Abstract
Purpose
Our study evaluates digital x-ray radiogrammetry (DXR) and Radiogrammetry Kit (RK) as a new diagnostic method for the measurement of disease-related osteoporosis including quantification of joint space narrowing dependent on the severity of rheumatoid arthritis (RA).
Materials and Methods
A total of 172 unselected patients with RA underwent computerized measurements of bone mineral density (BMD) and metacarpal index (MCI) by DXR, as well as a semiautomated measurement of joint space distances at the metacarpal–phalangeal articulation (JSD-MCP 2–5), both were analyzed from plain radiographs of the nondominant hand.
Results
Correlations between DXR-BMD and DXR-MCI vs. parameters of RK were all significant (0.34 < R < 0.61; p < 0.01). An expected negative association was observed between RK parameters and the different scoring methods (−0.27 < R < −0.59). The maximum relative decrease in BMD vs. MCI as measured by DXR between the highest and lowest RA severity group was −27.7% vs. −27.5% (p < 0.01) for the modified Larsen Score, whereas the minimal value of relative DXR-BMD and DXR-MCI reduction could be documented for the Sharp Erosion Score (−20.8% vs. −26.8%; p < 0.01). The relative reduction of mean JSD-MCP using RK significantly varied from −25.0% (Sharp Erosion Score) to −41.2% (modified Larsen Score). In addition, an excellent reproducibility of DXR and RK could be verified.
Conclusion
DXR in combination with RK could be a promising, widely available diagnostic tool to supplement the different scoring methods of RA with quantitative data, allowing an earlier and improved diagnosis and more precision in determining disease progression.
Key Words: Digital x-ray radiogrammetry, rheumatoid arthritis, joint space width, bone mineral density, metacarpal index, Larsen Score, Sharp Score
Introduction
Rheumatoid arthritis (RA) is a systemic and chronic disease characterized by inflammation in and around soft tissue, cartilage, and bone tissue of the joints, frequently resulting in impairment of function and destruction of the affected small joints of the hand.1 The characteristic pattern of juxtaarticular inflammatory affection includes both cartilage destruction and bone erosion,2 which distinguishes RA from other forms of chronic arthritis. Besides bone erosion, the process of joint destruction is further accelerated by the degradation and dissolution of the cartilage caused by the direct effects of enzymes and other synovial cell products that accumulate in the synovial fluid,3 which results in a narrowing of the joint spaces.
In addition to inflammatory-related joint alteration (i.e., bone erosion and cartilage destruction), osteoporosis is another significant clinical complication in RA and occurs in two forms: periarticular osteoporosis in near proximity to the inflamed joints, which is a typical phenomenon in early RA, and generalized osteoporosis affecting the axial and appendicular bones occurring during the course of rheumatoid arthritis.4
The hand shows the earliest manifestation of RA and the destruction of small joints correlates well with alterations seen on radiographs of large joints.1 The extent of joint destruction visualized on radiographs of the hands and feet varies widely in patients with RA, but is significantly associated with cumulative joint inflammation.
Conventional radiography, a widely available and cost-effective method, remains the standard of reference for the detection and quantification of joint destruction in the course of RA and for determining the success of antirheumatic therapy.5 The disadvantage of conventional imaging is its limited sensitivity in detecting an early narrowing of the peripheral joint space widths and in quantifying periarticular osteoporosis, which shows a high prevalence in the metacarpal bones. Generally, osteoporosis is only very imprecisely verified using radiographs; it is widely accepted that bone loss (i.e., reduction of bone mineral density) of less than 35% cannot be detected on plain radiographs.6
For earlier identification of inflammatory changes enabling a better prognosis and improved success of treatment strategies in patients suffering from RA, the use of digital techniques for acquisition and processing of radiographs has substantially increased and has continually gained wider acceptance in recent years. The availability of digital images provides the opportunity for quantitative measurements of radiographic features.7–9
The clinical application of radiogrammetry, which was initially introduced as a technique for evaluation of bone status by Barnett and Nordin,10 has undergone significant improvement as a result of further refinement, computerization, and the use of algorithms for automatic image analysis.11 Our study is performed with the Pronosco X-Posure System (Version 2.0; Sectra Pronosco A/S, Denmark), which uses a computerized radiogeometric analysis of the three middle metacarpal bones for the measurement of bone mineral density (BMD) and metacarpal index (MCI). The potential of digital x-ray radiogrammetry (DXR) for estimating cortical bone loss seems to be promising in clinical practice.11
Since its first approval by the US Food and Drug Administration in 1999, DXR has had a comprehensive record of clinical and technical studies evaluating and documenting its advantages in monitoring and supporting the diagnosis of various forms of osteoporosis. DXR-BMD was shown to be a significant indicator of fracture risk in healthy individuals and patients with osteoporosis and rheumatoid arthritis. In a prospective study, Bouxsein et al.12 documented that DXR showed an equal prediction value of fracture risk compared to single photon absorptiometry regarding fractures of the wrist, spine, and femur.
