Abstract
Fullerenes are sphere-like molecules with unique physico-chemical properties, which render them of particular interest in biomedical research, consumer products and industrial applications. Human and environmental exposure to fullerenes is not a new phenomenon, due to a long history of hydrocarbon-combustion sources, and will only increase in the future, as incorporation of fullerenes into consumer products becomes more widespread for use as anti-aging, anti-bacterial or anti-apoptotic agents.
An essential step in the determination of biological effects of fullerenes (and their surface-functionalized derivatives) is establishment of exposure-assessment techniques. However, in ecotoxicological studies, quantification of fullerenes is performed infrequently because robust, uniformly applicable analytical approaches have yet to be identified, due to the wide variety of sample types. Moreover, the unique physico-chemistry of fullerenes in aqueous matrices requires reassessment of conventional analytical approaches, especially in more complex biological matrices (e.g., urine, blood, plasma, milk, and tissue).
Here, we present a review of current analytical approaches for the quantification of fullerenes and propose a consensus approach for determination of these nanomaterials in a variety of environmental and biological matrices.
Keywords: Carbonaceous nanoparticles, Liquid chromatography, Environmental impact study, Liquid-liquid extraction, Mass spectrometry, Solid-phase extraction, Nanomaterial, Nanotoxicology, nC60, Toxicity assessment
1. Introduction
Fullerenes are molecules of pure carbon (e.g., C60 and C70) that are increasingly investigated for use in biomedical, cosmetic and industrial applications, ranging from drug delivery systems to anti-aging formulations to electrical components. Commercial and scientific interest is spawned by their unique physico-chemical properties that make them both robust and versatile. Fullerenes possess a thermodynamically-stable carbon shell of about 10 Å in diameter (for C60) that can withstand heat, pressure and radiation [1–3] but, due to their unique electron-hybridization pattern of sp2 bonds, they are also highly configurable. These electron double bonds allow pristine fullerenes, which are intrinsically hydrophobic [4], to become readily derivatized and water soluble through the addition of various functional groups {e.g., oxygen-, hydroxyl-, polyvinylpyrrolidone (PVP)- [5], and phenyl Cn-butyric acid methyl-ester moieties [6]}. This functionalization enables fullerene derivatives to permeate through cellular membranes [7,8], where they may interact with and inhibit active sites of enzymes [9]. Hence, potential biomedical applications are numerous and have been comprehensively reviewed [10]. Briefly, fullerenes and their derivatives were found to show potential as antiviral and antibacterial agents, slow-release drug- or gene-delivery systems, electron-transfer shuttles, and antioxidant and radical scavengers, although they also can generate reactive oxygen species {e.g., singlet oxygen, superoxide and hydroxyl radicals (references in [10]).
The marketable value of fullerenes is anticipated to lead to increased human and environmental exposure on a global scale. This process is already under way, most notably in the widespread dermal application of cosmetic products [11], inhalation of dust particles [12] or soot from combustion processes [13], and discharge of fullerene-containing products into waterways [14]. To unravel the biological effects of fullerenes, numerous groups have initiated ecotoxicological [15–18], pharmacokinetic [19–21], environmental-fate [14] and environmental-impact studies [1,22–26].
To interpret the toxicological data accurately, establish the pharmacokinetics, or determine the body burden, the concentrations that organisms or cells are (intentionally) exposed to during experiments should be unambiguously determined using a robust methodology. Moreover, the availability of robust, overarching methods would further improve exposure assessments, which, along with biological effects, are required for meaningful risk assessments, and can be a prerequisite for considering potential regulations for limiting environmental discharge and human exposure. The scientific community has therefore initiated an effort to deliver such methods, which are reviewed below and are extrapolated to human and ecological risk-assessment studies.
A tremendous amount of data has been generated regarding the environmental implications of fullerenes. For readers specifically interested in the field of environmental exposure to fullerenes, we would like to refer to recently published comprehensive reviews on:
the quantitative analysis of fullerenes in environmental samples [27];
the colloidal properties of fullerenes [28];
the behavior and ecotoxicity of carbon nanoparticles in the aquatic environment [29];
the physico-chemical interactions between nanoparticles and biological systems [30]; and,
the mechanisms driving toxicity of fullerenes [31].
In the past few years, significant advances have been made in the analytical quantification of fullerenes [18,20,27,32]. Traditional detection strategies include visualization techniques (e.g., transmission electron microscopy) to ascertain the presence of fullerenes [23,33], or use of radioactively or fluorescently labeled fullerenes for fate studies of carbonaceous nanoparticles in vivo and in vitro [34–36]. Since each method comes with its own benefits and limitations, it is frequently desirable to use two or more strategies for fullerene tracking. For example, labeling techniques offer convenient ease of use but may also affect the behavior of the nanomaterials during the course of the experiment. While these methods are valid and yield valuable information, future investigations on human subjects (e.g., to determine the body burden) will require a less invasive, more quantitative approach. More recent approaches therefore employ liquid chromatography coupled to ultraviolet spectroscopy (LC/UV) or mass spectrometry (LC/MS) to quantify fullerenes in plasma or skin [37].
In this article, we review analytical quantification approaches for pristine C60 fullerenes, and place them in the framework of studies in environmental health, ecotoxicology and pharmacokinetics. Most of the manuscripts discussed are readily available through scientific databases (e.g., NCBI PubMed and ISI Web of Knowledge). Our goal was to elucidate and discuss three phases in the workflow process that are pivotal for human health and ecotoxicological studies involving fullerenes and that have been shown to affect significantly the outcome of nanometrology, which we identified as:
nC60-stock suspension preparation;
spiking and extraction; and,
detection, quantification and recovery determination.
2. Water-soluble fullerene dispersions and their detection
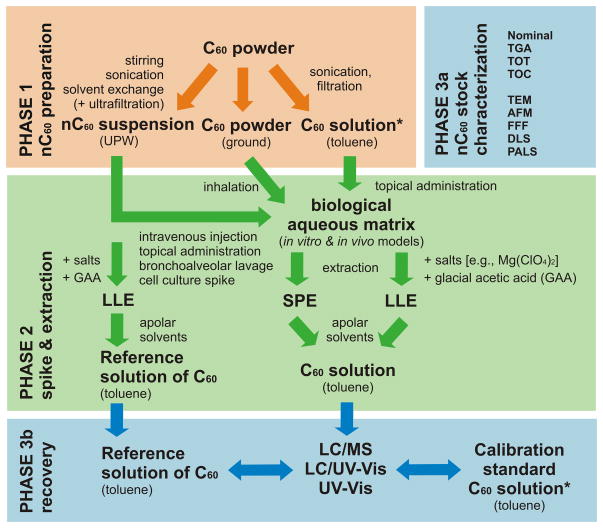
Experimental protocols for the quantification of fullerenes in biological systems encompass three distinct phases that can significantly affect the outcome of the ecotoxicological and analytical findings (Fig. 1):
Figure 1.
The three phases of a fullerene-exposure experiment. In phase 1, the C60 solution and nC60 aggregate suspension are prepared, and both can be used to administer fullerenes to a biological matrix. In phase 2, the fullerenes are spiked in a biological matrix and then extracted using liquid–liquid extraction (LLE) or solid-phase extraction (SPE). The nC60 dispersion in ultrapure water (UPW) is used as a reference for the extraction efficiency. In phase 3, the C60 and nC60 stocks are characterized and the recovery from the biological matrix is determined by comparing to the calibration standard of C60 in toluene or the reference solution of C60 after exhaustive LLE. TGA: thermogravimetric analysis; TOT: thermal/optical transmission analysis; TOC: total organic carbon analysis; TEM: transmission electron microscopy; AFM: atomic force microscopy; FFF: field flow fractionation; DLS: dynamic light scattering; PALS: phase analysis light scattering. The asterisk indicates the same C60 solution.
During the initial phase, the C60-calibration standards and the aqueous nC60-stock suspension are prepared. While the former is used exclusively for quantification (with some exceptions [37]), the latter is used for both quantification [22] and spiking of fullerene aggregates into aqueous and biological matrices. Preparing an aqueous-stock suspension of the hydrophobic C60 molecules has proven to be a pivotal step in assessing the toxicity of fullerenes and is required for, e.g., intravenous injections, topical administrations, bronchoalveolar lavages, and in vitro toxicity assays [20,36,38,39]. The dispersion of C60 in the air has also been reported, mainly to assess the impact of inhalation of nanomaterials during in vivo exposure studies [39].
