Abstract
A brief exposure to isoflurane prior to brain ischemia reduces ischemic brain injury in rodents. Here we showed that exposure of rat cerebral cortical neuronal cultures to 2% isoflurane for 30 min at 24 h before a 2-h oxygen-glucose deprivation (OGD) reduced the OGD-induced cell injury. This effect was abolished by HA14-1, a selective inhibitor of B-cell lymphoma 2 (Bcl-2) protein. Bcl-2 is well-known for its anti-apoptotic property. HA14-1 alone did not change OGD-induced cell injury. OGD reduced the expression of Bcl-2 in these neurons. This reduction was attenuated by isoflurane preconditioning. These results suggest that isoflurane preconditioning-induced neuroprotection is mediated by Bcl-2.
Keywords: Bcl-2 protein, isoflurane, neurons, preconditioning
1. Introduction
Ischemic brain injury is a fundamental pathophysiological process underlying various diseases, such as stroke and brain trauma. Thus, identifying methods to reduce ischemic brain injury has been a research focus in the scientific community. One of these methods is preconditioning that involves application of a preconditioning stimulus or drugs prior to an insult to reduce the insult-induced cell injury (Gidday 2006). We and others have shown that commonly used volatile anesthetics can induce a preconditioning effect in animal brains (Kapinya et al., 2002; Li et al., 2008; Li and Zuo 2009; Zheng and Zuo 2004). This form of neuroprotection may have a significant implication in the perioperative period if this protection can be demonstrated in humans.
Our previous studies have suggested a role of B-cell lymphoma 2 (Bcl-2) protein (Li et al. 2008; Li and Zuo 2009; Zhao et al., 2007), an anti-apoptotic protein (Yang et al., 1997), in the isoflurane preconditioning-induced neuroprotection because isoflurane increased the Bcl-2 expression in rat brain. We designed this study to test the hypothesis that Bcl-2 mediates isoflurane preconditioning-induced neuroprotection.
2. Materials and Methods
The animal protocol was approved by the institutional Animal Care and Use Committee of the University of Virginia (Charlottesville, VA, USA). All animal experiments were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (National Institutes of Health publication No. 80-23) revised in 1996.
2.1. Primary neuronal culture
Cerebral cortical neuron cultures were prepared as we described previously (Zuo et al., 1999). Briefly, cerebral hemispheres of 16- to 18-day gestation Sprague–Dawley rat fetuses were isolated and finely minced in Dulbecco’s phosphate-buffered saline (D-PBS) on ice. They were digested by 5 mg/ml trypsin in Dulbecco’s modified Eagle’s medium (DMEM) for 25 min at 37°C and then triturated with a “fire-polished” Pasteur pipette into DMEM containing 1 M N-2-hydroxyethylpiperazine-N-2-ethanesulfonic acid (HEPES), 10% Ham’s F12 medium, 10% heat-inactivated fetal calf serum, 100 μg/ml streptomycin and 100 U/ml penicillin. Cells were plated in the same medium at a density of 3×106 cells/well of six-well tissue culture plates that had been precoated with 20 μg/ml poly-L-lysine. The culture plates were placed in a humidified incubator at 37°C in 5% CO2 in air. The medium was changed every two or three days. Cytosine β-D-arabino-furenoside (10 μM) was added to thecultures for 24 h to inhibit glial cell proliferation on day 4 or 5. They then were cultured in the same medium but without Ham’s F-12 to eliminate glutamate in the medium. Cells were used for experiments after 8 days in culture.
2.2. Isoflurane preconditioning and OGD
Isoflurane preconditioning was performed in an airtight chamber with 2% isoflurane for 30 min in 5% CO2-95% air at 37°C as we described before (Xu et al., 2008). Cells were treated identically but without isoflurane for the groups that did not need isoflurane preconditioning.
OGD was performed at 24 h after isoflurane preconditioning. As we described before (Kim et al., 2009b), OGD was applied by replacing the culture medium with glucose-free buffer containing 154 mM NaCl, 5.6 mM KCl, 3.6 mM NaHCO3, 3.6 mM CaCl2 and 5.0 mM HEPES at pH 7.4. This buffer was pregassed with 100% N2 for 5 min. The cells were placed in an airtight chamber that was gassed with 100% N2 for 10 min. After confirmation of hypoxia (< 1% oxygen content) in the chamber gas, the chamber was closed and kept at 37°C for 2 h. Cells in the groups that did not need OGD were incubated in the same solution but containing 4.5 g/L glucose under normoxic conditions. After OGD, the buffer was replaced with fresh culture medium. The cultures were maintained under their normal culture conditions for 24 h before they were used for lactate dehydrogenase (LDH) release assay or 3 h before they were harvested for western analysis.