Several studies have reported a close correlation between peripheral and axial measured data based on dual energy x-ray absorptiometry (DXA) and DXR-BMD;11 additionally, normative values have been reported for DXR.13–16
Moreover, Hyldstrup et al.17 verified an increase in DXR-BMD and DXR-MCI in combination with a reduction of cortical porosity in postmenopausal women after treatment with bisphosphonates and also using hormone replacement therapy.
Radiogrammetry Kit (RK) is a separate software program implemented for semiautomated measurement of joint space distances of the metacarpal– phalangeal articulation (JSD-MCP) of the second to the fifth finger. Previous techniques used for joint space analysis have not been able to provide consistently accurate estimates for affected finger joints caused by distinctive disease-related abnormalities.9 Furthermore, the proximal interphalangeal joint should be not considered, because these joints show a bicompartmental configuration resulting in a varied width of each compartment dependent on minor rotation of the hand. Therefore establishing normative data and estimating minimal disease-related changes is limited in this articulation.9
Using digital hand radiographs, this study evaluated the ability of making precise measurements of the radiographic visible joint space distances of the metacarpal–phalangeal articulation in any grade of RA via the Radiogrammetry Kit, and of quantifying the periarticular demineralization via DXR in the course of RA. Our technique should help to identify those patients with RA who are going to develop joint damage before major erosions occur and to consecutively optimize the individual therapeutic strategies.
Materials and Methods
Patients
One hundred seventy-two Caucasian patients recruited from our outpatient clinic (138 female and 34 male) were enrolled in this study. These patients were 18–80 years old (mean age = 61.6 ± 11.5 years) and fulfilled the American College of Rheumatology 1987 revised classification criteria for RA.18 Disease duration varied from 6 months up to 40 years. No preselection relating to the severity of RA or steroid therapy was performed. Thirty-two patients were treated with methotrexate and 85 subjects with a combination consisted of disease-modifying antirheumatic drugs (DMARDs) and nonsteroidal anti-inflammatory drugs (NSAIDs). Twenty-five patients had been on long-term low-dose prednisolone therapy (<5 mg/day over a 6-month period). The remaining 30 patients had neither received systemic corticosteroids and DMARDs nor immune modulating drugs. Subjects with abnormal renal function (serum creatinine >130 μmol/L), or are on hormone replacement therapy/biphosphonates, or having other conditions known to affect bone metabolism were excluded.
All examinations were performed in accordance with the rules and regulations laid down by the local research and ethics committee. As a special note, the authors emphasize that all radiographs used for DXR calculations and analysis of joint space distances were performed as part of routine clinical care; no additional radiographs were obtained for study purposes.
Exclusion criteria were signs of fracture and visible osteosynthetic material in the right and left upper extremities (including ulna, radius and hand).
Digital radiographs of both hands and both feet were obtained from all subjects at the time of the study. These radiographs were independently and blindly scored by two musculoskeletal radiologists (J.B. and A.P.) using the modified Larsen Score and the two parts of the Sharp Score defined as follows:
Modified Larsen Score19 evaluates 32 joints of feet and hands (total sum of points: 160) and comprises a new definition of grade 1 without provision for soft tissue swelling and demineralization.
Sharp Erosion Score20 evaluates 34 joints of the hands (total sum of points: 170).
Sharp Joint Space Narrowing Score20 evaluates 36 joints of the hands (total sum of points: 144).
Each evaluator scored all plain radiographs (including grading of the feet for the modified Larsen Score) of each patient, whereas each scoring method mentioned above was considered by both observers, respectively. Both readers interdependently proceeded as recommended by Sharp et al.20: The individual sum of scoring points of each patient and for each separate score was divided by the evaluated joints of the used score, and patients were subdivided into different groups of grading (i.e., RA severity groups of each scoring method) according to the appropriate scoring method. In cases of ambiguity, a third musculoskeletal radiologist (A.M.) reviewed the radiographs in question. The independent and separate consideration of three different scoring methods, comprising a different main focus in the rating of joint destruction, has ensured the reliability and objectivity of our data in differentiating the disease-related alterations quantified by DXR and RK techniques during the course of rheumatoid arthritis.
Calculation of BMD and MCI (Digital X-ray Radiogrammetry)
The Pronosco X-Posure System™ (Version 2.0, Sectra Pronosco A/S, Vedbaek, Denmark, which includes a radiogeometric and textural analysis of the three middle metacarpal bones, was used to determine BMD and MCI estimated by DXR, requiring digital performed radiographs of the nondominant hand.
All plain radiographs were acquired via Polydoros SX 80 (Siemens, Munich, Germany) under the following conditions: filter with 1.0 mm thickness in relation to aluminum 80, tube voltage 42 kV, exposure level 4 mAs, film focus distance 100 cm, Scopix Laser 2 B 400 (Agfa GmbH and Cie. KG, Cologne, Germany).
The digital radiographs were sent into the system in high-resolution quality, corresponding to 118 pixels/cm. The system itself checked the quality of the scanned images to identify the necessary bone structures (i.e., metacarpals) of the nondominant hand and interrupted the examination in case of inadequate identification. In this context, the Pronosco X-Posure System™ can only identify bone structures for patients who are more than 6 years old. Computer algorithms automatically defined regions of interest (ROIs) around the narrowest bone parts of the metacarpalia II, III, and IV, and subsequently determined the outer and inner cortical edges of the studied cortical bone parts.
