the actual administration of services decrease patient anxiety, increase patient compliance, and decrease cost.7–10
The relatively high recall rates of screened women (5–15%) to supplement information that was not ascertained during the initial visit (e.g., spot compression, magnification views) strain all facets of breast cancer screening practices.10–12 More importantly, perhaps it necessitates that the recalled woman spends additional time and effort before receiving a diagnosis, and there are clear indications that this process adds emotional stress as well, particularly in remote locations or underserved locations.7,8,12 Enabling physicians to remotely “monitor” and “manage” potential recall-related issues could aid in the diagnostic decision making in “almost real time” while the woman remains in the clinic. The remote management concept could exploit the reported abilities of trained mammography technologists to provide limited nonclinical interpretation of screening mammograms13,14 to identify women who may need additional imaging procedures and obtain a physician's decision regarding the actual need (or not) for such procedures while the woman remains in the clinic.
Early attempts to develop and implement a practical telemammography system failed because of several technical problems associated with acquisition, transmission, management, and display of the mammographic images.15,16 Management of the large amount of acquired image data (35–55 MB/image) is but one challenge to effective implementation of telemammography-based practices. Many of these technical issues have been resolved in recent years, but some remain.16–18 Although an adequate communication infrastructure for high-quality telemammography is available in many large urban medical centers, where it may be needed most (i.e., remote, nonurban locations), two-way communication systems are often limited to the Plain Old Telephone System (POTS). Other communication technologies (e.g., satellites) are being evaluated for this purpose, but it is unlikely that these will replace POTS in many underserved areas.19 Hence, the problem of cost-effective, timely, remote patient management in many of these areas is not a simple one. Using a unique data-handling scheme and a variety of image-processing techniques, we have demonstrated that high-quality, multisite telemammography systems can be developed under these acquisition and communication constraints.20,21
This study describes the capability of a telemammography system designed to enable an “almost real-time” remote patient monitoring and management, specifically to reduce the number of women who would physically need to return to the clinic for additional imaging procedures (termed “recall”) as part of a breast cancer screening protocol. Under the investigated scenarios, remote patient management would be typically initiated when a mammography technologist at a remote site identifies a woman that may require additional image procedures to supplement the “routine” mammographic imaging being performed. The technologists then transmit pertinent patient data and communicate their (the technologist's) concerns to experienced radiologists at the central site. The telemammography system was evaluated under several clinically simulated paradigms with an evolution of relevant clinical information being transmitted by the technologists. Radiologists' performance during a series of “off-line” clinically simulated studies that included reviewing and rating of screening examinations using the telemammography system was compared with the actual clinical interpretations of the same cases.
Materials and Methods
The high-quality, multisite telemammography system was designed to acquire, process, transmit, and display mammographic image data and other relevant information (e.g., prior reports) in a reasonable time across a low-level communication system as well as to provide a two-way messaging system. Several key image processing and transmission techniques were implemented to allow the timely transmission of the large amount of digitized mammographic image data. Computer programs at the remote and central sites are designed and implemented using multithreading to permit each task to be completed in a timely manner, yet allow the system to be responsive to user input (Fig. 1).
Fig. 1.
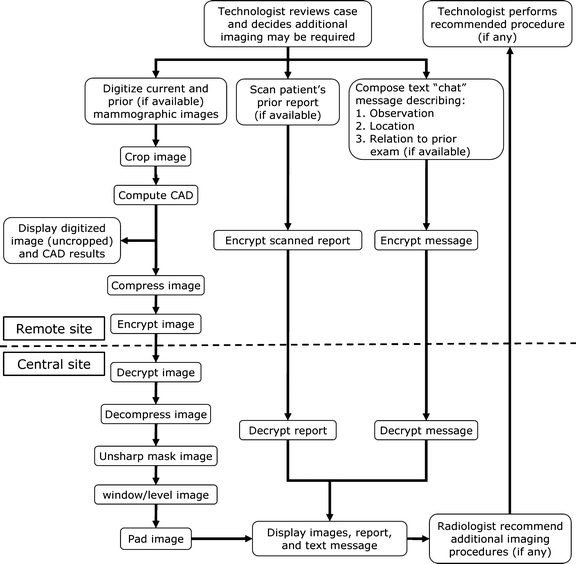
Telemammography system organizational chart illustrating the flow of digitizing and processing image data and intersite communication. The system software is implemented using multithreading that permits each task to be completed independently at the remote and central sites.