The second phase concentrates on the exposure experiment and encompasses the spiking/dosing and extraction of the nanomaterials from the environmental and biological samples. In this section, the matrix-dependent flexibility of the experimental design is reviewed. As discussed in more detail below (sub-section 2.2.), the selection of the spiking strategies and extraction protocols from blood, milk, urine and tissue is driven mostly by the availability of source materials and by existing analytical limitations.
The final phase comprises the actual quantification of the fullerenes. Various protocols have been described to determine the recovery efficiency of fullerenes in biological matrices. Those approaches are reviewed in the framework of the type of exposure experiment conducted.
2.1. Phase 1 – Preparation of the nC60 source material
2.1.1. Dispersion of C60 fullerenes in water
Pristine C60 fullerenes are highly hydrophobic (log Kow = 6.67), due to their composition of pure carbon [4]. As a result, they are only poorly dissolvable in aqueous matrices (solubility in water 7.96 ng/L – 11 µg/L) [4, 40]. When C60 powder is introduced into water (Fig. 1), it can take between two weeks and several months of stirring to obtain a stable aqueous suspension [38,41–44]. As they slowly transition from the bulk phase to the water phase, pristine fullerenes form stable aggregates, which exhibit colloidal properties and are generally referred to as nano-C60 (nC60). These water-stable nC60 particles exhibit physico-chemical duality, as they form aggregates with a hydrophobic core surrounded by a hydrophilic shell or cluster of water molecules [45]. This polar cloak is stabilized by weak donor-acceptor type bonds between the hydrogen of the water molecules [45], and is potentially the source of the typical electronegative surface of the nC60 aggregates [46,47].
Direct transfer of fullerene powder into water by stirring or sonication is considered the most representative method to create nC60 [24], as it approaches the real-world transition of C60 from the particulate or commercialized form to the aqueous form. nC60 prepared by direct transfer into water is denoted as aqu/nC60 and it was shown to be the most stable form of nC60 nanoparticles compared to the aggregates generated with other methods (see sub-section 2.1.3) [48]. The direct dissolution method avoids the use of solvents (e.g., THF) that could impart artifacts to (eco)toxicological studies, but the preparation can be extremely time consuming [49].
Electrophoretic mobility, electrokinetic potential, surface charge or zeta potential is commonly measured using phase-analysis light scattering (PALS) instrumentation [48,50]. The size range of the colloids can be determined directly in suspension using dynamic light scattering (DLS) [44] or field flow fractionation (FFF) [51]. These data can then be validated after evaporation of the sample, using transmission electron microscopy (TEM) [44,52] and atomic force microscopy (AFM) [53]. The size ranges of spherical and hexagonal-shaped nC60 particles are often mono-dispersed and change only slightly over time [38,44,54,55], but some have reported polydispersity of the nC60 particles [47,51]. The reported sizes vary in the ranges from ~10–500 nm in ultrapure water [52,56] to ~500–1200 nm in the presence of ionic compounds (i.e. salts) (Table 1).
Table 1.
Overview of preparation approaches for nC60-stock suspensions and characteristics of the colloidal fullerenes
| Ref. | Preparation |
Characterization |
Quantification |
|||
|---|---|---|---|---|---|---|
| Stock suspension | Filter | Size (nm) | Charge (mV) | Conc. (mg/L) | Method | |
| [62] | Solvent exchange | NR | 250–350 | NR | 1.512 | NR |
| ben/C60 + THF + acetone + UPW | ||||||
| [63] | Solvent exchange + co-solubilization | NR | NR | NR | 400 | Nominal |
| ben/C60 + CLF + PVP + UPW + son. | ||||||
| [56] | Solvent exchangea | 0.45 μm, c | 62.8 | −30 | NRd | LLE-UV |
| THF/C60 + UPW | PTFE | |||||
| [55] | Solvent exchange | |||||
| Tol/C60 + THF + acetone + UPW | NR | 20–168 | −50f | 151–1 498 | NR | |
| THF/C60 | NR | 249 | NR | NR | NR | |
| [22] | Solvent exchange | 0.22 μm, cellulose acetate | 60–120e | −36 (at pH 5.0) | NR | LLE-LC/UV |
| THF/C60 + UPW + rotary evap. | NR | TGA | ||||
| [24] | Solvent exchangea | 0.22 μm, c Nylon & cellulose | 50–150 | NR | 2–15 | LLE-UV |
| THF/C60 + UPW + rotary evap. | ||||||
| Sonication | 0.45 & 0.22 μm, nylon & cellulose | <10–25 | NR | 2–15 | LLE-UV | |
| tol/C60 + UPW | ||||||
| Stirring | 0.45 & 0.22 μm, nylon & cellulose | 30–100 | NR | 2–15 | UV | |
| 2–4 weeks, in UPW at 40°C | ||||||
| Co-solubilization | 0.45 & 0.22 μm, nylon & cellulose | <10–25 | NR | 2–15 | UV | |
| tol/C60 + CLF + PVP + UPW | ||||||
| [37] | Solvent exchange | 0.22 μm, c nylon | NR | NR | 3.10 | LLE-LC/UV |
| THF/C60 + UPW + rotary evap. | ||||||
| [47] | Solvent exchange | |||||
| tol/C60 + THF + acetone + UPW + dist. | NR | 170±20 | −30 | 3.5 | NR | |
| THF/C60 + UPW + rotary evap. | 0.22 μm, c Nylon | 160±20 | −50 | 6 | NR | |
| tol/C60 + UPW + son. | 0.45 μm, Type NR | 160±20 | −30 | 9 | NR | |
| Stirring | 0.45 μm, Type NR | 180±20 | −30 | 5 | NR | |
| 2 weeks | ||||||
| [42] | Grinding | NR | 235 | −39.0 ±1.4 | 473 | NR |
| Ground C60 + UPW + SDS + son. | ||||||
| [60] | Solvent exchange | NR | NR | NR | 50 (31b) | Nominal (LLE-LC/MS) |
| DMSO/C60 + UPW + son. | ||||||
| [44] | Solvent exchange | 0.45 μm, Type NR | 121.8 ±0.8 | −31.6 ±2.3 | 4.20 ±0.86 | LLE- LC-MS |
| EtOH/C60 + UPW + son. +rotary evap. | ||||||
| Stirring | 0.45 μm, Type NR | 178.6 ±1.2 | −13.5 ±1.1 | 0.22 ±0.07 | Evap-UV | |
| 2 weeks – 11 months | 211.8 ±1.9 | −44.5 ±0.4 | 0.23 ±0.05 | UF-UV | ||
| [41] | Stirring | NR | 513–1270 | −61.8 to −43 | 250 | NR |
| 13 days in 10 mM NaCl, pH = 4,7,10 | ||||||
| [64] | Solvent exchange | 0.7 μm, glass fiber | 60–70 | −44 (at pH 7.2) | 87.3 | TGA |
| tol/C60 + UPW + EtOH + son. | ||||||
| [43] | Solvent exchange | NR | 120–250 | −54 to −48 (at pH 7.0) | 50 | Nominal |
| tol/C60 + UPW + N2 purging | ||||||
| Stirring | Settling + 10 μm, type NR | 380–580g | NR | 50 | Nominal | |
| 4 weeks in UPW, SDS, or NOMg | ||||||
| [1] | Stirring | NR | 360 ±210 | NR | NR | NR |
| 5 weeks in UPW | ||||||
| [48] | Sonication | 0.45 & 0.22 μm, | 50.5 ±1.0 | −40 to −20 (at pH 5.5) | 11.62 | TOC |
| tol/C60 +UPW + EtOH + son. | Cellulose | |||||
| Stirring | 0.45 & 0.22 μm, cellulose acetate & cellulose nitrate | 83.1 | −60 to −30 (at pH 5.5) | 3.34 | TOC | |
| 40 days | ||||||
| [15] | Sonication | |||||
| Corn oil/nC60 | NR | 234–3124 | NR | NR | NR | |
| Saline/nC60 | NR | 407–5117 | NR | NR | NR | |
| [65] | Solvent exchange | 0.45 μm, type NR | 73–191 | −40 | NR | LLE-UV/Vis |
| tol/C60 + UPW + EtOH + son. | ||||||
| [51] | Sonication | 0.45 μm, cellulose acetate | 130 ±1 | −40 ±1 | 1.7 ±0.12 | LLE-LC/MS |
| Ground C60 + son. | ||||||
ben, benzene; CLF, chloroform; Conc., concentration; dist., distillation; DMSO, dimethylsulfoxide; EtOH, ethanol; Evap., evaporation; LC-UV, online ultraviolet spectroscopy following liquid chromatography; LLE, exhaustive liquid-liquid extraction; 13C NMR, nuclear magnetic resonance targeting 13C; NOM, natural organic matter; Nominal, weighed mass per added volume of solvent; NR, information was not reported; MS, mass spectrometry; PTFE, polytetrafluoroethylene; PVP, polyvinylpyrrolidone; rotary evap., rotary evaporation; SDS, sodium dodecyl sulfate; son., sonication; TGA, thermogravimetric analysis; THF, tetrahydrofuran; tol, toluene; TOC, total organic carbon analysis; UF, ultrafiltration; UPW, ultrapure water (18.2 MΩ/cm); UV, ultraviolet spectroscopy measurement in 1 cm cuvette.
under anoxic conditions;
after exhaustive LLE the concentration was 60% of the nominal concentration;
filtration prior to addition of water;
more than 75% of C60 remained in water, a yellow film was observed sticking to the glass wall of the vial;
pH 10.25–3.75;
at 10 μM NaCl pH 7.0;
in the presence of 50 and 5 mg/L-C NOM, respectively.