Ethyl 2-amino-6-bromo-4-(1-cyano-2-ethoxy-2-oxoethyl)-4H-chromene-3-carboxylate) (HA 14-1), a selective Bcl-2 inhibitor (Wang et al., 2000), was added to make a final concentration of 7.5 μM just before the OGD and was present throughout the 24-h after the OGD.
2.3. Lactate dehydrogenase activity assay
The LDH activity assay was based on the cleavage of a tetrazolium salt by LDH. The assay was performed as we described before (Kim et al., 2009a). The amount of LDH in the culture medium (released LDH) and in the cells (intracellular LDH that was measured after the cells were lyzed by 0.5% Triton X-100™ solution) was quantified. The percentage of released LDH in the total LDH (released LDH plus intracellular LDH) was calculated and used to reflect the cell injury.
2.4. Western analysis
Twenty microgram proteins per lane of cell lysates were subjected to 15% sodium dodecylsulfate-polyacrylamide gel electrophoresis and then transferred onto a polyvinylidene fluoride membrane. After incubation with PBS containing 5% nonfat milk and 0.1% Tween-20 for 1 h at room temperature, the membrane was probed with various primary antibodies in PBS containing 0.1% Tween-20 overnight at 4°C. The primary antibodies used were mouse monoclonal anti-Bcl-2 antibody (1:200 dilution, catalog number: ab16904, Abcam, Cambridge, MA), mouse monoclonal anti-Bax antibody (1:200 dilution, catalog number: sc-23959, Santa Cruz Biotechnology, Santa Cruz, CA) and rabbit polyclonal anti-actin antibody (1:1000 dilution, Catalog number: A2066, Sigma Chemical, St Louis, MI). Appropriate secondary antibodies were used and the protein bands were visualized with the enhanced chemiluminescence methods using a Genomic and Proteomic Gel Documentation (Gel Doc) Systems from Syngene (Frederick, MD). The densities of Bcl-2 and Bax protein bands were normalized to those of actin in the same sample to control for variations in protein loading and transferring during western analysis. The results from cells exposed to OGD or isoflurane preconditioning plus OGD were normalized to the corresponding data of control cells in the same experiment.
2.5. Statistical analysis
Data are presented as means ± SD and were analyzed by one-way analysis of variance followed by the Student-Newman-Keul’s test for post hoc comparison. A P < 0.05 was considered as significant.
3. Results
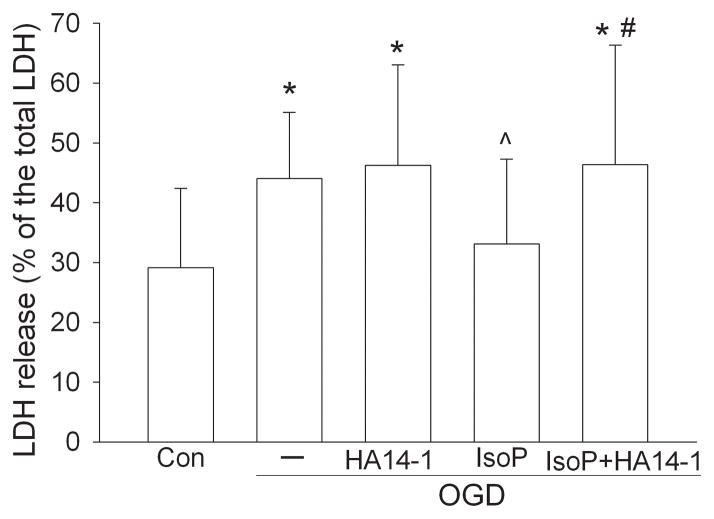
A 2-h exposure to OGD significantly increased LDH release from the neuronal cultures, suggesting that OGD caused cell injury. This injury was reduced significantly by isoflurane preconditioning. Although the degree of cell injury was not different between OGD alone and OGD plus HA14-1, HA14-1 abolished isoflurane preconditioning-induced neuroprotection (Fig. 1).
Fig. 1. Isoflurane preconditioning-induced protection.
Rat cerebral cortical neuronal cultures were exposed to or not exposed to 2% isoflurane for 30 min at 24 h before a 2-h oxygen-glucose deprivation (OGD). Lactate dehydrogenase release was assayed at 24 h after the OGD. Results are means ± SD (n = 30 – 35). * < 0.05 compared with control. ^ P < 0.05 compared with OGD only. # P < 0.05 compared with isoflurane preconditioning and OGD. Con: control; IsoP: Isoflurane preconditioning.