In short, a technologist at a remote facility (women's imaging clinic) identifies a woman who may need additional imaging procedures to complete a screening mammography examination during a review of the mammographic films for quality assurance (QA) purposes. The technologist digitizes the current and prior (when available) mammographic films, inputs patient data into the computer data form, composes a message (text and graphic) that describes the type of finding and location of concern, and scans the patient's prior report (when available). This information and the results of computer-assisted detection (CAD) scheme to detected suspicious regions are transmitted to the central site. At the central site, a radiologist reviews the mammographic image data, the technologist's message (text and graphic), and patient reports using a three-monitor workstation that was specifically designed for this purpose. The radiologist then responds to the technologist with the recommended additional procedures (if any). The technical description of the system has been provided in several reports.20,21 The following is a brief summary of the system and its functionality.
Remote Site
Hardware
The remote site hardware includes a personal computer (Athlon 900, Advanced Micro Device, Sunnyvale, CA, USA) operating under Microsoft Windows 2000 Workstation and connected to a high-resolution, laser film digitizer (Lumiscan 85, Eastman Kodak, Rochester, NY, USA) and document scanner (HP Scanjet 5470c scanner, Hewlett-Packard Company, Palo Alto, CA, USA). The computer is equipped with both a 56K modem for data transfer across the POTS analog lines and an Ethernet network card for data transfer across the local area network (LAN). The film digitizer and document scanner are equipped with automatic film and document feeders, respectively, for scanning multiple films and multiple page reports, respectively.
Mammographic Image Digitization and Image Processing
At the remote sites, mammographic films are digitized at a 50-μm pixel size over optical densities ranging from 0 to 4.0 OD (useful range 0.15–3.7 OD). The digital images are automatically cropped to reduce the nontissue areas and compressed at a ratio of 75:1 using the irreversible (lossy), 9/7 transform, wavelet-based JPEG 2000 method.22 A CAD scheme is applied to the noncompressed images. Patient reports are scanned and converted to 1 bit per pixel portable network graphic (PNG) images. Application of a CAD scheme to the original digitized images warranted the high-spatial resolution digitization for the depiction and detection of microcalcifications. There are several methods to reduce the file size (e.g., subsampling, image compression) prior to transmission subsequent to application of the CAD scheme. Lossy, wavelet-based image compression was chosen to decrease file size while preserving much of the high-spatial frequency information.
Intersite communication
Technologists (remote site) and radiologists (central site) communicate using a message window that features text and graphics containing five sections: (1) patient demographics, (2) message display area, (3) pull-down menus, (4) interactive generic image (chart) of the right and left breasts, and (5) free text typing window (Fig. 2). At the remote site, technologists construct a chat message using five pull-down menus to focus communication on possible actionable items that indicate the following:
breast: left or right
view: craniocaudal and/or mediolateral oblique
finding: mass, calcifications, tissue asymmetry, palpable lump, or nodule
comparison with prior exam: baseline exam, new finding, slight change, moderate change, or remarkable change
other findings stable
possible additional procedure needed: additional views and/or ultrasound
Fig. 2.
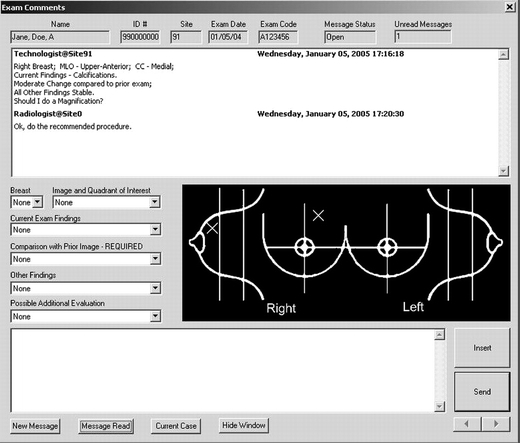
Intersite communication messaging window depicted as it would appear at the remote sites used by the technologists to send messages to the central site (radiologists).
The free text window permits description of suspicious regions or pertinent patient information not listed in the pull-down menus. The interactive generic image of the breasts allows the technologists to place an “X” mark on the region of suspicion, which appears on the central site display messaging window.
Data transfer
The mammographic image data, chat message (text and graphic components), patient report image, and CAD results are parsed and then encrypted using strong 128-bit Microsoft Point-to-Point Encryption. The data packets are prioritized and sent to the central site using a handshaking protocol.
Central Site
Hardware and Software
The central site dual processor telemammography workstation (Athlon MP, dual 1.2-GHz multiprocessor, Advanced Micro Device) operates under Microsoft Windows 2000 Server and communicates with the remote sites via 56K modems and Ethernet network cards. The workstation display consists of three high-resolution (2048 × 2560), 8-bit grayscale, portrait monitors at a nominal setting of 80 ftL: two Dome C5i flat-panel monitors (Planar Systems, Beaverton, OR, USA) for image display and one Clinton DS5100P cathode ray tube monitor (Clinton Electronics, Rockford, IL, USA) for text.