To locate the bones in the radiograph, the Pronosco X-Posure System™ applies a model-based algorithm known as Active Shape Model (ASM).11 The ASM algorithm has been adapted to find the diaphysis of the three middle metacarpals in the hand. After each diaphysis has been identified, ROIs are automatically placed for the three middle metacarpals.
In detail, the algorithm places the three ROIs in a coupled fashion by sliding them in a partly fixed configuration along the bone shafts to a position identified by the minimum combined bone width. The height of ROIs is fixed to 2.0, 1.8, and 1.6 cm for the second, third, and fourth metacarpals, respectively.
Within each ROI, the endosteal (inner) and periosteal (outer) edges are automatically found, thereby segmenting the bone into two cortical regions and one endosteal region. Along a profile across the bone, the two endosteal edge points are associated with the points of maximal intensity as illustrated in Figure 1. The periosteal edge points are associated with the points of maximal curvature, which, in this case, is similar to the gradient.
Fig 1.

Principles for determination of the basic radiogrammetrical quantities by DXR. (L: longitude of the specific region of interest; T: cortical thickness; W: outer bone diameter; R: radius of total outer bone diameter; r: radius of endosteal region).
There is otherwise no operator interaction connected to DXR measurement. The analyzed images and their ROIs were displayed on the computer monitor so the observer could check the correct position of the ROIs (see Figure 2).
Fig 2.

Automatically ROI positioning for analysis of DXR parameters by the Pronosco X-posure System™. The algorithm places the three ROIs in a coupled fashion by sliding them in a partly fixed configuration along the bone shafts to a position identified by the minimum combined bone width. The height of ROIs is fixed to 2.0, 1.8, and 1.6 cm for the second, third, and fourth metacarpals, respectively.
The mean of cortical thickness and overall bone cortical thickness (CT) of the second, third, and fourth metacarpals were estimated as described by Rosholm et al.11
Subsequently, the cortical volume per area (VPA, in cm) is computed for each metacarpal assuming a cylindrically shaped bone based on CT and mean outer bone diameter (W) as follows (see Fig. 1):
 |
or rather,
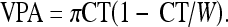 |
The total VPA for the metacarpals is defined as a weighted average:
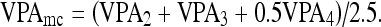 |
The fourth metacarpal bone is given a lower weight due to a lower precision and an inferior clinical importance.
The final DXR-BMD, based on the mean VPA, is then calculated with a correction mentioned below for the estimated porosity index (P).
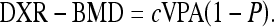 |
The scaling constant c is determined so that DXR-BMD, on average, is equal to that of the middistal forearm region of the Hologic QDR 2000 densitometer (Hologic, Waltham, MA, USA). The constant adapts VPA to both the volumetric mineral density of compact bone and the typical shape characteristics of the involved bones. Porosity index is a technical parameter with a value between 1 and 19, which is derived from the area percentage of local intensity minima found in the cortical part of the bone relative to the entire cortical area.11 Consequently, the porosity index represents an estimated three-dimensional cortical porosity, aimed to be the fraction of the cortical bone volume that is not occupied by bone.
For metacarpal index (DXR-MCI), the mean CT normalized with the mean outer bone diameter (W) for each bone part is obtained as follows:11
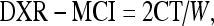 |
and the total DXR-MCI for the three metacarpals is also computed as the weighted above-mentioned average:
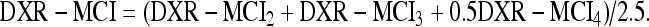 |
Measurement of Joint Spaces (Radiogrammetry Kit)
The Sectra Radiogrammetry Kit (Version 1.3.5; Sectra Pronosco A/S, Vedbaek, Denmark) performs a joint space analysis of a finger joint by detection of the joint edges within a rectangular ROI defined by the same user (i.e., musculoskeletal radiologist J.B.). User input to define the ROI is necessary to provide the correct selection of JSD-MCP. The software performs an edge filtering of the ROI and detects the tips of the two involved bones. A 1.5-cm-long edge path of across each bone is further determined and the distance between the two edges is calculated as a function of the horizontal position with the ROI. The mean average and standard deviation of the distance over a moving interval (horizontally across the ROI) of 0.8 cm is calculated (see Figure 3). The distance between the bones is defined to be over the interval, for which the standard deviation of the pixelwise edge distances is minimal. Specifically, the measurement of joint spaces is methodically established for the metacarpal–phalangeal articulation II–V and distances are given in centimeter.
Fig 3.

Computerized measurement of metacarpal–phalangeal joint spaces analyzed by Sectra Radiogrammetry Kit. Distance between bones is defined as the average distance between the two involved edges over an interval of 0.8 cm. The two bars mark this interval across the joint of a patient with RA (Larsen Score 2).
Statistical Analysis
Results are expressed as mean and standard deviation. Comparison of parameters between RK vs. scoring methods and DXR data was carried out via linear regression analysis (p < 0.05). For comparison of unmatched RA severity groups, Mann-Whitney U test was used. Statistical analysis was performed using SPSS Version 10.13.
Results
Comparison Between Parameters of DXR, RK and Scoring Methods (see Table 1)
Table 1.
