Abstract
In the literature, a few biological cells have been used as templates to form microcapsules of a variety of shapes and sizes. In this study, we proved the concept that living cells like platelets can be encapsulated with polyelectrolytes using electrostatic layer-by-layer self-assembly (LBL), and, most importantly, the encapsulation process did not induce activation of the platelets. Glycol-chitosan and poly-L-glutamic acid were electrostatically deposited onto platelets, and the encapsulation was confirmed using confocal laser scanning microscopy and scanning electron microscopy. Transmission electron microscopy observation further confirmed that the encapsulation process was mild and the activation of platelets was negligible. The encapsulation of living biological cells like platelets can serve as a model system in a wide range of biomedical applications including local and sustained drug delivery, immune protection of artificial tissues, and versatile artificial blood.
Keywords: Electrostatic layer-by-layer self-assembly, cell encapsulation, polyelectrolyte, platelet
I. INTRODUCTION
Encapsulation of living biological cells has broad potential applications to treat major human diseases, including encapsulating allogeneic islets to treat diabetes, encapsulating bovine adrenal chromaffin cells to treat chronic pain, and encapsulating genetically engineered cells that produce clotting factor IX to treat hemophilia.1 In the latter, sustained delivery of factor IX via cell encapsulation would circumvent the hemorrhagic crises associated with the disease thus providing a much improved and more economic therapy. The potential economic impacts of cell encapsulation as therapeutics are enormous due to the fact that nearly one-half trillion dollars are spent every year in the United States alone to care for patients suffering from tissue loss or dysfunction.
One promising technique for cell encapsulation is called electrostatic layer-by-layer self-assembly (LBL), which is based on the attachment of oppositely-charged polyions onto charged surfaces in a self-assembly process.2 A variety of polyelectrolytes have been used to modify surfaces of implants or cells. Our laboratory demonstrated that LBL could be used to prepare antibacterial implant surfaces that provide controlled local release of cytokines3,4 and antibiotics.5–7 Work by others showed that LBL surface modification could lead to targeting of cancer cells8 and prolonged in vivo circulation time.9 Moreover, living Saccharomyces cerevisiae yeast was encapsulated with polyelectrolyte multilayers using LBL technology, and the encapsulated cells preserved their metabolic activities.10 Human pancreatic islets were encapsulated with polyelectrolyte multilayers and maintained their functionality,11 which could be promising for immunoisolated transplantation for patients with diabetes. In addition, cells (e.g. blood cell, Escherichia coli) were simply used as templates to fabricate microcapsules of a wide variety of shapes and sizes;12–14 the cells were fixed in paraformaldehyde before encapsulation and lost their biological functions. It is expected that the LBL technique could, in the future, allow for encapsulation of cells and tissues that may prevent graft rejection and macrophage attacks and meanwhile obtain sustained release of biological molecules.
In this study, we demonstrated the possibility of using LBL technology to encapsulate a single living platelet without activation of the cell. Platelets were used as a living biological cell model.
II. MATERIALS AND METHOD
Glycol-chitosan (degree of polymerization ≥ 400), poly-L-glutamic acid (Poly-L-Glu, MW = 50,000–100,000) and fluorescein isothiocyanate (FITC) were purchased from Sigma-Aldrich (St. Louis, MO). FITC labeled glycol-chitosan was prepared based on the reaction between the isothiocyanate group of FITC and the primary amine group on glycol-chitosan.15 0.1 M phosphate buffered saline (PBS, pH 7.4) without Ca2+ and Mg2+ was prepared. Polyelectrolytes, including glycol-chitosan and Poly-L-Glu, were dissolved separately in PBS at a concentration of 1 mg/ml. Platelets were isolated from the venous blood of a rabbit according to our established protocol.16 Platelet concentration was determined using hemocytometry.5
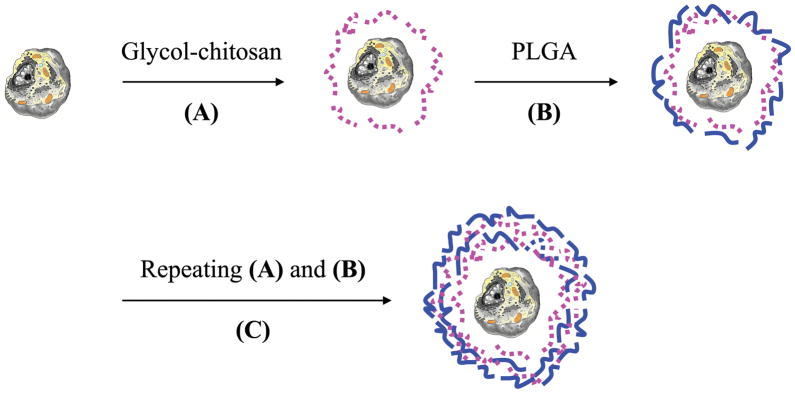
Cell encapsulation was carried out by self-assembly of polyelectrolytes on platelets using LBL technology. Briefly, platelets with a concentration of 2.5 × 107/ml were rinsed with ice cold PBS thrice, and were incubated at ambient temperature in 1 ml glycol-chitosan (1 mg/ml) solution for 10 min with gentle shaking. Platelets were then centrifuged (Eppendorf 5415D, Westbury, NY) at 4200 rpm for 7 min. The supernatant was discarded and the platelets received 3 washings with ice cold PBS. In the next step, platelets were incubated in 1 ml Poly-L-Glu (1 mg/ml) solution for 10 min with gentle shaking. Three centrifugation/washings were followed to remove loosely attached polymers. The deposition of glycol-chitosan and Poly-L-Glu was repeated to obtain the desired number of polymer layers (Figure 1). In most cases, four polymer layers were obtained and designated as (glycol-chitosan/Poly-L-Glu)2.