“Salting out” of fullerenes occurs when the electron double layers of the fullerenes are compressed by increasing ionic strength, and intermolecular repulsion is decreased, so the fullerenes form larger colloidal structures due to the attractive Van der Waals forces [55]. Nonetheless, the size distribution can be skewed due to the presence of elongated nC60 particles 300–860 nm long [44]. Also, in some cases, TEM can be used to screen for even smaller particles in the range <10–20 nm, often not captured by DLS [44]. However, neither approach is powerful enough to confirm or deny the presence of single hydrated C60 molecules or aggregates of 3.4 nm [45,57]. Accurately characterizing the colloidal properties of the fullerene-nanoparticle dispersions will prove important for interpretation and interlaboratory comparison of ecotoxicological findings.
2.1.2. Impact of nC60 preparation method on nC60 chemistry
During the nC60 preparation, extended contact times or high-energy sonication procedures may influence the interactions between the C60 molecules and the matrix components [48,58] (e.g., water molecules, ions, dissolved gasses, (non-)ionic surfactants, and biological macromolecules). Prolonged exposure of fullerenes to these matrix components, in combination with the high energy provided through sonication, was shown to yield novel covalently-bound polar moieties [59] or increase the measured oxygen content in a suspension of pure-carbon nC60 particles [48]. Hence, the nC60 particles could comprise a hydrophobic core of pristine fullerenes surrounded and stabilized by a small population of polar and amphiphilic C60-derivative molecules [22,48]. Because these hydrophilic C60 derivatives remain unidentified to date, C60 remains, within our ability to detect with 13C NMR, underivatized in these aggregates [22]. Nevertheless, several groups have observed that the elemental composition of aqu/nC60 contained 0.3% oxygen [1] or increased from 1.6% in C60 powder to 2.9–5.0% after being stirred for 40 days in water [48]. These findings could indicate the presence of oxidized C60 derivatives. In the same framework, other authors have confirmed the presence of ions with an m/z ratio corresponding to C60O [60,61], with a peak area of approximately 2.6% of the C60 peak (m/z 720) [62], while others reported its absence [44]. Even though polar C60 derivatives will not interfere with the m/z-based quantification of pristine fullerenes [62], they might remain undetected in some cases because the extraction methods are based on the use of non-polar solvents only. In addition, while the oxidized forms are currently detected to a lesser extent (due to the above-mentioned analytical challenges), cells or organisms used for in vitro assays and during in vivo experiments will be co-exposed to these derivatives. Because their relative contribution to the toxicity of fullerenes has not been established to date, this co-exposure should be considered in future ecotoxicological assays. The oxidized derivatives may possess inherent toxicity or may alter that of the fullerenes under investigation.
2.1.3. Alternative nC60-preparation methods
Alternative approaches have been devised to facilitate or accelerate the transition of fullerenes into the water phase for research purposes only:
The first two [(1) and (2)] are by far the most commonly-reported approaches in laboratory-based investigations (e.g., oral gavage in rats, exposure of in vitro cell cultures, detection in porcine plasma, skin samples, and wastewater and surface-water analogs), as they are less time-consuming than direct dissolution in water [41–43]. By contrast, the next two approaches [(3) and (4)] are used in the search for applications of fullerenes [6, 36]. For this reason, they will not be discussed further and are not shown in Fig. 1.
There are many variations of the solvent-exchange protocol to generate nC60 particles. Table 1 reveals that 19 papers reported four different approaches, most often with a small modification of the previous protocol (yielding about 26 variations). In general, C60 powder is dissolved in an organic solvent {e.g., benzene [62,63], toluene [24,47], tetrahydrofuran (THF) [55,56], dimethylsulfoxide (DMSO) [60,67], or dimethylformamide (DMF) [67]}, then, in some cases, filtered through membranes of varying pore sizes (0.2–0.45 μm). Thereafter, the C60 solution (tol/C60, ben/C60, or THF/C60) is transferred to ultrapure water at a given rate, in some cases in the presence of a co-solvent (e.g., acetone, ethanol, chloroform) or a co-solubilization substrate (e.g., PVP) [55,62–64]. Research showed that the rate at which C60 is transferred from the organic to the aqueous phase can significantly affect the size of the particles [22]. The organic solvents are ultimately removed either by boiling or rotary evaporation (Table 1).
The nC60 suspension obtained can be sonicated, allowed to settle, and/or be filtered using membranes with pore sizes of 0.22–10 μm [22,43,64,65] (Table 1). Varying the transfer solvent, temperature, C60 concentration, and mixing regime appears to affect the colloidal properties {e.g., size [52,68], structure [45,52,56,57], and charge [46,56] of the nC60 particles}. However, the reported size and zeta potential of the different nC60 suspensions in ultrapure water are similar (Table 1). Nonetheless, one must consider how the different preparation methods can affect other less frequently reported colloidal properties (e.g., surface area and particle shape) or the outcome of the toxicological assays. The colloidal properties are of particular importance, as they will determine the target, the mechanism and the strength of interaction between nanoparticles and biological interfaces [30].
2.1.4. Impact of nC60 solvent-exchange method on downstream ecotoxicological assays
Even though it is conventional to use organic solvents to prepare stock solutions of hydrophobic small molecules (e.g., endocrine-disrupting compounds) to spike cell cultures accurately, this approach is not so straightforward for fullerenes. Despite the characteristically high vapor pressures of the solvents (most often THF, Table 1), they are intercalated in the crystalline-like lattice of the nC60 or are adsorbed to the nC60 particles [19,22,24,46,47,69], instead of completely evaporating from the aqueous sample. This causes nC60 to appear more toxic and reactive during toxicological studies when prepared with THF as opposed to water [18,22,24,38,44,70–72]. Moreover, these studies illustrate synergistic toxic effects between nC60 and residual THF [22,42,69] or THF-derivatives (i.e. THF-hydroxyperoxide, 2-tetrahydrofuranol, or γ-butyrolactone) [38,73]. As a convention, aqueous nC60 suspensions prepared using a specific transfer solvent are denoted as “solvent”/nC60, e.g., THF/nC60.
Ultrafiltration of the samples followed by multiple rinsing steps (Fig. 1) is efficient in removing the residual THF from the nC60 lattice and mitigates the observed nC60 toxicity for lung-cell cultures [38]. Moreover, solvent-free nC60 (prepared directly in water) was shown to be non-toxic to E. coli at concentrations of up to 500 ppb [56], while THF/nC60 completely inhibited microbial growth [22]. Nevertheless, the toxic effects might not be limited to residual solvent alone, as differences in colloidal properties between the aqu/nC60 and THF/nC60 have been shown [30,38,66,74,75], so these differences should not be overlooked when assessing the origin of the different biological effects.
The classic strategy of assessing the impact of the vehicle solvent (i.e. solvent control) is not ideal when working with fullerenes because it is difficult to quantify the residual transfer solvent in these nC60 dispersions. Moreover, the adverse effects of THF differ between directly applied THF and THF delivered as THF/nC60 [24,38]. To resolve the issues with THF in toxicological studies, ethanol has been chosen by some investigators as the sole transfer solvent because it was found not to influence a genotoxicity assay with human lymphocytes [44]. However, in another study, the cytotoxicity of EtOH/nC60 was intermediate to that of THF/nC60 and aqu/nC60 [70]. Hence, we recommend evaluating the transfer solvents case by case when it is not possible to use the more relevant aqu/nC60.