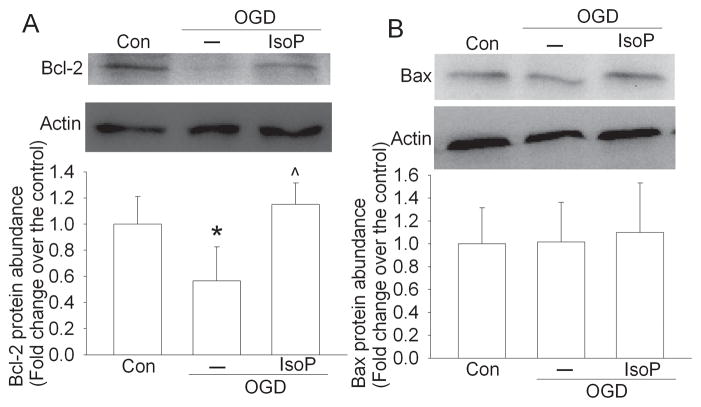
OGD significantly reduced Bcl-2 expression in these cortical neuronal cultures. This reduction was attenuated by isoflurane preconditioning (Fig. 2). The expression of Bax was not affected by OGD or isoflurane preconditioning (Fig. 2).
Fig. 2. Isoflurane preconditioning-induced perseveration of Bcl-2 expression.
Rat cerebral cortical neuronal cultures were exposed to or not exposed to 2% isoflurane for 30 min at 24 h before a 2-h oxygen-glucose deprivation (OGD). Cells were harvested 3 h after the OGD for western blotting. Results are means ± SD (n = 6) in the bar graphs. * P < 0.05 compared with control. ^ P < 0.05 compared with OGD only. Con: control; IsoP: Isoflurane preconditioning.
4. Discussion
We and others have shown that isoflurane preconditioning-induced neuroprotection in rodents under in vivo conditions (Kapinya et al. 2002; Li et al. 2008; Zheng and Zuo 2004). Our current results showed that isoflurane preconditioning also induced a protection in neuron-rich cultures, consistent with previous studies (Kapinya et al. 2002; McMurtrey and Zuo 2010). These results suggest that isoflurane may directly precondition the neurons to protect them against detrimental insults, such as ischemia/OGD.
Preconditioning-induced protection has two temporal phases (Dirnagl et al., 2003). The acute phase starts a few minutes after the preconditioning stimulus and lasts for a few hours. This phase of protection usually involves functional changes of existing proteins in the cells. The delayed phase occurs a few hours after the preconditioning stimulus and can last for days or even weeks. This protection is often mediated by increased expression of protective proteins (Dirnagl et al. 2003). We have shown that isoflurane can increase Bcl-2 expression in rat brain (Li et al. 2008; Li and Zuo 2009). Inhibition of inducible nitric oxide synthase inhibits isoflurane preconditioning-induced neuroprotection and increase of Bcl-2 expression (Zhao et al. 2007). These results, along with the knowledge that Bcl-2 is a protective protein (Yang et al. 1997), suggest a role of Bcl-2 in isoflurane preconditioning-induced neuroprotection. Our current study showed that OGD reduced Bcl-2 expression and that isoflurane preconditioning preserved Bcl-2 expression in cells subjected to OGD. In addition, HA14-1, a specific Bcl-2 inhibitor (Wang et al. 2000), abolished isoflurane preconditioning-induced neuroprotection in the neuronal cultures. These results provide stronger and more direct evidence for Bcl-2 to mediate the isoflurane preconditioning-induced neuroprotection.
One of the important mechanisms for Bcl-2 to provide protection is to block cytochrome c release from the mitochondria and, therefore, to prevent the activation of apoptotic pathways (Cahill et al., 2006; Ferrer and Planas 2003). This effect may be due to its binding to damaging proteins, such as Bax, to prevent these damaging proteins to form pores in the mitochondrial membrane (Cahill et al. 2006; Ferrer and Planas 2003). These pores can allow cytochrome c release from the mitochondria. HA14-1 is a small non-peptide ligand that can bind the Bcl-2 surface pocket. This binding blocks Bcl-2 to bind with the damaging proteins including Bax and, thus, inhibits the protective effects of Bcl-2 (Wang et al. 2000). In our study, OGD did not affect the Bax expression but decreased Bcl-2, suggesting a role of Bcl-2 decrease in the OGD-induced cell injury.