The key display features available on the workstation include manual lookup table (LUT) adjustments, magnification, quadrant viewing (images viewed one quadrant at a time), and multiple image display formats (one, two, or four images per monitor), which are all mouse-driven. The typical display resolution was approximately 200-μm pixel dimensions for two images/monitor (default). Images can be magnified by a free-moving magnification box or quadrant panning. The left and center monitors display the image data with the CAD results overlaid, and the right monitor displays the message windows, prior reports, case lists, etc.
The workstation is DICOM compatible and is capable of sending and printing selected images to a mammographic film printer (DryView 8610, Eastman Kodak). It also is capable of sending and receiving images to and from another DICOM device, such as full field digital mammography acquisition systems.
Intersite Communication
The radiologist can reply to the technologist's message after reviewing each case. His/her response includes the following: (1) do recommended procedure as suggested; (2) no additional procedures necessary; and (3) do not do the procedure recommended, but do X, Y, and Z. If the radiologist recommends additional procedures, the interactive generic image of the breast allows the radiologist to place a “square” mark precisely on the region that requires the additional workup, which appears on the remote site display messaging window. The central site messaging window is similar to the remote site window (Fig. 2) except that, for simplicity and efficiency, there is only one pull-down that contains the above responses.
Image Processing
At the central site, the data packets received are decrypted and reconstructed to their original file structure. The mammographic image data are decompressed. To reduce the visual effects of cropping, images are restored to full height (but not to full width) by padding (filling) prior to image display. Image display on the workstation is enhanced through minimal unsharp masking, and LUT values are automatically set. The CAD results are presented as an overlay.
Technical Assessments
The breast tissue was completely retained subsequent to the automated cropping, producing images that were adequate for review. The automated LUT settings for image display were acceptable for review, and manual adjustments were performed in only approximately 10% of the cases.
The average cycle time from initiation of digitization to availability for display at the central site was evaluated under expected operating conditions. The transmission time of a series of four cases (back to back) each consisting of four images per case was reliably less than 7 min/case for all three sites. The combination of image cropping and 75:1 data compression decreased image file size to allow cycle times that were adequate for implementation of the telemammography concept and met our planned technical specifications.
There were no complaints during case review of noticeable image artifacts or poor image quality because of the high image compression used. At extremely high magnifications, there were some visually detectable differences between noncompressed digitized mammographic images and images compressed at a 75:1 ratio, but based on several assessments we performed, these differences should not have affected the diagnostic image quality. In a two-alternative forced choice discrimination experiment, when displayed side-by-side, radiologists could not accurately or reliably discriminate between noncompressed images and those compressed to 50:1 and 75:1 compression levels.22
Telemammography System Operation
The telemammography system has been operational for more than 2 1/2 years, and to date, over 2400 different cases (some more than once) have been successfully transmitted and received. Currently, sites 1, 2, and 3 are 15, 20, and 15 mi from the central site, respectively. However, in the past, we successfully implemented and tested the system at a site located 90 mi from the central site. Sites 1 and 2 transmit data across analog POTS lines. Site 3 transmits data across the LAN.
The image quality, image display configurations, effects of the image processing, and telemammography system features are generally well received and considered more than adequate for reviewing screening mammography examinations by participating radiologists. The magnification features provide a detailed review of the breast tissue patterns at full resolution (i.e., 50 μm/pixel), particularly microcalcifications.
The message window provides effective communication between the technologists (remote site) and radiologists (central site) and operates in almost real time (Fig. 2). Typically, a message window is sent with each case and communication performed in one cycle. The technologist sends a message with each case, and the radiologist responds directly to the message.
The technical performance of the telemammography system was evaluated during a field test after the software was modified to include mammographic images from the patient's current and prior screening examinations. The field tests involved the simultaneous transmission of multiple examinations (with and without prior images) from the three sites to the central site.
Three separate “live studies” were performed to evaluate clinical feasibility of the concept and the potential for a “real-time” use of the telemammography system. The remote site technologists sequestered screening exams the day prior to the live studies that they (the technologists) believed needed additional imaging procedures. These cases intermixed with all applicable cases from the day of the experiment were transmitted serially (back to back) to the central site and reviewed and rated immediately by radiologists dedicated to the telemammography workstation during the experiments. Eight to 17 cases were successfully transmitted, interpreted, and responded to by the radiologist during each study. We emphasize that these studies were performed “off-line” in terms of the clinical practice and had no impact on actual patient care.