Coefficients of Correlation Between Modified Larsen Score, Sharp Erosion Score, Sharp Joint Space Narrowing Score and DXR Parameters Versus Estimated Joint Spaces of Metacarpal–Phalangeal Articulations by RK
| Joint Space Distances Estimated by Radiogrammetry Kit | |||||
|---|---|---|---|---|---|
| JSD-MCP II | JSD-MCP III | JSD-MCP IV | JSD-MCP V | JSD-MCP (mean) | |
| DXR-BMD | 0.53* | 0.56* | 0.54* | 0.50* | 0.61* |
| DXR-MCI | 0.45* | 0.42* | 0.34** | 0.37** | 0.45* |
| Larsen Score modified | −0.58* | −0.54* | −0.47* | −0.43* | −0.59* |
| Sharp Erosion Score | −0.47* | −0.47* | −0.27** | −0.43* | −0.47* |
| Sharp Joint Space Narrowing Score | −0.55* | −0.57* | −0.35** | −0.49* | −0.56* |
JSD-MCP = joint space distance–metacarpal phalangeal joint; DXR-BMD = digital x-ray radiogrammetry-bone mineral density; DXR-MCI = digital x-ray radiogrammetry-metacarpal index.
*p < 0.01; **p < 0.05.
Measurements of DXR-BMD and DXR-MCI as well as JSD-MCP parameters could be carried out in all patients.
All moderate correlations between the two DXR parameters and the JSD-MCP parameters were significant, although the latter had consistently higher correlation with DXR-BMD than with DXR-MCI. The highest correlation was observed between JSD-MCP (mean) and DXR-BMD (R = 0.61, p < 0.01), whereas the R2 value showed that only 37% of the variance in one variable was accounted for by the other variable.
A significant negative association was observed between the different JSD-MCP parameters and all scoring methods (−0.59 < R < −0.27); pronounced associations could be documented for modified Larsen Score and the second/third fingers, whereas the Sharp Joint Space Narrowing Score surpassed the Sharp Erosion Score.
Severity-Dependent Periarticular Demineralization and Joint Space Narrowing
Modified Larsen Score
DXR-BMD showed a significant difference (−27.7%) from 0.55 g/cm2 ± 0.09 in the RA severity group 1 to 0.40 g/cm2 ± 0.08 in the RA severity group 5 (Table 2; Figure 4a). DXR-MCI also revealed a significant difference (−27.5%) from 0.40 ± 0.09 (RA severity group 1) to 0.29 ± 0.07 (RA severity group 5). The similar difference for joint space narrowing ranged from −26.7% (JSD-MCP V) to −50.0% (JSD-MCP II). The mean JSD-MCP decreased between RA severity group 1 and RA severity group 5 at an extent of −41.2%.
Table 2.
Reduction of DXR and RK Parameters from RA Severity Group 1 to 5 Regarding Modified Larsen Score
| Score, n = 172 | 1 Mean (SD), n = 33 | 2 Mean (SD), n = 37 | 3 Mean (SD), n = 31 | 4 Mean (SD), n = 33 | 5 Mean (SD), n = 38 | Relative Reduction from Grade 1 to Grade 5 |
|---|---|---|---|---|---|---|
| JSD-MCP II (cm) | 0.18 (0.04) | 0.18 (0.04) | 0.14 (0.06) | 0.10 (0.08) | 0.09 (0.08) | −50.0% (p < 0.01) |
| JSD-MCP III (cm) | 0.17 (0.03) | 0.16 (0.03) | 0.14 (0.06) | 0.10 (0.07) | 0.09 (0.07) | −47.1% (p < 0.01) |
| JSD-MCP IV (cm) | 0.16 (0.02) | 0.15 (0.03) | 0.15 (0.03) | 0.13 (0.04) | 0.11 (0.05) | −31.3% (p < 0.01) |
| JSD-MCP V (cm) | 0.15 (0.02) | 0.15 (0.03) | 0.13 (0.02) | 0.10 (0.08) | 0.11 (0.06) | −26.7% (p < 0.01) |
| JSD-MCP mean (cm) | 0.17 (0.02) | 0.16 (0.03) | 0.14 (0.03) | 0.11 (0.06) | 0.10 (0.06) | −41.2% (p < 0.01) |
JSD-MCP = joint space distance–metacarpal phalangeal joint.
Fig 4.



(a) Reduction of DXR parameters from RA severity group 1 to 5 regarding the modified Larsen Score. (b) Reduction of DXR parameters from RA severity group 0 to 5 regarding the Sharp Erosion Score. (c) Reduction of DXR parameters from RA severity group 0 to 4 regarding the Sharp Joint Space Narrowing Score.
Sharp Scores
For both Sharp Score principles, DXR-BMD decreased markedly (−27.3% vs. −20.8%); a similar level of reduction was also noted for DXR-MCI (−26.8%). In this context, JSD-MCP (mean) showed an expected narrowing of −35.3% for the Joint Space Narrowing segment, whereas the Sharp Erosion Score revealed a significantly lower decline in JSD-MCP (mean) with −25.0% (Tables 3 and 4; Figure 4b,c). For the other joints; there was a relative reduction between −13.3% (JSD-MCP IV, Sharp Erosion part) and −47.4% (JSD-MCP II, Sharp Joint Space Narrowing part).