2.2. Phase 2 – nC60 extraction from aqueous media
The following section describes liquid-liquid extraction (LLE) and solid-phase extraction (SPE) methods that are used for nC60 in environmental and biological samples.
2.2.1. Liquid-liquid extraction
LLE is the most commonly used technique for extracting C60 from aqueous samples. LLE is a traditional method for extracting non-polar molecules from aqueous phases into non-polar solvents where the choice of solvent is paramount to the extraction efficiency. In one case, benzene, heptane-isoamyl alcohol, and chloroform-methanol were all inefficient for extracting C60 solubilized with PVP from rat plasma [76], perhaps due to solubility in that solvent. Summaries of solvents with high C60 solubility are available [77], and toluene has become the most widely-used solvent for LLE.
After discovering that nC60 is not extracted in non-polar solvents alone [22,45,62], addition of salt to the aqueous nC60 solution was shown to be essential to destabilize the electric double layer of the nC60 aggregates and facilitate extraction into the toluene phase with high recoveries [22]. Many salts have been investigated for this purpose, including Mg(ClO4)2 [22,37,41], KCl [37], and NaCl [64,65] in ranges up to 3.4 M [64]. At least 10 mM Mg(ClO4)2 was found to be required to extract trace amounts (60 ng) of nC60 from water [37] (Fig. 2). The use of these various salts would suggest that destabilization of nC60 occurs as a result of increased ionic strength [37], although oxidative effects of salts [e.g., Mg(ClO4)2] have not been thoroughly investigated. After all, oxidation of nC60 would produce more polar fullerene molecules, which are less soluble in toluene [22,78] (see sub-section 2.1.2.). The fact that salts improve extraction of aqueous fullerenes is an interesting observation for the future investigation of human and murine urine.
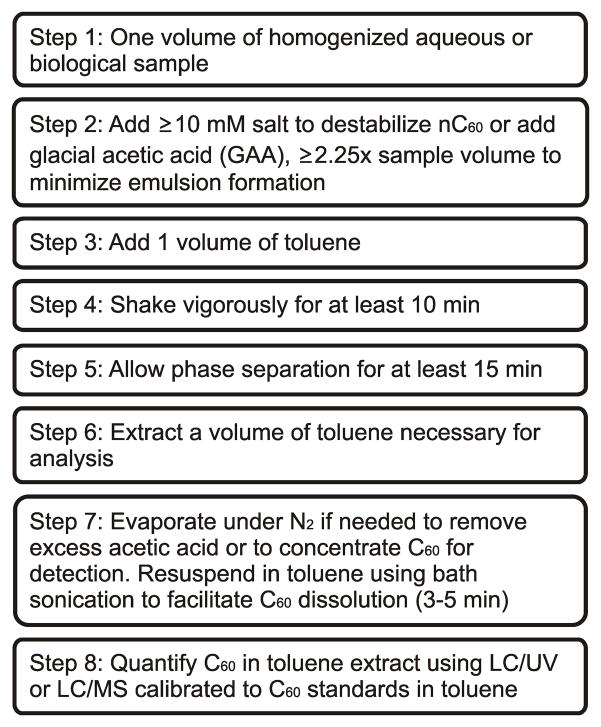
Figure 2.
Consensus liquid-liquid extraction protocol based on a comprehensive review of the literature on quantification of nC60 fullerenes in various biological and environmental matrices.
LLE requires thorough mixing of the aqueous and solvent phases, which has been done in various ways. Horizontal and rotary shakers have been used to promote mixing over times ranging from 10 min [37] to 4 h [65]. A simplified, non-exhaustive LLE was shown to be effective for just 10 min of mixing on a rotary shaker at 500 rpm [37]. This vigorous mixing can cause formation of emulsions between the two phases in the presence of organic material (e.g., proteins and lipids) and/or surfactants. In extractions of nC60 from bovine serum albumin (BSA) solutions and porcine plasma, it was found that a ratio of glacial acetic acid (GAA) to sample volume of greater than 2.25 [37] was necessary to eliminate formation of emulsions. This addition of GAA was also found to eliminate the need for addition of Mg(ClO4)2, possibly due to the low pH and subsequent lower zeta potential of the nC60 aggregates [48].
2.2.2. Solid-phase extraction
SPE is a separation technique in which molecules extracted from aqueous solution through sorption onto a solid matrix, after which the target compound is eluted into the desired solvent. SPE has been used previously to extract nC60 (Table 2). Reversed-phase SPE is most often used in which a hydrocarbon sorbent removes nC60 from the aqueous phase to be eluted into a non-polar solvent. Various types of SPE columns, including Sep-Pak C18 cartridges [41,76], 500 mg/6mL Strata C18-E [64], and 500 mg/6mL Strata SDB-L [65], have been used with varying results. Column-preparation protocols have also varied with a range of conditioning solvents (e.g., methanol, acetonitrile, and toluene) and the use of salts (KCl and Mg(ClO4)2) in the nC60 aqueous sample [41,76].
Table 2.
Published nC60 extraction and quantification protocols and respective limits of detection in various aqueous matrices
| Ref. | Spike |
Extraction |
Analytical |
Whole-method LOD (µg/L) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| nC60 type | nC60 conc. (mg/L) | Matrix |
Concentration factor |
Injection Volume (μL) | Detection | λ (nm) or Ions (m/z) | |||||
| LLE | SPE | Evap./Filtr. | |||||||||
| [76] | PVP/C60 | 10 000 | Plasma | 0.05 | - | - | 5 | UV-Vis | - | 256 nm (hex) | 500b |
| SAA | SAA | SAA | SAA | - | - | 10 | - | APCI-MS | TIC, m/z 720 | 5 000b | |
| [32] | tol/nC60 | 50–200 000 | Blood | 0.03–0.3 | - | - | 50 | UV-Vis | APCI-MS | 333 nm, m/z 150–1 000 | NRg |
| SAA | NAe | Liver | NAe | - | - | SAA | UV-Vis | SAA | SAA | SAA | |
| SAA | NAe | Spleen | NAe | - | - | SAA | UV-Vis | SAA | SAA | SAA | |
| [37] | THF/nC60 | 3.1 | 4.5% BSA | 10 | - | NR | UV-Vis | - | 333 nm | 0.34 | |
| SAA | SAA | 50% plasma | SAA | - | - | SAA | SAA | - | SAA | 0.68 | |
| tol/C60 | 40 μg | Skin | NR | - | - | SAA | SAA | - | SAA | NR | |
| [60] | DMSO/nC60 | 100–400 | Fish embryos | 1 | - | - | 500 | - | ESI-MS | m/z 720, 728–740, 736 | 0.02c |
| SAA | SAA | 1% DMSO | 1 | - | SAA | - | SAA | SAA | |||
| [41] | aqu/nC60 | 63.4–8 770 | GW/SW | 0.5 | - | - | 100 | UV-Vis | - | 333 nm | 2.48 |
| SAA | SAA | SAA | 20 | - | - | SAA | SAA | - | SAA | 0.746 | |
| SAA | SAA | SAA | - | 12 | - | SAA | SAA | - | SAA | 0.746 | |
| [64] | tol/nC60 | 0.5–5 | UPW | - | - | 111 | NR | - | APCI-MS | TIC, m/z 720, 1 440, 360 | 2.8 |
| SAA | 10–25 | Tap water | 55.6 | - | - | SAA | - | SAA | SAA | 3.3 | |
| SAA | NR | SAA | - | 1000 | - | SAA | - | SAA | SAA | 0.30 | |
| [39] | Bulk C60 | NAf | Tissue | NAe | - | - | 5 | UV-Vis | - | 330 | NR |
| SAA | Lung | SAA | SAA | - | SAA | SAA | - | SAA | SAA | ||
| SAA | Blood | SAA | SAA | - | SAA | SAA | - | SAA | SAA | ||
| [43] | tol/nC60 | 5 000 | UPW | 1 | - | - | 100 | UV-Vis | - | NR | 250d |
| SAA | SAA | 5 mg/L-C NOM | 1 | - | - | SAA | SAA | - | SAA | NRd | |
| SAA | SAA | 50 mg/L-C NOM | 1 | - | - | SAA | SAA | - | SAA | NRd | |
| SAA | SAA | 1 % SDS | 1 | - | - | SAA | SAA | - | SAA | NRd | |
| [14] | NA | 0.5–1.5 | WWTP effluent | - | - | 133 | 150 | - | ESI-MS | m/z 720, 736, 840, 856 | 0.005 |
| [65] | tol/nC60 | 20–200 | UPW, WWa | 10 | - | - | 100 | UV-Vis | - | 332 nm | 3–4 |
| SAA | SAA | SAA | SAA | - | - | SAA | - | APCI-MS | m/z 720 | 4–11 | |
| SAA | 1–4 | Recl. WW | - | 1000 | - | SAA | UV-Vis | - | 332 nm | 0.42 | |
| SAA | SAA | Sec. effluent | - | SAA | - | SAA | SAA | - | SAA | 0.64 | |
APCI, atmospheric pressure chemical ionization; BSA, bovine serum albumin; Conc., concentration; DMSO, dimethyl sulfoxide; ESI, electrospray ionization; Evap., evaporation; Filtr., filtration; GW, groundwater; hex, hexane; LOD, limit of detection; MS, mass spectrometry; NA, not applicable; NOM, natural organic matter; NR, information is not reported; PVP, polyvinylpyrrolidone; Rec., reclaimed; SAA, same as above; SDS, sodium dodecyl sulfate; Sec. effluent, secondary effluent; SW, surface water; THF, tetrahydrofuran; TIC, total ion count; tol, toluene; UPW, ultrapure water; WW, wastewater; WWTP, wastewater treatment plant.