Clinically Simulated Studies
Retrospective, clinically simulated studies were conducted using cases accrued during normal operation of the telemammography system that originated from women who underwent breast cancer screening mammography at the three remote sites. All studies described herein were performed under an approved Institutional Review Board protocol. Registered mammography technologists selected examinations based on their belief (the technologists) of whether the woman in question would be recalled for additional imaging procedures to supplement the routine screening or not. The technologists prospectively selected the examinations to be transmitted and were unaware at the time of selection whether or not the woman would ultimately be recalled as a result of the actual clinical interpretation by an experienced radiologist. The four routine mammographic films acquired at our centers are the left and right craniocaudal views and the left and right mediolateral oblique views. In study 1, the technologists transmitted an equal number of cases that they believed needed and did not need to be recalled. In studies 2–5, the technologists transmitted only cases that they believed needed to be recalled. The actual, subsequent clinical interpretation was used to categorize each examination using the Breast Imaging Reporting and Data System (BIRADS; Table 1). In this study, the term recall is represented by BIRADS category 0. Patients classified as BIRADS 0 due exclusively to technical deficiency during the acquisition of the images were excluded from the study.
Table 1.
Distribution of BIRADS Categories as a Result of the Actual Clinical Interpretation
| BIRADS category | 0 (recall) | 1 | 2 | Total |
|---|---|---|---|---|
| Study 1 (independent set) | 42 (13.7%) | 206 (67.3%) | 58 (19.0%) | 306 |
| Study 2 (independent set) | 51 (39.2%) | 34 (26.2%) | 45 (34.6%) | 130 |
| Study 3 (subset of Study 2) | 38 (38.4%) | 25 (25.2%) | 36 (36.4%) | 99 |
| Study 4 (independent set) | 47 (40.9%) | 41 (35.6%) | 27 (23.5%) | 115 |
| Study 5 (independent set) | 122 (34.6%) | 153 (43.3%) | 78 (22.1%) | 353 |
Number of cases (percentage).
Ten radiologists specializing in breast imaging (performing or reading more than 2000 breast imaging procedures per year) participated in five multimode observer performance studies in which they reviewed and rated over 900 breast cancer screening examinations with incrementally progressive levels (amounts) of patient information supplied by the remote site technologist (Table 2). The radiologists were informed of the case origination and selection criteria, but not the mix of “recall” and “no-recall” cases. Cases were randomly presented in each session, and during the interpretation of an individual case, all modes performed were presented and completed serially. The radiologists used a computer mouse to complete a computerized scoring form that included the technologist's chat message and graphic marks for each examination case as appropriate for the specific study mode (Fig. 3). On the scoring form, the radiologists indicated: (1) if additional procedures were recommended; (2) if images from prior examination were reviewed; (3) when appropriate, which breast depicted an abnormality that need additional imaging; and (4) when appropriate, the recommended procedures. The examinations in studies 1–4 were read by all participating radiologists, whereas the examinations for study 5 were read only by one radiologist. The number of examinations read by the individual radiologists in study 5 ranged from 45 to 154.
Table 2.
Patient Information Present for Interpretation during Studies and Modes
| Mode 1 | Mode 2 | Mode 3 | Readers | |
|---|---|---|---|---|
| Study 1 | I | n/a | n/a | 5 |
| Study 2 | I | I, M | n/a | 4 |
| Study 3 | I, M | I, M, R | n/a | 4 |
| Study 4 | I, M, R | I, M, R, G | I, M, R, G, C | 5 |
| Study 5 | I, M, R, G, C, P | n/a | n/a | 5 |
n/a—not applicable.
I—images from the current mammography exam.
M—technologist's text message.
R—patient's report from the prior mammography exam (when available).
G—technologist's graphic marks on a generic breast image.
C—CAD results.
P—images from the prior mammography exam (when available).
Fig. 3.
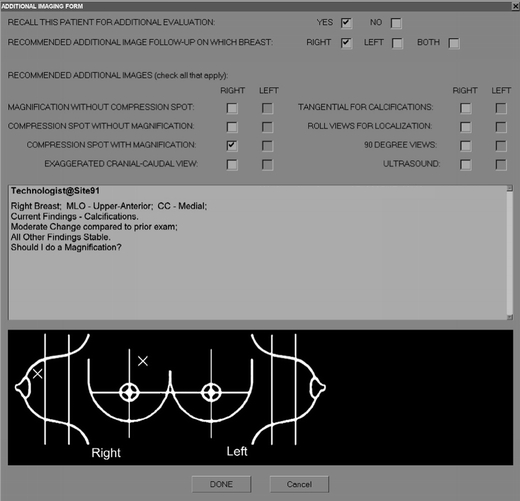
Computerized scoring form completed by the radiologists during the clinically simulated studies depicted as it would appear during mode 3 of study 3.