Table 3.
Reduction of DXR and RK Parameters from RA Severity Group 0 to 5 Regarding Sharp Erosion Score
| Score, n = 172 | 0 Mean (SD), n = 26 | 1 Mean (SD), n = 27 | 2 Mean (SD), n = 26 | 3 Mean (SD),n = 29 | 4 Mean (SD), n = 25 | 5 Mean (SD), n = 39 | Relative Reduction from Grade 0 to Grade5 |
|---|---|---|---|---|---|---|---|
| JSD-MCP II (cm) | 0.18 (0.02) | 0.18 (0.04) | 0.18 (0.06) | 0.20 (0.05) | 0.15 (0.03) | 0.11 (0.07) | −38.8% (p < 0.01) |
| JSD-MCPIII (cm) | 0.17 (0.02) | 0.16 (0.02) | 0.17 (0.03) | 0.17 (0.03) | 0.14 (0.03) | 0.12 (0.07) | −29.4% (p < 0.01) |
| JSD-MCP IV (cm) | 0.15 (0.02) | 0.15 (0.02) | 0.15 (0.02) | 0.16 (0.04) | 0.12 (0.02) | 0.13 (0.04) | −13.3% (p < 0.05) |
| JSD-MCP V (cm) | 0.15 (0.02) | 0.15 (0.02) | 0.16 (0.02) | 0.16 (0.04) | 0.12 (0.03) | 0.11 (0.06) | −26.7% (p < 0.01) |
| JSD-MCP mean (cm) | 0.16 (0.02) | 0.16 (0.02) | 0.16 (0.03) | 0.17 (0.04) | 0.13 (0.02) | 0.12 (0.05) | −25.0% (p < 0.01) |
JSD-MCP = joint space distance–metacarpal phalangeal joint.
Table 4.
Reduction of DXR and RK Parameters from RA Severity Group 0 to 4 Regarding Sharp Joint Space Narrowing Score
| Score, n = 172 | 0 Mean (SD), n = 30 | 1 Mean (SD), n = 33 | 2 Mean (SD), n = 36 | 3 Mean (SD), n = 34 | 4 Mean (SD), n = 39 | Relative reduction from grade 0 to grade 4 |
|---|---|---|---|---|---|---|
| JSD-MCP II (cm) | 0.19 (0.02) | 0.18 (0.04) | 0.18 (0.05) | 0.15 (0.06) | 0.10 (0.08) | −47.4% (p < 0.01) |
| JSD-MCP III (cm) | 0.17 (0.02) | 0.16 (0.03) | 0.15 (0.03) | 0.10 (0.02) | 0.10 (0.07) | −41.2% (p < 0.01) |
| JSD-MCP IV (cm) | 0.16 (0.02) | 0.15 (0.02) | 0.15 (0.04) | 0.15 (0.02) | 0.13 (0.04) | −18.8% (p < 0.05) |
| JSD-MCP V (cm) | 0.16 (0.02) | 0.15 (0.02) | 0.15 (0.05) | 0.14 (0.03) | 0.10 (0.07) | −37.5% (p < 0.01) |
| JSD-MCP mean (cm) | 0.17 (0.02) | 0.16 (0.02) | 0.15 (0.04) | 0.15 (0.02) | 0.11 (0.06) | −35.3% (p < 0.01) |
JSD-MCP = joint space distance–metacarpal phalangeal joint.
The short-term precision (i.e., intraradiograph reproducibility with ten repeated measurements ofthe same digital image) of DXR and RK parameters shows the following coefficients of variation (CV):
| DXR-BMD: 0.19% | DXR-MCI: 0.24% |
| JSD-MCP II: 0.59% | JSD-MCP III: 0.85% |
| JSD-MCP IV: 0.99% | JSD-MCP V: 0.98% |
Discussion
Osteoporosis occurs more frequently in patients with RA than in healthy individuals.4,21 Periarticular osteoporosis was shown to be closely associated to level of disease activity, and, to a lesser extent, to disease duration, even indicating a maximal demineralization in early RA.22–24 Because of a frequent and severe involvement of metacarpal joints in the rheumatoid inflammatory process, Alenfeld et al.23 observed a higher degree of bone loss in the subregions of phalanges and metacarpals in comparison with whole-hand BMD reduction.
Our data revealed a significant reduction in periarticular cortical bone mass depending on the severity of RA using DXR (see Tables 2, 3, and 4), comparable to recently published results.25 Böttcher et al.26 revealed a marked severity-dependent DXR-BMD decline for patients in different stages of RA, and no significant loss of BMD as estimated by DXA in total femur and the lumbar spine.
DXR is ideal for quantification of periarticular demineralization without an interference from soft tissue, because this technique utilizes metacarpals as the measurement site. The earliest and most extensive inflammatory activity occurs here in RA. The influence of disease-related bony defects and erosions in the DXR calculations can be minimized because of DXR measurements on the diaphyseal part of the metacarpal bones.
The fact that DXR technology is based on a standard hand radiograph implies the advantage of providing BMD estimates from existing radiographs without exposing the patient to additional radiation. This is most relevant in the context of RA, in which hand radiographs are already acquired as part of the standard examination during the clinical care of these patients.