five different wastewater matrices;
instrument detection limit, 0.05 ng at S, N=2;
Detection limit, 0.0002 ng and quantification limit, 0.001 ng;
Methodology for whole-method LOD determination is not reported;
After inhalation and distribution among the organs via blood circulation;
2.22 mg/m3 C60 at an inhalation rate of 140 mL/min;
instrument detection limit = 0.1 ng of C60 injected
SPE is especially advantageous for extractions of large sample volumes, as it conserves solvent use (compared to LLE), and the samples can be concentrated from the L range down to hundreds of µL for analysis [64,65]. C60 is often eluted from SPE cartridges with 10–15 mL of toluene, although 2.5 mL were used for C18 Sep-Paks with groundwater and surface water [41]. The extraction of fullerenes from large-sample volumes into small-solvent volumes allows SPE to have high concentration factors more economically compared to LLE (Table 2). Although a common method in analytical chemistry is to evaporate to dryness and reconstitute into a known volume of solvent, this technique can lower the recovery of C60 [37]. This decrease in efficiency could be due to aggregate formation and/or adsorption to the glassware [37]. These various methods of applying SPE to aqueous samples (with varying success, see sub-section 2.3.) demonstrate an opportunity to standardize SPE protocols for nC60 extraction based on experience with environmental samples.
Despite the popularity of (automated) SPE to extract other organic compounds from biological and environmental matrices in “real-world” concentrations, the use of SPE for the recovery of nC60 dispersions from environmental and biological samples has been limited, probably due to the early successes with LLE [22] and the matrix-dependent recoveries with a simplified SPE protocol [64]. Hence, the potential of an optimized SPE protocol seems not to have been investigated to date. Nevertheless, most of the SPE-related issues could be resolved with some basic optimization steps, by:
diluting the sample to improve sample flow-through rates;
adding solvents to the sample to destabilize emulsions (e.g., in milk or blood);
adding a sample-homogenization step and avoiding filtration to overcome loss of C60 retained in the biomass or the filter itself [65];
investigating the best cartridge types, volumes, pore sizes, cleaning solvents or solvent mixtures;
adding a protein- and lipid-removal step; and,
investigating the ideal elution conditions for C60 and the different fullerene derivatives.
Once all these factors have been taken into account, SPE could prove a valuable approach due to its high reproducibility (when automated), low solvent consumption, high sample-volume-loading capacity, and its known capacity to separate analytes from interferences at high efficiency.
2.3. Phase 3 – nC60 quantification and recovery determination
2.3.1. Analytical separation
Chromatographic separation of fullerenes has been thoroughly investigated since the 1990s [77,79]. Detection of C60 in environmental samples has occurred rather recently, and chromatographic conditions are similar among researchers (Table 3). LC columns for fullerene analysis are commonly 3.9–4.6 mm i.d. by 150–250 mm packed with 5 μm particles of C18, pyrenylpropyl, pyrenylethyl, or related hydrophobic stationary phases [4,41,51,60,65]. Toluene is the most common eluent for Buckyprep columns because of its high solubility for C60, and mixtures with acetonitrile (20–45% ACN) have improved separation on C18 columns. Flow is often isocratic at rates around 1 mL/min, and elution of C60 can be achieved in as little as 3 min [64].
Table 3.
Approaches for recovery determination of nC60 extraction from aqueous and biological matrices
| Ref. | nC60 type | Matrix | Extraction | Calibration | Recoveries (%) | Precision (%) |
|---|---|---|---|---|---|---|
| [76] | PVP/C60 | Plasma | LLE | nC60 LLE | ND–12.5 | NR |
| SAA | SPE | SAA | 62 | 4 | ||
| [32] | tol/nC60 | Blood | LLE | nC60 in blood LLE | 87–92 | 1.1 |
| Liver | LLE | nC60 in liver LLE | 85–91 | 15.2 | ||
| Spleen | LLE | nC60 in spleen LLE | 84–90 | 11.5 | ||
| [22] | THF/nC60 | UPW+salts | LLE | nC60 LLE, TGA, rel. PAe | 94–101g | NR |
| [37] | THF/nC60 | BSA/plasma | LLE | nC60 in UPW LLE | 94–≥100 a | 15 |
| [60] | tol/nC60 | Fish embryos | LLE | tol/C60 | 90b | 2 |
| SAA | Culture water | SAA | SAA | 93–110b | 7–10 | |
| [41] | aqu/nC60 | UPW | LLE | nC60 in UPW LLE | 98.5f | 3.17 |
| SAA | GW or SW | LLE/SPE | SAA | ~78/~78f | ~3/~3 | |
| [64] | tol/nC60 | Tap W/UPW | Evap | tol/C60 | 2.1/35 | 0.4/4.9 |
| SAA | SAA/SAA | LLE | SAA | 32/39 | 7.0/3.2 | |
| SAA | SAA/SAA | SPE | SAA | 33/42 | 2.6/2.9 | |
| [43] | THF/nC60 | 5 mg/L–C NOM | LLE | NR | >98 | NR |
| [14] | NA | SW | Filtration | tol/C60 | 70–77 | 2–5 |
| SAA | WW | SAA | SAA | 63–67 | 7–8 | |
| [65] | tol/nC60 | UPW/WW | LLE | nC60 in ≠ matrices LLE | 89–94c | 2 |
| SAA | Reclaimed WW | SPE | SAA | 18d | 1 | |
| SAA | WWTP effluent | SPE | SAA | 9d | 1 |
aqu/nC60: nC60 suspension prepared without transfer solvent; BSA: bovine serum albumin; Evap: evaporation; GW: groundwater; LLE: liquid-liquid extraction; NA: not applicable; ND: not detectable; NOM: natural organic matter; NR: information not reported; PVP: polyvinylpyrrolidone; rel. PA: relative peak area; SAA, same as above; SPE: solid-phase extraction; SW: surface water; TGA: thermogravimetric analysis; THF: tetrahydrofuran; tol: toluene; Tap W: tap water; UPW: ultrapure water; WW: wastewater; WWTP: wastewater-treatment plant.
Recovery is calculated compared to the extraction from UPW using the simplified LLE method (same as samples);
since no matrix interferences were observed upon standard addition, 0.1–150 ppb, quantification based on ratio of analyte peak area to the 13C60 peak area;
the ratio of the amount extracted to the amount that was spiked;
C60 sorbed to particulates were filtered out;
the C60 peak area/total peak area = ≥98% (wavelength not reported);
extraction recoveries can be best determined through exhaustive suspension extraction to determine the total analyte mass in suspension;
recovery was verified through comparison with a thermogravimetric procedure.
2.3.2. Analytical detection
Following extraction into solvent, detection of C60 can be carried out by UV-Vis spectrometry, LC/UV-Vis or LC/MS (Fig. 1). For environmental and biological samples, UV-Vis spectrometry can be impaired by interfering organic compounds. For these samples, chromatography offers separation of C60 from background interferences enabling detection with UV-Vis spectrometry or MS.