The radiologists' recommendations when using the telemammography workstation were compared with the actual interpretation during the clinical review. Performance was evaluated in terms of the fraction (percent) of exams recommended for recall, percent agreement, and kappa between both types of interpretations (i.e., telemammography and actual clinical). The cost of potentially “eliminating” the need for women to physically return to the clinic for additional imaging procedures was measured by the performance of presumed “unnecessary” additional procedures.
Results
Technologists were able to identify women whose screening mammography examinations were suspicious in terms that additional imaging procedures may be required, but the number (fraction) of women they recommended for additional procedures was substantially larger than the actual clinical interpretation. Radiologists at the central site were able to reduce the number of women the technologists recommended for presumably “unnecessary” additional procedures using the telemammography system to interpret examinations in question. The addition of the mammographic images from the prior screening examination to the images from the current examination had the greatest positive effect on the radiologists' performance when interpreting cases using the telemammography system compared to the inclusion of other pertinent information (e.g., patient history, technologist's observations).
The recall rates for radiologists interpreting breast cancer screening examinations using the telemammography system during all modes of studies 1–4 (images from the prior exam not transmitted) were significantly higher (at least 1.8-fold) than the actual clinical interpretation of the same cases (Table 3). The inclusion of images from the prior examination (when available) in study 5 reduced the difference in recall rates to 1.3-fold. The different case selection criteria for study 1 resulted in the lowest actual clinical recall rate, but the image-only telemammography interpretation produced the largest difference in recommended recall rates (telemammography vs. actual clinical interpretations). As the level of patient information increased across studies and modes, the ratio (cost) of eliminating a recommendation for a physical recall for additional imaging procedures vs. performing “unnecessary” additional procedures during the same visit aided by remote management decreased (Table 4).
Table 3.
Mean Percent Recall Rates During Studies and Modes
| Mode 1 | Mode 2 | Mode 3 | |
|---|---|---|---|
| Study 1 | 38.2% (±11.6) | n/a | n/a |
| Study 2 | 73.3% (±17.9) | 82.5% (±16.2) | n/a |
| Study 3 | 79.6% (±12.3) | 77.5% (±13.8) | n/a |
| Study 4 | 72.3% (±9.3) | 72.3% (±9.3) | 72.7% (±9.2) |
| Study 5 | 46.1% (±8.4) | n/a | n/a |
Mean percent (standard deviation).
n/a—not applicable.
Table 4.
Ratio of When the Telemammography Interpretation was to “Recall” and the Actual Clinical Interpretation was “No-Recall” Vs. When both the Telemammography and Actual Clinical Interpretations were to “Recall”
| Mode 1 | Mode 2 | Mode 3 | |
|---|---|---|---|
| Study 1 | 2.87 (433/151) | n/a | n/a |
| Study 2 | 1.28 (214/167) | 1.34 (246/183) | n/a |
| Study 3 | 1.27 (176/139) | 1.26 (171/136) | n/a |
| Study 4 | 1.05 (213/203) | 1.06 (214/202) | 1.07 (216/202) |
| Study 5 | 0.94 (81/86) | n/a | n/a |
Ratios for all reads (total counts).
n/a—not applicable.
The agreement between the telemammography readings and actual clinical interpretations was poor for studies 2–4 and modest for studies 1 and 5 (Tables 5 and 6). However, study 1 had a disproportionately high number (86.3%) of no-recall cases during the actual clinical interpretation, and the agreement between telemammography and actual clinical interpretations for cases recommended as “no-recall” accounted for the majority of the agreement cases (85.4%) in study 1. Agreement between the telemammography and actual clinical interpretations in study 5 was more balanced between cases recommended for recall (36.4%) and no-recall (63.6%). Agreement between the telemammography and actual clinical interpretations increased across studies 2–5 as the level of patient information increased (Tables 5 and 6). However, within a study, agreement remained relatively consistent as the level of patient information increased.
Table 5.
Mean Percent Agreement During Studies and Modes
| Mode 1 | Mode 2 | Mode 3 | |
|---|---|---|---|
| Study 1 | 67.8% (±10.0) | n/a | n/a |
| Study 2 | 51.7% (±5.5) | 48.7% (±6.3) | n/a |
| Study 3 | 52.3% (±6.7) | 52.8% (±7.0) | n/a |
| Study 4 | 57.4% (±4.6) | 57.1% (±3.9) | 56.7% (±3.9) |
| Study 5 | 66.6% (±5.3) | n/a | n/a |
Mean percent (standard deviation).
n/a—not applicable.