In addition, the short-term precision of DXR at approximately 0.40% is at a very low level,27 indicating that estimated demineralization is in fact disease-related and not based on the precision error of the densitometric method itself. Beside the operator-independent function of DXR, the high precision of DXR may also be explained by the procedure to detect the inner cortical edges of a bone, in particular for patients with RA-related cortical thinning.27 Additionally, a high-quality radiography with standardized imaging capture conditions preserves excellent reproducibility data.27 Constancy of tube voltage and the assortment of appropriate digital image devices are essential to achieve reliable results.27 Furthermore, the DXR software must adapt to the x-ray equipment, because DXR technology is dependent on a robust and consistent detection of edges. Therefore, image enhancement procedures nonlinearly affecting gray levels must not be applied on images intended to be measured with DXR.
This is ensured by defining a standard image processing protocol for each modality and subsequently verifying compliance to the protocol through DICOM tags before analyzing an image.27 This is referred to as image processing protocol, which is normally integrated in the compatible software of DXR, and comprises edge enhancement, noise reduction, and other similar procedures that depend on image contents.
DXR technology recently provided a calculation of BMD and MCI by using original digital images for direct analysis with an internal batch program version of the X-Posure System. This software applies its own calibration constants to adapt to the different resolutions. All noise-related interaction from the printing process, unintended image enhancement, variations based on different scanner resolutions, and slight position alterations during the scanning process of radiographs could be avoided, which consequently improve the precision of DXR.27
Besides the lack in our study design regarding a comparison between our techniques (i.e., DXR and RK) and an established method as gold standard, a further limitation of DXR may be the measurement of only the cortical partition of BMD. Otherwise, cortical thinning of periarticular bone, enhanced by the inflammation process, is a typical phenomenon of bone destruction in rheumatoid arthritis, which can be assumed because of very high bone turnover on the inner bone surface.28
Monitoring of inflammatory progression in the joints of hands and feet during the course of RA seems to be as relevant as assessing new destruction in previously nonaffected joints. Therefore, different scoring methods have been validated and established 19,20,29 that are based on conventional radiography as the common imaging technique to evaluate the progression of RA. Scoring methods are designed to semiquantitatively measure radiographically visible degeneration, in particular, erosions and joint space narrowing caused by cartilage damage.
The first scoring method, as devised by Steinbroker et al.,29 divided radiological changes in the hand skeleton into four stages, and grades only the most serious joint destruction. The Larsen Score evaluates 32 joints of hands and feet with a sixfold graduation (scoring device). Within the Larsen Score, the definition of grade 1 by soft tissue swelling and especially osteoporosis is disadvantageous, because both periarticular osteoporosis and soft tissue changes are unable to be effectively quantified on radiographs. The Larsen method was recently modified and now has a new definition of grade 1.19 A separate evaluation of erosions and joint space narrowing of hand and finger joints can be performed by the Sharp Score.20 A limitation in the scoring methods of assessing joint alterations by small lesions is that these lesions frequently can not be visualized if the location of erosion is not at the margin of the bone or if it is superimposed by other bony structures.30 The cross-sectional study reported by Jawaid et al.31 revealed that DXR estimates showed a better precision (i.e., smallest detectable difference) compared to the Sharp score.
Stewart et al.32 verified that DXR significantly predicted the erosive status of individuals; in this prospective longitudinal study, the reduction of DXR-BMD after 1 year was very specific (100%) and highly sensitive (63%) in detecting patients who developed an accelerated progress of RA with occurrence of erosions after a 4-year observation period.
In another cross-sectional study, DXR-BMD was independently associated with radiographic hand joint damage.33
Estimation of joint space narrowing is hindered, as inflammatory involved joints often show asymmetry, subluxation, and overlying soft tissue. The computerized technique for estimating joint space distances, presented and evaluated for the first time in this study, reveals severity-dependent narrowing of the JSD-MCP with high reproducibility. Independent of the scoring method, our results show a preference of narrowing regarding the JSD-MCP of the second and third fingers.
The usual disadvantages of joint space measurements using serial filming, such as different position of the hands during imaging, variation in x-ray beam angle, and disease-related limitations (in particular, destruction and subluxation of articulations), can be avoided by sampling a large portion of each joint space, which was done in our new technique as recommended by Sharp et al.9 To achieve reliable data, our study uses the metacarpal–phalangeal articulation for joint space calculation, which facilitates the edge detection via more effective visualization of the ridge of the joint margin by the computerized program and which shows the most inflammatory alterations caused by RA.5 Finally, this computerized, operator-independent calculation of the four JSD-MCP, DXR-MCI, and DXR-BMD for one hand requires only up to 4 min, thereby using less time compared to the scoring methods.
The combined application of DXR and the Radiogrammetry Kit seems to be promising for the initial detection and quantification of joint space narrowing and periarticular disease-related osteoporosis in RA patients. Further prospective studies will evaluate, in more detail, this technique and its advantages in obtaining quantitative information on reparative and therapy-induced changes.