The ionization of fullerenes (mainly yielding m/z 720 in negative mode) has been achieved using different approaches [e.g., electrospray ionization (ESI), atmospheric pressure chemical ionization (APCI), atmospheric pressure photoionization (APPI), matrix-assisted laser desorption/ionization (MALDI), laser desorption/ionization (LDI)] reviewed previously [27], with the first two being the most frequently used (Table 2). MS has been used to detect C60 down to 0.2 pg of injected mass [60] while UV-Vis has been reported to achieve a limit of detection (LOD) of 50 pg [76].
In one case, the LOD was extremely low due to the high injection volume of 150 µL (Table 2) [14]. In that study, a remarkably high ESI source temperature (725°C) was also reported [14] compared to the commonly used temperature ranges of 180–220°C and 200–450°C for APCI [32,64,65,76] and 125°C and 250°C for ESI [60] as source and desolvation temperatures, respectively. Whole-method LODs are a function of numerous parameters including LC injection volumes, initial sample volume, and sample pre-concentration (Table 2).
2.3.3. Establishing the reference/calibration standard
The most common way to quantify fullerenes in standard ultrapure-water dispersions (i.e. spike suspensions) is to conduct exhaustive LLE into toluene, and then to quantify the concentrated extract using UV-Vis, LC/UV, or LC-MS with nominal calibration standards of C60 dissolved in toluene [22,37,41,60,65,76] (Fig. 1). To validate this approach as a reference method, gravimetric analyses have been performed by measuring the residual dry mass from nC60 stock dispersions after complete evaporation of the aqueous phase [22]. Thermal optical transmittance (TOT) analysis has been used to quantify the total-carbon content of multi-walled carbon-nanotube-stock solutions [80] and could be applied as a method of verification of the C60 concentrations in nC60-stock solutions obtained by LLE with LC/UV or LC/MS detection. Similarly, total organic carbon (TOC) analyses can be used to provide verification of nC60-stock concentrations [66]. Finally, filtration or ultrafiltration followed by resuspension of the filter cake in toluene can also be applied to isolate fullerenes {even in matrices with low concentrations of hydrophobic/amphiphilic biosolids (e.g., urine) [42,44,62]}.
Spike and recovery experiments are challenging because of the uncertainty in concentrations of the nC60-stock solutions. Nominal concentrations of the nC60-stock dispersions that are based on the ratio of a weighed mass of dry C60 powder added to a volume of ultrapure water can be up to 33% higher than quantification based on the reference method (i.e. exhaustive LLE followed by LC/UV or LC/MS analysis) [60]. This discrepancy between the nominal and measured values could be a result of loss of C60 during nC60-preparative steps {e.g., filtration, adsorption to glassware [27,37,64], or sonication in an unsealed vessel [31]}, or due to aggregation and settling of nC60 in aqueous matrices [56,65]. Identifying the source and the extent of this disparity could be of significance for ecotoxicological studies, as cells are presumably exposed to higher fullerene concentrations than we are able to recover and to measure.
Table 3 summarizes many ways in which researchers have quantified C60 concentrations in nC60 dispersions, and determined recovery efficiencies after spiking of sample matrices.
2.3.4. Calculating C60 recovery
To calculate C60 recovery, the amount of nC60 that is recovered from the sample matrix is compared to the amount of C60 recovered from ultrapure water or in a nominal calibration standard in toluene (see sub-section 2.3.1.). As shown in Table 3, recoveries can be 2–110%. To understand why recoveries can vary to such an extent, we need to investigate the method of recovery determination in every cited study. First, it is necessary to determine with respect to which type of calibration curve the recoveries are being calculated. When nC60 is spiked into distilled or ultrapure water and recovered using effective LLE protocols with LC/UV detection, recoveries are 92–101% compared to a nominal C60 standard in toluene (Table 3). LLE was also efficient at recovering 88–97% of nC60 spiked into raw and treated wastewater samples when compared to an extracted nC60 ultrapure-water standard [65].
2.3.5. Factors affecting the recovery
Beside the recovery-calculation approach, the efficiency of the chosen extraction procedure itself can also influence the recovery, as not all extraction procedures are suitable for all sample matrices. In one case, SPE was not applicable for wastewater samples [65] because C60 adsorbed to the biomass and recoveries of 9–18% were obtained, while, in complex water matrices with less biosolids (e.g., surface water and groundwater), recoveries for LLE and SPE were comparable (75–81%) [41].
Similar to recovery from various environmental samples, recoveries of nC60 from biological samples (e.g., tissues, blood, and plasma) can be very efficient. Fullerenes spiked at 10 ng/mL into BSA solutions and porcine plasma were recovered at 94–100% using LLE [37]. Recoveries of nC60 from rat liver, spleen, and blood were 84–92% using a liquid-liquid tissue-extraction method into toluene with LC/UV detection (compared to an nC60 in ultrapure-water standard) [32]. Similarly, nC60 was recovered from an embryonic zebrafish homogenate at 90 ± 3% [60]. These extraction and detection protocols have improved in recent years, as an early attempt at extracting nC60 (solubilized with polyvinylpyrrolidone) from rat plasma achieved 12.5%, 7.7% and 0% recoveries using LLE into benzene, heptane-isoamylalcohol, and chloroform-methanol, respectively, with LC/UV detection [76]. SPE recovery was increased with a benzene elution, although it remained relatively low at 62.1% [76].
Two important caveats to successful LLE are the use of a destabilizing agent [e.g., Mg(ClO4)2] and avoiding sample evaporation to dryness when salts are present [37,60] (Fig. 2). Acetic acid is added in LLE approaches to prevent emulsion formation and can also be used as a destabilizing agent [41]. However, our personal experience has indicated that residual GAA in the toluene extract can suppress MS detection of C60 using APCI and a toluene/acetonitrile (55:45) eluent flowing at 1 mL/min. Bringing the samples to dryness mitigates GAA-induced interference by volatilizing GAA and yields higher recoveries (unpublished results), despite the previously described issues with evaporation to dryness [37] that is due to the presence of salts [64].
The use of SPE for extraction of spiked C60 has yielded more varied results compared to LLE. C60 recoveries were increased from plasma by using SPE instead of LLE, although toluene was not used as the extraction solvent [76]. Similar recoveries were obtained between SPE and LLE for recovering C60 from surface water and groundwater [41]. However, dramatically lower recoveries from wastewater samples were obtained using SPE, although this was partly due to the removal of nC60 during sample pre-filtering [65]. Low recoveries were acquired using SPE in tap-water samples, although recoveries were also low for LLE (32–42%) [64].
Even though SPE has not been successfully applied across a variety of aqueous and biological matrices by many researchers, it can be advantageous for treating larger sample volumes [64,65] with lower solvent use, a high degree of automation, and ease of field use compared to LLE [41], so it can lead to lower LODs and better reproducibility. We believe that SPE protocols may simply have not been sufficiently optimized for universal application, but it is possible that SPE is more susceptible to matrix variations (e.g., the presence of biosolids).
Currently, many questions remain regarding the appropriate SPE cartridge (type and size), sample matrix pretreatment (e.g., digestion of matrix components), conditioning-rinse-elution solvents, and sample loading rate, to name a few. Therefore, we propose the protocol described in Fig. 2 for extraction of nC60 from biological samples and detection of C60, based on the review of LLE techniques found in the literature (references in Table 2).
3. Conclusions
Fullerenes are unique chemicals with potential for many beneficial applications in biomedical and technological fields. If fullerenes are to become widely used, analytical techniques that are able to detect their presence in various environmental and biological matrices are indispensable, so we may conduct the necessary assays to determine their environmental occurrence, pharmacokinetics, and body burden, and ultimately evaluate the potential hazard for human health.
Past studies have illustrated the implications of different nC60-preparation methods on the colloidal properties and have evaluated the impact of transfer solvents in ecotoxicological assays. In-depth material characterization (based on, e.g., FFF, TEM, or zeta-potential analysis) in the wide spectrum of environmental and biological matrices will no doubt be the subject of many future investigations.
Our ability to quantify fullerenes in biological samples largely depends on our ability to extract these compounds from these complex matrices. LLE has been optimized to isolate fullerenes from a wide range of matrices using a simple consensus protocol. LLE is therefore currently considered the most robust method for fullerene extraction. However, as LLE is now widely used for the extraction of fullerenes from small-volume samples only, a drawback will ultimately be the LOD (and the large volumes of solvents that LLE requires). For this reason, we see an opportunity for (automated) SPE to become a crucial route to allow detection of fullerenes in “real-world” concentrations, especially for population studies, where hundreds of small volumes can be pooled, concentrated, and analyzed using LC/UV or LC/MS.
