Abstract
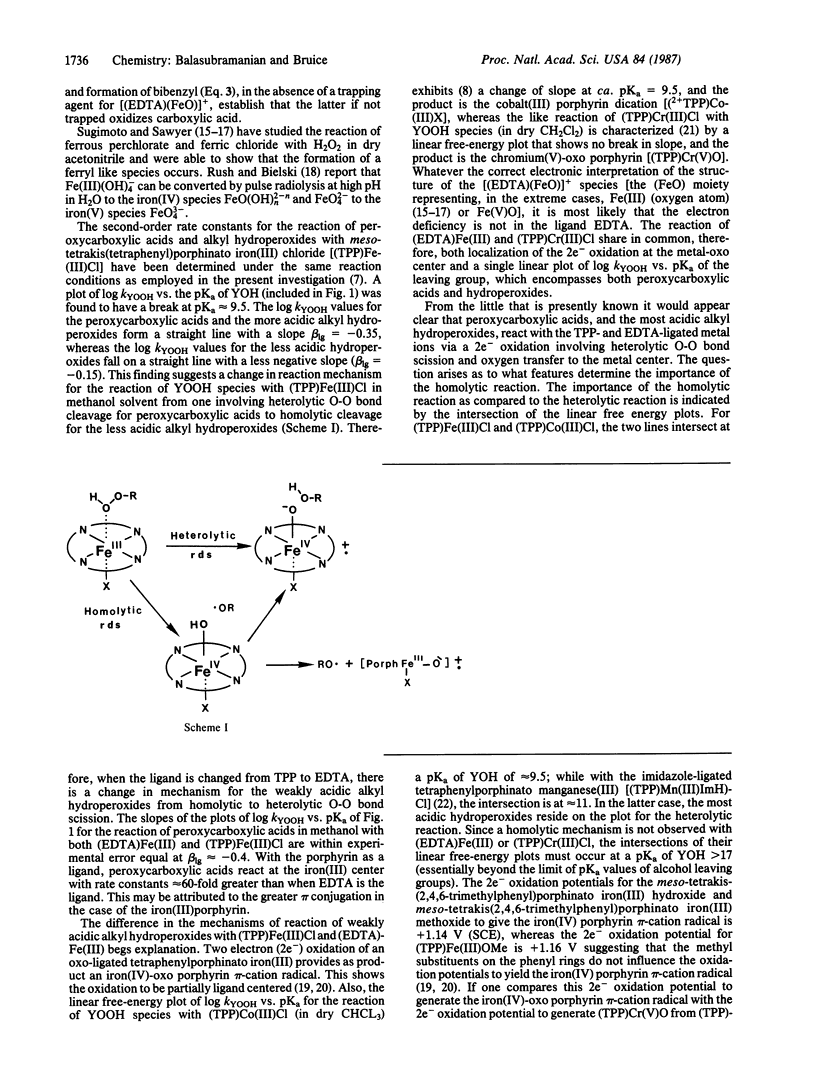
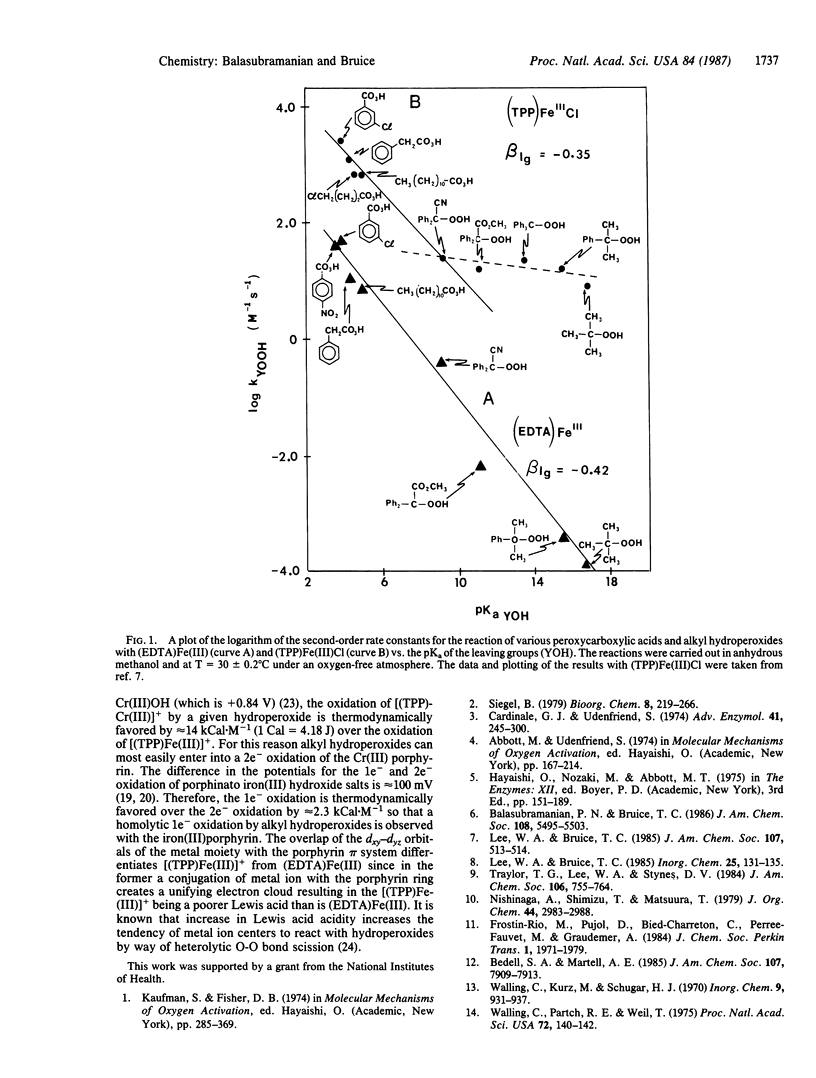
Ethylenediaminetetraacetato iron(III) [(EDTA)Fe(III)] has been shown to react with a series of four peroxycarboxylic acids and four alkyl hydroperoxides (YOOH; dry methanol solvent, 30 degrees C) by heterolytic O-O bond scission that accompanies the transfer of an oxygen atom to the iron(III) moiety [(EDTA)Fe(III) + YOOH----[(EDTA)(FeO)]+ + YOH]. A single linear free-energy relationship exists for both peroxycarboxylic acids and alkyl hydroperoxides when the logarithm of the second-order rate constant (kYOOH) for reaction of YOOH species with (EDTA)Fe(III) is plotted vs. the pKa of the YOH leaving group.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cardinale G. J., Udenfriend S. Prolyl hydroxylase. Adv Enzymol Relat Areas Mol Biol. 1974;41(0):245–300. doi: 10.1002/9780470122860.ch6. [DOI] [PubMed] [Google Scholar]
- Lee W. A., Calderwood T. S., Bruice T. C. Stabilization of higher-valent states of iron porphyrin by hydroxide and methoxide ligands: electrochemical generation of iron(IV)-oxo porphyrins. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4301–4305. doi: 10.1073/pnas.82.13.4301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walling C., Partch R. E., Weil T. Kinetics of the decomposition of hydrogen peroxide catalyzed by ferric ethylenediaminetetraacetate complex. Proc Natl Acad Sci U S A. 1975 Jan;72(1):140–142. doi: 10.1073/pnas.72.1.140. [DOI] [PMC free article] [PubMed] [Google Scholar]