References
- 1.Blair WF. An approach to complex rheumatoid arthritis hand and wrist problems. Hand Clin. 1996;12:615–628. [PubMed] [Google Scholar]
- 2.Sugimoto H, Takeda A, Masuyama J, Furuse M. Early-stage rheumatoid arthritis diagnositic accuracy of MR imaging. Radiology. 1996;198:185–192. doi: 10.1148/radiology.198.1.8539375. [DOI] [PubMed] [Google Scholar]
- 3.Tak PP, Bresnihan B. The pathogenesis and prevention of joint damage in rheumatoid arthritis. Advances from synovial biopsy and tissue analysis. Arthritis Rheum. 2000;43:2619–2633. doi: 10.1002/1529-0131(200012)43:12<2619::AID-ANR1>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 4.Lodder MC, Haugeberg G, Lems WF, Uhlig T, Orstavik RE, Kostense PJ, Dijkmans PA, Kvien TK, Woolf AD. Radiographic damage associated with low bone mineral density and vertebral deformities in rheumatoid arthritis: The Oslo–Truro–Amsterdam (OSTRA) collaborative study. Arthritis Rheum. 2003;49:209–215. doi: 10.1002/art.10996. [DOI] [PubMed] [Google Scholar]
- 5.Backhaus M, Kamradt T, Sandrock D. Arthritis of the finger joints: A comprehensive approach comparing radiography, scintigraphy, ultrasound, and contrast-enhanced magnetic resonance imaging. Arthritis Rheum. 1999;42:1232–1245. doi: 10.1002/1529-0131(199906)42:6<1232::AID-ANR21>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 6.Jergas M, Uffmann M, Escher H, Gluer CC, Young KC, Grampp S, Koster O, Genant HK. Interobserver variation in the detection of osteopenia by radiography and comparison with dual X-ray absorptiometry of the lumbar spine. Skeletal Radiol. 1994;23:195–199. doi: 10.1007/BF00197459. [DOI] [PubMed] [Google Scholar]
- 7.Pietka E, Gertych A, Pospiech-Kurkowska S, Cao F, Huang HK, Gilzanz V. Computer-assisted bone age assessment: Graphical user interface for image processing and comparison. J Digit Imaging. 2004;17:175–188. doi: 10.1007/s10278-004-1006-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Malich A, Fischer DR, Fascius M, Petrovitch A, Böttcher J, Marx C, Hansch A, Kaiser WA. Effect of breast density on computer aided detection. J Digit Imaging. 2005;19:1871–1889. doi: 10.1007/s10278-004-1047-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sharp JT, Gardner JC, Bennett EM. Computed-based methods for measuring joint space and estimating erosion volume in the finger and wrist joints of patients with rheumatoid arthritis. Arthritis Rheum. 2000;43:1378–1386. doi: 10.1002/1529-0131(200006)43:6<1378::AID-ANR23>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 10.Barnett E, Nordin B. The radiological diagnosis of osteoporosis: A new approach. Clin Radiol. 1960;11:166–174. doi: 10.1016/S0009-9260(60)80012-8. [DOI] [PubMed] [Google Scholar]
- 11.Rosholm A, Hylsdrup L, Baeksgaard L, Grunkin M, Thodberg HH. Estimation of bone mineral density by digital X-ray radiogrammetry: Theoretical background and clinical testing. Osteoporos Int. 2001;12:961–969. doi: 10.1007/s001980170026. [DOI] [PubMed] [Google Scholar]
- 12.Bouxsein ML, Palermo L, Yeung C, Black DM. Digital X-ray radiogrammetry predicts hip, wirst and vertebral fracture in elderly women: A prospective analysis from the study of osteoporotic fractures. Osteoporos Int. 2002;13:358–365. doi: 10.1007/s001980200040. [DOI] [PubMed] [Google Scholar]
- 13.Maffei L, Venarotti H, Treviso J, Sorensen TK, Nissen D. Digital X-ray radiogrammetry: A Hispanic normative reference database for the Pronosco X-Posure system. J Bone Miner Res. 2000;15:304. [Google Scholar]
- 14.Malich A, Freesmeyer MG, Mentzel HJ, Sauner D, Böttcher J, Petrovitch A, Behrendt W, Kaiser WA. Normative values of bone parameters of children and adolescents using digital computer-assisted radiogrammetry (DXR) J Clin Densitom. 2003;6:103–112. doi: 10.1385/JCD:6:2:103. [DOI] [PubMed] [Google Scholar]
- 15.Shepherd JA, Meta M, Landau J, Sherrer YSR, Goddard DH, Ovalle MI, Rosholm A, Genant HK. Metacarpal index and bone mineral density in healthy African-American women. Osteoporos Int. 2005;16:1621–1626. doi: 10.1007/s00198-005-1885-5. [DOI] [PubMed] [Google Scholar]
- 16.Wüster C, Wenzler M, Kappes J, Rehm C, Gühring T, Arnbjerg C. Digital X-ray radiogrammetry as a clinical method for estimating bone mineral density—a German reference database. J Bone Miner Res. 2000;15:298. [Google Scholar]