Acknowledgments
This research was supported by grants 1RC2ES018801 and 1R01ES015445 of the National Institute of Environmental Health Sciences (NIEHS). This content is solely the responsibility of the authors and does not necessarily represent the official views of the NIEHS or the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Benny F.G. Pycke, Center for Environmental Biotechnology, The Biodesign Institute at Arizona State University, Tempe, AZ 85287, USA
Troy M. Benn, School of Sustainable Engineering and the Built Environment, Arizona State University, Tempe, AZ 85287, USA
Pierre Herckes, Department of Chemistry and Biochemistry, Arizona State University, Tempe, AZ, 85287, USA.
Paul Westerhoff, School of Sustainable Engineering and the Built Environment, Arizona State University, Tempe, AZ 85287, USA.
Rolf U. Halden, Center for Environmental Biotechnology, The Biodesign Institute at Arizona State University, Tempe, AZ 85287, USA, School of Sustainable Engineering and the Built Environment, Arizona State University, Tempe, AZ 85287, USA, Department of Environmental Health Sciences, Johns Hopkins University, Bloomberg School of Public Health, Baltimore, MD 21205, USA.
References
- 1.Kang S, Mauter MS, Elimelech M. Environ Sci Technol. 2009;43:2648. doi: 10.1021/es8031506. [DOI] [PubMed] [Google Scholar]
- 2.Duclos SJ, Brister K, Haddon RC, Kortan AR, Thiel FA. Nature (London) 1991;351:380. [Google Scholar]
- 3.Albarran G, Basiuk VA, Basiuk EV, Saniger JM. Adv Space Res. 2004;33:72. [Google Scholar]
- 4.Jafvert CT, Kulkarni PP. Environ Sci Technol. 2008;42:5945. doi: 10.1021/es702809a. [DOI] [PubMed] [Google Scholar]
- 5.Khairullin II, Chen YH, Hwang LP. Chem Phys Lett. 1997;275:1. [Google Scholar]
- 6.Hummelen JC, Knight BW, LePeq F, Wudl F. J Org Chem. 1995;60:532. [Google Scholar]
- 7.Foley S, Crowley C, Smaihi M, Bonfils C, Erlanger BF, Seta P, Larroque C. Biochem Biophys Res Commun. 2002;294:116. doi: 10.1016/S0006-291X(02)00445-X. [DOI] [PubMed] [Google Scholar]
- 8.Qiao R, Roberts AP, Mount AS, Klaine SJ, Ke PC. Nano Lett. 2007;7:614. doi: 10.1021/nl062515f. [DOI] [PubMed] [Google Scholar]
- 9.Friedman SH, DeCamp DL, Sijbesma RP, Srdanov G, Wudl F, Kenyon GL. J Am Chem Soc. 1993;115:6506. [Google Scholar]
- 10.Bakry R, Vallant RM, Najam-ul-Haq M, Rainer M, Szabo Z, Huck CW, Bonn GK. Int J Nanomed. 2007;2:639. [PMC free article] [PubMed] [Google Scholar]
- 11.Halford B. Chem Eng News. 2006;84:47. [Google Scholar]
- 12.Ebbesen TW, Hiura H, Hedenquist JW, de Ronde CEJ, Andersen A, Often M, Melezhik VA. Science (Washington, DC) 1995;268:1634. doi: 10.1126/science.268.5217.1634. [DOI] [PubMed] [Google Scholar]
- 13.Lee TH, Yao N, Chen TJ, Hsu WK. Carbon. 2002;40:2275. [Google Scholar]
- 14.Farre M, Perez S, Gajda-Schrantz K, Osorio V, Kantiani L, Ginebreda A, Barcelo D. J Hydrol. 2010;383:44. [Google Scholar]
- 15.Folkmann JK, Risom L, Jacobsen NR, Wallin H, Loft S, Moller P. Environ Health Perspect. 2009;117:703. doi: 10.1289/ehp.11922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jia G, Wang H, Yan L, Wang X, Pei R, Yan T, Zhao Y, Guo X. Environ Sci Technol. 2005;39:1378. doi: 10.1021/es048729l. [DOI] [PubMed] [Google Scholar]
- 17.Leavens TL, Xia XR, Lee HA, Monteiro-Riviere NA, Brooks JD, Riviere JE. Toxicol Lett. 2010;197:1. doi: 10.1016/j.toxlet.2010.03.1119. [DOI] [PubMed] [Google Scholar]
- 18.Sayes CM, Fortner JD, Guo W, Lyon D, Boyd AM, Ausman KD, Tao YJ, Sitharaman B, Wilson LJ, Hughes JB, West JL, Colvin VL. Nano Lett. 2004;4:1881. [Google Scholar]
- 19.Nikolic N, Vranjes-Ethuric S, Jankovic D, Ethokic D, Mirkovic M, Bibic N, Trajkovic V. Nanotechnol. 2009;20:385102. doi: 10.1088/0957-4484/20/38/385102. [DOI] [PubMed] [Google Scholar]
- 20.Rajagopalan P, Wudl F, Schinazi RF, Boudinot FD. Antimicrob Agents Chemother. 1996;40:2262. doi: 10.1128/aac.40.10.2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Riviere JE. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2009;1:26. doi: 10.1002/wnan.24. [DOI] [PubMed] [Google Scholar]
- 22.Fortner JD, Lyon DY, Sayes CM, Boyd AM, Falkner JC, Hotze EM, Alemany LB, Tao YJ, Guo W, Ausman KD, Colvin VL, Hughes JB. Environ Sci Technol. 2005;39:4307. doi: 10.1021/es048099n. [DOI] [PubMed] [Google Scholar]
- 23.Lovern SB, Klaper R. Environ Toxicol Chem. 2006;25:1132. doi: 10.1897/05-278r.1. [DOI] [PubMed] [Google Scholar]
- 24.Lyon DY, Adams LK, Falkner JC, Alvarez PJ. Environ Sci Technol. 2006;40:4360. doi: 10.1021/es0603655. [DOI] [PubMed] [Google Scholar]
- 25.Oberdorster E, Zhu SQ, Blickley TM, McClellan-Green P, Haasch ML. Carbon. 2006;44:1112. [Google Scholar]
- 26.Ringwood AH, Levi-Polyachenko N, Carroll DL. Environ Sci Technol. 2009;43:7136. doi: 10.1021/es900621j. [DOI] [PubMed] [Google Scholar]
- 27.Isaacson CW, Kleber M, Field JA. Environ Sci Technol. 2009;43:6463. doi: 10.1021/es900692e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen KL, Smith BA, Ball WP, Fairbrother DH. Environ Chem. 2010;7:10. [Google Scholar]
- 29.Perez S, Farre M, Barcelo D. Trends Anal Chem. 2009;28:820. [Google Scholar]
- 30.Nel AE, Madler L, Velegol D, Xia T, Hoek EM, Somasundaran P, Klaessig F, Castranova V, Thompson M. Nat Mater. 2009;8:543. doi: 10.1038/nmat2442. [DOI] [PubMed] [Google Scholar]
- 31.Johnston HJ, Hutchison GR, Christensen FM, Aschberger K, Stone V. Toxicol Sci. 2010;114:162. doi: 10.1093/toxsci/kfp265. [DOI] [PubMed] [Google Scholar]
- 32.Moussa F, Pressac M, Genin E, Roux S, Trivin F, Rassat A, Ceolin R, Szwarc H. J Chromatogr, B. 1997;696:153. doi: 10.1016/s0378-4347(97)00228-4. [DOI] [PubMed] [Google Scholar]
- 33.Pauluhn J. Toxicol Sci. 2010;113:226. doi: 10.1093/toxsci/kfp247. [DOI] [PubMed] [Google Scholar]
- 34.Cagle DW, Kennel SJ, Mirzadeh S, Alford JM, Wilson LJ. Proc Natl Acad Sci USA. 1999;96:5182. doi: 10.1073/pnas.96.9.5182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gulson B, Wong H. Environ Health Perspect. 2006;114:1486. doi: 10.1289/ehp.9277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yamago S, Tokuyama H, Nakamura E, Kikuchi K, Kananishi S, Sueki K, Nakahara H, Enomoto S, Ambe F. Chem Biol. 1995;2:385. doi: 10.1016/1074-5521(95)90219-8. [DOI] [PubMed] [Google Scholar]