Table 6.
Mean Kappa During Studies and Modes
| Mode 1 | Mode 2 | Mode 3 | |
|---|---|---|---|
| Study 1 | 0.237 (±0.082) | n/a | n/a |
| Study 2 | 0.125 (±0.041) | 0.102 (±0.059) | n/a |
| Study 3 | 0.163 (±0.077) | 0.165 (±0.081) | n/a |
| Study 4 | 0.213 (±0.072) | 0.206 (±0.060) | 0.201 (±0.061) |
| Study 5 | 0.315 (±0.107) | n/a | n/a |
Mean (standard deviation).
n/a—not applicable.
The majority of disagreement between the telemammography readings and actual clinical interpretations for all study modes resulted when the telemammography review recommended additional procedures and the actual clinical interpretation recommended no additional procedures. These disagreements (telemammography recall and actual clinical no-recall) accounted for 86.5% of the disagreements on average when images from prior examinations were not included (studies 1–4). The inclusion of prior images (when available) in study 5 reduced this disparity to 66.1%.
Discussion
The “proof of concept” that a high-quality, multisite telemammography system implemented with low-level data connections can provide remote “underserved” clinics (e.g., a location where a radiologist is not physically present) “almost real-time” access to an experienced radiologist to evaluate breast cancer screening examinations was accomplished during this study. Particularly, we focused on identifying women recommended for additional imaging procedures as part of screening protocol while the woman remains in the clinic, and it was demonstrated that the number of women who would need to physically return to the clinic for additional imaging procedures could be reduced substantially. The two-way communication implemented in the telemammography system between the technologists (remote site) and radiologists (central) using a messaging window (Fig. 2) that contained text and graphics was sufficient and was treated as a welcomed increase in communication by both radiologists and technologists.
This study indicates that prior images (when available) along with the ensemble of tools and information provided by the telemammography allowed the radiologist to successfully manage a large fraction of cases that technologists consider to be “difficult” or those likely to require additional imaging procedures. In studies 2, 4, and 5 (598 unique cases), the technologists recommend additional imaging procedures at 2.7 times the actual clinical recall rate (220 cases) for the same cases (Table 1). Using the telemammography system and implementing the remote management concept, radiologists reduced this “recall” rate by more than 50% during study 5. For every potential saving of an actual recall, approximately one presumably unnecessary additional procedure (Table 4) would have been performed.
This study supports the results of other studies advocating utilization of mammography technologists as physician extenders in breast cancer screening practices,13,14 which may be accomplished through implementation of a telemammography system. This study demonstrated that technologists are reasonably sensitive, if not specific, to mammography features and changes that may lead to recall of women for additional imaging procedures. Of the 598 unique screening mammography exams used in studies 2, 4, and 5 that technologists believed needed additional imaging procedures, 63.4% (378/598) were not recalled during the actual clinical interpretation (Table 1). One hundred fifty of the 378 not recalled (39.7%) were rated as a BIRADS category of 2. This suggests that the technologists were able to detect benign findings during the screening mammography exam, but were not skilled at classifying the depicted findings as benign. Appropriate training may be required for this purpose.
The high recall rates for additional imaging procedures during this study when interpretation was conducted without prior images (studies 2–4) were similar to those previously reported.5 The high recall rates could be partially attributed to the radiologists' expectation of an “enriched” sample population. Additionally, in our studies, the radiologists may have deferred to the technologists' observations during limited review (e.g., lack of images from a prior examination) of examinations using telemammography system.
The short cycle time of the system was realized because of image-processing techniques that reduced the image file size through automated image cropping and image data compression and the efficient multitasking software approach based on a synchronized multithreading design.21 The image-processing techniques produced images without a significant degradation of the diagnostic image quality, which were well received by the radiologists. Despite the initial uncertainty regarding high level of image compression (75:1), the implementation of wavelet compression permits high levels of compression without a significant degradation in diagnostic image quality.22–24
There are several limitations to the current study. First, this study did not affect clinical management, and the knowledge that this was carried out “off-line” may have resulted in the observed overreading. Second, the technologists were not trained in selecting suspected examinations resulting in a relatively low threshold of selection, in particular as related to benign findings. Finally, we were “breaking ground” in several respects that include, but are not limited to, the involvement of technologists in the decision-making process (namely, which cases to send to the central site and why) and possibly the increased “reliance” of the radiologists on the technologists' judgments. Our breast imaging practice is a very well established one, and it is not clear whether this “reliance” and “trust” would exist in other practices. We note that this study was designed to assess the effectiveness of the telemammography system for reviewing screening examinations for management purposes rather than primary diagnosis.