- 17.Hyldstrup L, Jorgensen JT, Sorensen TK, Baeksgaard L. Response of cortical bone to antiresorptive treatment. Calcif Tissue Int. 2001;68:135–139. doi: 10.1007/s002230001204. [DOI] [PubMed] [Google Scholar]
- 18.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fies FJ, Cooper NS. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 19.Larsen A. How to apply Larsen score in evaluating radiographs of rheumatoid arthritis in long-term studies? J Rheumatol. 1995;22:1974–1975. [PubMed] [Google Scholar]
- 20.Sharp JT, Young DY, Bluhm GB. How many joints in the hands and wirst should be included in a score of radiologic abnormalities used to assess rheumatoid arthritis? Arthritis Rheum. 1985;28:1326–1335. doi: 10.1002/art.1780281203. [DOI] [PubMed] [Google Scholar]
- 21.Haugeberg G, Orstavik RE, Uhlig T, Falch JA, Halse JI, Kvien TK. Bone loss in patients with rheumatoid arthritis. Results from a population-based cohort of 366 patients followed up to two years. Arthritis Rheum. 2002;46:1720–1728. doi: 10.1002/art.10408. [DOI] [PubMed] [Google Scholar]
- 22.Deodhar AA, Brabyn J, Jones PW, Davis MJ, Woolf AD. Longitudinal study of hand bone densitometry in rheumatoid arthritis. Arthritis Rheum. 1995;38:1204–1210. doi: 10.1002/art.1780380905. [DOI] [PubMed] [Google Scholar]
- 23.Alenfeld FE, Diessel E, Brezger M, Sieper J, Felsenberg D, Braun J. Detailed analyses of periarticular osteoporosis in rheumatoid arthritis. Osteoporos Int. 2000;11:400–407. doi: 10.1007/s001980070106. [DOI] [PubMed] [Google Scholar]
- 24.Devlin J, Lilley J, Gough A, Huissoon A, Holder R, Reece R, Perkins P, Emery P. Clinical associations of dual-energy X-ray absorptiometry measurement of hand bone mass in rheumatoid arthritis. Br J Rheumatol. 1996;35:1256–1262. doi: 10.1093/rheumatology/35.12.1256. [DOI] [PubMed] [Google Scholar]
- 25.Jensen T, Klarlund M, Hansen M, Jensen KE, Podenphant J, Hansen TM. Bone loss in unclassified polyarthritis and early rheumatoid arthritis is better detected by digital radiogrammetry than dual X-ray absorptiometry: Relationship with disease activity and radiographic outcome. Ann Rheum Dis. 2004;63:15–22. doi: 10.1136/ard.2003.013888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Böttcher J, Malich A, Pfeil A, Petrovitch A, Lehmann G, Heyne JP, Hein G, Kaiser WA. Potential clinical relevance of digital radiogrammetry for quantification of periarticular bone demineralization in patients suffering from rheumatoid arthritis depending on severity and compared with DXA. Eur Radiol. 2004;14:631–637. doi: 10.1007/s00330-003-2087-1. [DOI] [PubMed] [Google Scholar]
- 27.Böttcher J, Pfeil A, Rosholm A, Malich A, Petrovitch A, Heinrich B, Lehmann G, Mentzel HJ, Hein G, Linß W, Kaiser WA. Influence of image-capturing parameters on digital X-ray radiogrammetry. J Clin Densitom. 2005;8:87–94. doi: 10.1385/JCD:8:1:087. [DOI] [PubMed] [Google Scholar]
- 28.Jergas M. Conventional radiographs and basic quantitative methods. In: Grampp S, editor. Radiology of Osteoporosis. Berlin: Springer; 2003. pp. 62–64. [Google Scholar]
- 29.Steinbroker O, Traeger CH, Batterman RC. Therapeutic criteria in rheumatoid arthritis. JAMA. 1949;140:659–662. doi: 10.1001/jama.1949.02900430001001. [DOI] [PubMed] [Google Scholar]
- 30.Berglin E, Lorentzon R, Nordmark L, Nilsson-Sojka B, Rantapaa Dahlqvist S. Predictors of radiological progression and changes in hand bone density in early rheumatoid arthritis. Rheumatology. 2003;42:268–275. doi: 10.1093/rheumatology/keg077. [DOI] [PubMed] [Google Scholar]
- 31.Jawaid WB, Crosbie D, Shotton J, Reid DM, Stewart A. The use of digital X-ray radiogrammetry in the assessment of joint damage in rheumatoid arthritis. Ann Rheum Dis. 2006;65:459–464. doi: 10.1136/ard.2005.039792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stewart A, Mackenzie LM, Black AJ, Reid DM. Predicting erosive disease in rheumatoid arthritis. A longitudinal study of changes in bone density using digital X-ray radiogrammetry: a pilot study. Rheumatology. 2004;43:1561–1564. doi: 10.1093/rheumatology/keh385. [DOI] [PubMed] [Google Scholar]
- 33.Haugeberg G, Lodder MC, Lems WF. Hand cortical bone mass and its associations with radiographic joint damage and fractures in 50–70 year old female patients with rheumatoid arthritis: Cross sectional Oslo–Truro–Amsterdam (OSTRA) collaborative study. Ann Rheum Dis. 2004;63:1331–1334. doi: 10.1136/ard.2003.015065. [DOI] [PMC free article] [PubMed] [Google Scholar]