Acknowledgments
This study was supported by a grant (R01 GM065211 to Z Zuo) from the National Institutes of Health, Bethesda, MD, by a grant from the International Anesthesia Research Society (2007 Frontiers in Anesthesia Research Award to Z Zuo), San Francisco, CA and the Department of Anesthesiology, University of Virginia
Footnotes
Conflicts of interest
We have no conflicting or competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Cahill J, Calvert JW, Zhang JH. Mechanisms of early brain injury after subarachnoid hemorrhage. J Cereb Blood Flow Metab. 2006;26(11):1341–53. doi: 10.1038/sj.jcbfm.9600283. [DOI] [PubMed] [Google Scholar]
- Dirnagl U, Simon RP, Hallenbeck JM. Ischemic tolerance and endogenous neuroprotection. Trends Neurosci. 2003;26(5):248–54. doi: 10.1016/S0166-2236(03)00071-7. [DOI] [PubMed] [Google Scholar]
- Ferrer I, Planas AM. Signaling of cell death and cell survival following focal cerebral ischemia: life and death struggle in the penumbra. J Neuropathol Exp Neurol. 2003;62(4):329–39. doi: 10.1093/jnen/62.4.329. [DOI] [PubMed] [Google Scholar]
- Gidday JM. Cerebral preconditioning and ischaemic tolerance. Nat Rev Neurosci. 2006;7(6):437–48. doi: 10.1038/nrn1927. [DOI] [PubMed] [Google Scholar]
- Kapinya KJ, Lowl D, Futterer C, Maurer M, Waschke K, Isaev NK, Dirnagl U. Tolerance Against Ischemic Neuronal Injury Can Be Induced by Volatile Anesthetics and Is Inducible NO Synthase Dependent. Stroke. 2002;33:1889–1898. doi: 10.1161/01.str.0000020092.41820.58. [DOI] [PubMed] [Google Scholar]
- Kim J, Li L, Z Z. Delayed treatment with isoflurane attenuates lipopolysaccharide and interferon ?-induced activation and injury of mouse microglial cells. Anesthesiology. 2009a;111:566–73. doi: 10.1097/ALN.0b013e3181af5b3d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JA, Li L, Zuo Z. Isoflurane induces a postconditioning effect on bovine pulmonary arterial endothelial cells exposed to oxygen-glucose deprivation. Eur J Pharmacol. 2009b;615(1–3):144–9. doi: 10.1016/j.ejphar.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Peng L, Zuo Z. Isoflurane preconditioning increases B-cell lymphoma-2 expression and reduces cytochrome c release from the mitochondria in the ischemic penumbra of rat brain. Eur J Pharmacol. 2008;586:106–113. doi: 10.1016/j.ejphar.2008.02.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Zuo Z. Isoflurane preconditioning improves short-term and long-term neurological outcome after focal brain ischemia in adult rats. Neuroscience. 2009;164:497–506. doi: 10.1016/j.neuroscience.2009.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMurtrey RJ, Zuo Z. Isoflurane preconditioning and postconditioning in rat hippocampal neurons. Brain Res. 2010;1358:184–90. doi: 10.1016/j.brainres.2010.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JL, Liu D, Zhang ZJ, Shan S, Han X, Srinivasula SM, Croce CM, Alnemri ES, Huang Z. Structure-based discovery of an organic compound that binds Bcl-2 protein and induces apoptosis of tumor cells. Proc Natl Acad Sci U S A. 2000;97(13):7124–9. doi: 10.1073/pnas.97.13.7124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Feng J, Zuo Z. Isoflurane preconditioning reduces the rat NR8383 macrophage injury induced by lipopolysaccharide and interferon gamma. Anesthesiology. 2008;108(4):643–50. doi: 10.1097/ALN.0b013e318167aeb4. [DOI] [PubMed] [Google Scholar]
- Yang J, Liu X, Bhalla K, Kim CN, Ibrado AM, Cai J, Peng TI, Jones DP, Wang X. Prevention of apoptosis by Bcl-2: release of cytochrome c from mitochondria blocked. Science. 1997;275(5303):1129–32. doi: 10.1126/science.275.5303.1129. [DOI] [PubMed] [Google Scholar]
- Zhao P, Peng L, Li L, xu X, Zuo Z. Isoflurane preconditioning improves long-term neurological outcome after hypoxic-ischemic brain injury in neonatal rats. Anesthesiology. 2007;107(6):963–70. doi: 10.1097/01.anes.0000291447.21046.4d. [DOI] [PubMed] [Google Scholar]
- Zheng S, Zuo Z. Isoflurane preconditioning induces neuroprotection against ischemia via activation of p38 mitogen-activated protein kinase. Mol Pharmacol. 2004;65:1172–1180. doi: 10.1124/mol.65.5.1172. [DOI] [PubMed] [Google Scholar]
- Zuo Z, Tichotsky A, Johns RA. Inhibition of excitatory neurotransmitter-nitric oxide signaling pathway by inhalational anesthetics. Neuroscience. 1999;93(3):1167–72. doi: 10.1016/s0306-4522(99)00194-3. [DOI] [PubMed] [Google Scholar]