Conclusion
This study demonstrated that the concept of remote patient management using a telemammography system can potentially reduce the number of women who need to physically return to the clinic for additional image procedures (e.g., spot compression, magnification views) without performing an unreasonable number of presumed unnecessary imaging procedures. This could be accomplished by exploiting the ability of experienced technologists to identify suspicious findings during QA procedures and the use of a telemammography system to transmit all pertinent information to a radiologist (decision maker) at a central site. Even with a low-level communication system, the complete review process can be accomplished while the woman remains in the clinic. Implementation of the remote management concept requires a high “comfort level” within the team (radiologists and technologists). Our estimates are that this type of practice could reduce actual recall rates by as much as 50%.
Acknowledgments
This work is supported in part by the US Army Medical Research Acquisition Center, 820 Chandler Street, Fort Detrick, MD 21702-5014, under contract DAMD17-00-1-0410. The content of the information contained herein does not necessarily reflect the position or the policy of the government, and no official endorsement should be inferred.
References
- 1.Taplin SH, Ichikawa L, Buist DS, Seger D, White E. Evaluating organized breast cancer screening implementation: the prevention of late-stage disease? Cancer Epidemiol Biomark Prev. 2004;13(2):225–234. doi: 10.1158/1055-9965.EPI-03-0206. [DOI] [PubMed] [Google Scholar]
- 2.Humphrey LL, Helfand M, Chan BKS, Woolf SH. Breast cancer screening: a summary of the evidence for the U.S. preventive services task force. Ann Intern Med. 2002;137(5, Part 1):E347–E367. doi: 10.7326/0003-4819-137-5_part_1-200209030-00012. [DOI] [PubMed] [Google Scholar]
- 3.Elmore JG, Reisch LM, Barton MB, Barlow WE, Rolnick S, Harris EL, Herrinton LJ, Geiger AM, Beverly RK, Hart G, Yu O, Greene SM, Weiss NS, Fletcher SW. Efficacy of breast cancer screening in the community according to risk level. J Natl Cancer Inst. 2005;97(14):1035–1043. doi: 10.1093/jnci/dji183. [DOI] [PubMed] [Google Scholar]
- 4.Miller AB, To T, Baines CJ, Wall C. The Canadian Nation Breast Screening Study-1: breast cancer mortality after 11 to 16 years of follow-up: a randomized screening trial of mammography in women age 40 to 49 years. Ann Intern Med. 2002;137(5, Part 1):E305–E315. doi: 10.7326/0003-4819-137-5_part_1-200209030-00005. [DOI] [PubMed] [Google Scholar]
- 5.Elmore JG, Wells CK, Lee CH, Howard DH, Feinstein AR. Variability in radiologists' interpretations of mammograms. N Engl J Med. 1994;331(22):1493–1499. doi: 10.1056/NEJM199412013312206. [DOI] [PubMed] [Google Scholar]
- 6.Warren RML, Duffy SW. Comparison of single reading with double reading of mammograms and change in effectiveness with experience. Br J Radiol. 1995;68(813):958–962. doi: 10.1259/0007-1285-68-813-958. [DOI] [PubMed] [Google Scholar]
- 7.Sandin B, Chorot P, Valiente RM, Lostao L, Santed MA. Adverse psychological effects in women attending a second-stage breast cancer screening. J Psychosom Res. 2002;52(5):303–309. doi: 10.1016/S0022-3999(01)00227-6. [DOI] [PubMed] [Google Scholar]
- 8.Lindfors KK, O'Connor J, Parker RA. False-positive screening mammograms: effect of immediate versus later work-up on patient stress. Radiology. 2001;218(1):247–253. doi: 10.1148/radiology.218.1.r01ja35247. [DOI] [PubMed] [Google Scholar]
- 9.Dolan NC, McDermott MM, Morrow M, Venta L, Martin GJ. Impact of same-day screening mammography availability: results of a controlled clinical trial. Arch Intern Med. 1999;159(4):393–398. doi: 10.1001/archinte.159.4.393. [DOI] [PubMed] [Google Scholar]