- 37.Xia XR, Monteiro-Riviere NA, Riviere JE. J Chromatogr, A. 2006;1129:216. doi: 10.1016/j.chroma.2006.07.030. [DOI] [PubMed] [Google Scholar]
- 38.Spohn P, Hirsch C, Hasler F, Bruinink A, Krug HF, Wick P. Environ Pollut. 2009;157:1134. doi: 10.1016/j.envpol.2008.08.013. [DOI] [PubMed] [Google Scholar]
- 39.Baker GL, Gupta A, Clark ML, Valenzuela BR, Staska LM, Harbo SJ, Pierce JT, Dill JA. Toxicol Sci. 2008;101:122. doi: 10.1093/toxsci/kfm243. [DOI] [PubMed] [Google Scholar]
- 40.Heymann D. Fullerene Sci Technol. 1996;4:509. [Google Scholar]
- 41.Bouchard D, Ma X. J Chromatogr, A. 2008;1203:153. doi: 10.1016/j.chroma.2008.07.068. [DOI] [PubMed] [Google Scholar]
- 42.Deguchi S, Mukai S, Tsudome M, Horikoshi K. Adv Mater. 2006;18:729. [Google Scholar]
- 43.Hyung H, Kim JH. Water Res. 2009;43:2463. doi: 10.1016/j.watres.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 44.Dhawan A, Taurozzi JS, Pandey AK, Shan W, Miller SM, Hashsham SA, Tarabara VV. Environ Sci Technol. 2006;40:7394. doi: 10.1021/es0609708. [DOI] [PubMed] [Google Scholar]
- 45.Andrievsky GV, Klochkov VK, Bordyuh AB, Dovbeshko GI. Chem Phys Lett. 2002;364:8. [Google Scholar]
- 46.Brant J, Lecoanet H, Hotze M, Wiesner M. Environ Sci Technol. 2005;39:6343. doi: 10.1021/es050090d. [DOI] [PubMed] [Google Scholar]
- 47.Brant JA, Labille J, Bottero JY, Wiesner MR. Langmuir. 2006;22:3878. doi: 10.1021/la053293o. [DOI] [PubMed] [Google Scholar]
- 48.Chen KL, Elimelech M. Environ Sci Technol. 2009;43:7270. doi: 10.1021/es900185p. [DOI] [PubMed] [Google Scholar]
- 49.Zhang B, Cho M, Fortner JD, Lee J, Huang CH, Hughes JB, Kim JH. Environ Sci Technol. 2009;43:108. doi: 10.1021/es8019066. [DOI] [PubMed] [Google Scholar]
- 50.Bouchard D, Ma X, Isaacson C. Environ Sci Technol. 2009;43:6597. doi: 10.1021/es901354r. [DOI] [PubMed] [Google Scholar]
- 51.Isaacson CW, Bouchard D. J Chromatogr, A. 2010;1217:1506. doi: 10.1016/j.chroma.2009.12.060. [DOI] [PubMed] [Google Scholar]
- 52.Andrievsky GV, Klochkov VK, Karyakina EL, Mchedlov-Petrossyan NO. Chem Phys Lett. 1999;300:392. [Google Scholar]
- 53.Velzeboer I, Hendriks AJ, Ragas AM, Van de Meent D. Environ Toxicol Chem. 2008;27:1942. doi: 10.1897/07-509.1. [DOI] [PubMed] [Google Scholar]
- 54.Lee J, Fortner JD, Hughes JB, Kim JH. Environ Sci Technol. 2007;41:2529. doi: 10.1021/es062066l. [DOI] [PubMed] [Google Scholar]
- 55.Brant J, Lecoanet H, Wiesner MR. J Nanoparticle Res. 2005;7:545. [Google Scholar]
- 56.Deguchi S, Alargova RG, Tsujii K. Langmuir. 2001;17:6013. [Google Scholar]
- 57.Avdeev MV, Khokhryakov AA, Tropin TV, Andrievsky GV, Klochkov VK, Derevyanchenko LI, Rosta L, Garamus VM, Priezzhev VB, Korobov MV, Aksenov VL. Langmuir. 2004;20:4363. doi: 10.1021/la0361969. [DOI] [PubMed] [Google Scholar]
- 58.Arrais A, Diana E, Rossetti R, Boccaleri E. Carbon. 2007;2007:2502. [Google Scholar]
- 59.Ko WB, Park YH, Jeong MK. Ultrasonics. 2006;44(Suppl 1):e367. doi: 10.1016/j.ultras.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 60.Isaacson CW, Usenko CY, Tanguay RL, Field JA. Anal Chem. 2007;79:9091. doi: 10.1021/ac0712289. [DOI] [PubMed] [Google Scholar]
- 61.Creegan KM, Robbins JL, Robbins WK, Millar JM, Sherwood RD, Tindall PJ, Cox DM, Smith AB, Mccauley JP, Jones DR, Gallagher RT. J Am Chem Soc. 1992;114:1103. [Google Scholar]
- 62.Scrivens WA, Tour JM, Creek KE, Pirisi L. J Am Chem Soc. 1994;116:4517. [Google Scholar]
- 63.Yamakoshi YN, Yagami T, Fukuhara K, Sueyoshi S, Miyata N. J Chem Soc Chem Commun. 1994:517. [Google Scholar]
- 64.Chen Z, Westerhoff P, Herckes P. Environ Toxicol Chem. 2008;27:1852. doi: 10.1897/07-560.1. [DOI] [PubMed] [Google Scholar]
- 65.Wang C, Shang C, Westerhoff P. Chemosphere. 2010;80:334. doi: 10.1016/j.chemosphere.2010.03.052. [DOI] [PubMed] [Google Scholar]
- 66.Xie B, Xu Z, Guo W, Li Q. Environ Sci Technol. 2008;42:2853. doi: 10.1021/es702231g. [DOI] [PubMed] [Google Scholar]
- 67.Wei X, Wu M, Qi L, Xu Z. J Chem Soc, Perkin Trans. 1997;2:1389. [Google Scholar]
- 68.Ying QC, Marecek J, Chu B. Chem Phys Lett. 1994;219:214. [Google Scholar]
- 69.Skokan EV, Privalov VI, Arkhangel’skii IV, Davydov VY, Tamm NB. J Phys Chem B. 1999;103:2050. [Google Scholar]
- 70.Markovic Z, Todorovic-Markovic B, Kleut D, Nikolic N, Vranjes-Djuric S, Misirkic M, Vucicevic L, Janjetovic K, Isakovic A, Harhaji L, Babic-Stojic B, Dramicanin M, Trajkovic V. Biomaterials. 2007;28:5437. doi: 10.1016/j.biomaterials.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 71.Gharbi N, Pressac M, Hadchouel M, Szwarc H, Wilson SR, Moussa F. Nano Lett. 2005;5:2578. doi: 10.1021/nl051866b. [DOI] [PubMed] [Google Scholar]
- 72.Lyon DY, Fortner JD, Sayes CM, Colvin VL, Hughes JB. Environ Toxicol Chem. 2005;24:2757. doi: 10.1897/04-649r.1. [DOI] [PubMed] [Google Scholar]
- 73.Henry TB, Menn FM, Fleming JT, Wilgus J, Compton RN, Sayler GS. Environ Health Perspect. 2007;115:1059. doi: 10.1289/ehp.9757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Duncan LK, Jinschek JR, Vikesland PJ. Environ Sci Technol. 2008;42:173. doi: 10.1021/es071248s. [DOI] [PubMed] [Google Scholar]
- 75.Kulkarni PP, Jafvert CT. Environ Sci Technol. 2008;42:845. doi: 10.1021/es071062t. [DOI] [PubMed] [Google Scholar]
- 76.Santa T, Yoshioka D, Homma H, Imai K, Satoh M, Takayanagi I. Biol Pharm Bull. 1995;18:1171. doi: 10.1248/bpb.18.1171. [DOI] [PubMed] [Google Scholar]
- 77.Wu YQ, Sun YL, Gu ZN, Wang QW, Zhou XH, Xiong Y, Jin ZX. J Chromatogr, A. 1993;648:491. [Google Scholar]
- 78.Heymann D, Weisman RB. C R Chimie. 2006;9:1107. [Google Scholar]
- 79.Cox DM, Behal S, Disko M, Gorun SM, Greaney M, Hsu CS, Kollin EB, Millar J, Robbins J, Robbins W, Sherwood RD, Tindall P. J Am Chem Soc. 1991;113:2940. [Google Scholar]
- 80.Hyung H, Fortner JD, Hughes JB, Kim JH. Environ Sci Technol. 2007;41:179. doi: 10.1021/es061817g. [DOI] [PubMed] [Google Scholar]