- 10.Elmore JG, Barton MB, Moceri VM, Polk S, Arena PJ, Fletcher SW. Ten-year risk of false-positive screening mammograms and clinical breast examinations. N Engl J Med. 1998;338(16):1089–1096. doi: 10.1056/NEJM199804163381601. [DOI] [PubMed] [Google Scholar]
- 11.Yankaskas BC, Cleveland RJ, Schell MJ, Kozar R. Association of recall rates with sensitivity and positive predictive values of screening mammography. AJR Am J Roentgenol. 2001;177(3):543–549. doi: 10.2214/ajr.177.3.1770543. [DOI] [PubMed] [Google Scholar]
- 12.May DS, Lee NC, Nadel MR, Henson RM, Miller DS. The national breast and cervical cancer early detection program: report of the first 4 years of mammography provided to medically underserved women. AJR Am J Roentgenol. 1998;170(1):97–104. doi: 10.2214/ajr.170.1.9423608. [DOI] [PubMed] [Google Scholar]
- 13.Sumkin JH, Klaman HM, Graham M, Ruskauff T, Gennari RC, King JL, Klym AH, Ganott MA, Gur D. Prescreening mammography by technologists: a preliminary assessment. AJR Am J Roentgenol. 2003;180(1):253–256. doi: 10.2214/ajr.180.1.1800253. [DOI] [PubMed] [Google Scholar]
- 14.Tonita JM, Hillis JP, Lim CH. Medical radiologic technologist review: effects on a population-based breast cancer screening program. Radiology. 1999;211(2):529–533. doi: 10.1148/radiology.211.2.r99ma32529. [DOI] [PubMed] [Google Scholar]
- 15.Feig SA, Yaffe MJ. Digital mammography, computer-aided diagnosis, and telemammography. Radiol Clin North Am. 1995;33(6):1205–1228. [PubMed] [Google Scholar]
- 16.Goldberg MA, Dwyer SJ., 3rd Telemammography: implementation issues. Telemed J. 1995;1(3):215–226. doi: 10.1089/tmj.1.1995.1.215. [DOI] [PubMed] [Google Scholar]
- 17.Lou SL, Lin HD, Lin KP, Hoogstrate D. Automatic breast region extraction from digital mammograms for PACS and telemammography applications. Comput Med Imaging Graph. 2000;24(4):205–220. doi: 10.1016/S0895-6111(00)00009-4. [DOI] [PubMed] [Google Scholar]
- 18.Murphy JM, O'Hare NJ, Wheat D, McCarthy PA, Dowling A, Hayes R, Bowmer H, Wilson GF, Molloy MP. Digitized mammograms: a preliminary clinical evaluation and the potential for telemammography. J Telemed Telecare. 1999;5(3):193–197. doi: 10.1258/1357633991933620. [DOI] [PubMed] [Google Scholar]
- 19.Lou SL, Sickles EA, Huang HK, Hoogstrate D, Cao F, Wang J, Jahangiri M. Full-field direct digital telemammography: technical components, study protocols, and preliminary results. IEEE Trans Inf Technol Biomed. 1997;1(4):270–278. doi: 10.1109/4233.681171. [DOI] [PubMed] [Google Scholar]
- 20.Maitz GS, Chang TS, Sumkin JH, Wintz PW, Johns CM, Ganott M, Holbert BL, Hakim CM, Harris KM, Gur D, Herron JM. Preliminary clinical evaluation of a high-resolution telemammography system. Invest Radiol. 1997;32(4):236–240. doi: 10.1097/00004424-199704000-00007. [DOI] [PubMed] [Google Scholar]
- 21.Drescher JM, Maitz GS, Traylor C, Leader JK, Clearfield RJ, Shah R, Ganott MA, Pugliese F, Duffner D, Lockhart J, Gur D. A multi-site telemammography system: preliminary assessment of technical and operational issues. Proc SPIE. 2003;5033:360–369. [Google Scholar]
- 22.Leader JK, Sumkin JH, Ganott MA, Hakim C, Hardesty L, Shah R, Wallace L, Klym A, Drescher JM, Maitz GS, Gur D. Subjective assessment of high-level image compression of digitized mammograms. Proc SPIE. 2004;5372:415–422. [Google Scholar]
- 23.Lucier BJ, Kallergi M, Qian W, DeVore RA, Clark RA, Saff EB, Clarke LP. Wavelet compression and segmentation of digital mammograms. J Digit Imaging. 1994;7(1):27–38. doi: 10.1007/BF03168476. [DOI] [PubMed] [Google Scholar]
- 24.Perlmutter SM, Cosman PC, Gray RM, Olshen RA, Ikeda D, Adams CN, Betts BJ, Williams MB, Perlmutter KO, Li J, Aiyer A, Fajardo L, Birdwell R, Daniel BL. Image quality in lossy compressed digital mammograms. Signal Process. 1997;59(2):189–210. doi: 10.1016/S0165-1684(97)00046-7. [DOI] [Google Scholar]