FIG. 1.
Schematic illustration of nanoencapsulating a living cell using electrostatic layer-by-layer self-assembly. (A) Deposition of a layer of a polycation (i.e. glyco-chitosan). (B) Deposition of a layer of a polyanion (i.e. Poly-L-Glu). (C) Repeating the deposition of polycation and polyanion until a desired number of layers is achieved.  cell;
cell;  glycol-chitosan;
glycol-chitosan;  Poly-L-Glu.
Poly-L-Glu.
To visualize the encapsulated platelets under confocal laser scanning microscopy (CLSM, Zeiss LSM 510, Thornwood, NY), FITC labeled glycol-chitosan was used as the third layer adsorbed on platelets. The CLSM was equipped with a 100X oil immersion lens with an aperture of 1.44. Scanning electron microscopy (SEM, Hitachi S-4700, Tokyo, Japan) was used to observe the surface morphology of encapsulated platelets after they received critical point drying (critical CO2 dryer, Tousimis Autosamdri®-815, Rockville, MD). Samples were also prepared for transmission electron microscopy (TEM, JEOL 2000FX, Tokyo, Japan) observation: encapsulated platelets were fixed with 1 ml 4% ice cold formaldehyde in PBS for 15 min with intermittent gentle shaking, followed by washing with ice cold PBS 3 times; 1 min for each washing. The samples were then stained in 1% osmium tetraoxide (OeO4) for 4 h and rinsed 3 times with ice cold PBS. The samples were finally embedded in epoxy resin and ultramicrotomed. As a control, 1 ml freshly prepared platelets were activated by adding 50 μl 100 IU/ml bovine thrombin (Thrombin-JMI, King Pharmaceuticals, Inc., Bristol, TN) with 10% calcium chloride. The platelet suspension was pipetted up and down until it formed a firm clot which was then processed for TEM observation.
III. RESULTS AND DISCUSSION
In the past two decades, the encapsulation of cells with semi-permeable membranes has attracted great attention as a promising approach for treatment of Parkinson's, Alzheimer's, and Type I diabetes.1,17–19 In this study, we applied LBL nanotechnology to encapsulate a cell model (i.e. platelet) with a polyelectrolyte multilayer coating and to keep the integrity of the cell bioactivity during the deposition process. The multilayer coating is also expected to lead to sustained release of platelet contents upon activation. Advantages of using LBL technology include that the number of polyelectrolytes which can be used and combined to prepare multilayers is countless, the properties of the multilayer coatings can be tuned in the nanometer scale,20 and the multilayer coatings were found to be permeable to small molecules and ions but not to macromolecules.14
Platelets were selected for our study because platelets can be used as a cell model and, most importantly, they play an important biological role in blood vessel repair, clot formation, clot retraction, and clot dissolution. Platelets contain organelles such as mitochondria and microtubules, and have three major types of cytoplasmic granules (alpha, delta, and lambda). When tissue damage occurs, platelets aggregate at the injury site and rapidly change their morphology from a rounded shape to one that includes large sticky protuberances, or pseudopodia, and release a variety of bioactive molecules; this process is called platelet activation. It has been shown that, upon activation, more than 30 growth factors and other biologically active molecules are released, and activated platelets lose their biofunctions within a very short time (e.g. minutes). Therefore, cell encapsulation may lead to sustained release of growth factors and other biological molecules from activated platelets which could be advantageous for certain biomedical applications. Closely related, platelet-rich plasma (PRP), a mixture of concentrated platelets and leukocytes, has proved to be of great importance in bone and soft tissue healing.21 PRP releases a variety of growth factors and other biologically active molecules upon activation. In the current literature, the application of PRP requires multiple blood draws and only autologous PRP has been used to avoid rejection. Encapsulation of PRP cells including platelets would be desirable as it may reduce patient rejection and also lead to sustained release of growth factors and other contents thereby avoiding multiple blood draws.
Our cell encapsulation process is illustrated in Figure 1. Positively charged glycol-chitosan was first deposited on platelets, because of the negative surface charge of the outer cell membrane. Using the surface charges of cells as binding sites for polyions (i.e. glycol-chitosan) could ensure complete coverage with as few as two polyelectrolyte layers.11 As a result, LBL has been successfully applied to deposit multilayer polyelectrolyte coatings on cells.10,11,13 Diaspro et al. encapsulated Saccharomyces cerevisiae cells with four layers of polyelectrolytes,10 and Krol et al. encapsulated pancreatic islets with six layers of polyelectrolytes.11 Similarly, we encapsulated platelets with four layers of glycol-chitosan and Poly-L-Glu, i.e. (glycol-chitosan/Poly-L-Glu)2, and oppositely-charged polyelectrolytes (i.e. glycol-chitosan and Poly-L-Glu) were successfully deposited on platelets. The green fluorescence of FITC-glycol-chitosan was observed on platelets (Figure 2B), the glycol-chitosan/Poly-L-Glu coating on platelets was smooth (Figure 2C), and upon cell dissolution, the formed coating shells were visualized under SEM (Figure 2D) while no shell was observed in control samples without glycol-chitosan/Poly-L-Glu coatings. Further, TEM observation showed that the platelet contents were clearly observed from the cross section view of platelets before and after encapsulation, and the internal cell structures and contents were preserved during polyelectrolyte encapsulation (Figures 3A and B); activated platelets underwent a rapid shape change, formed pseudopodia, and released most, if not all, of their contents (Figure 3C).22,23 It should be noted that some polyelectrolytes may lead to activation and dissolution of platelets. We found in this study that polyelectrolytes of positively-charged polymers including poly-L-lysine, poly(allylamine hydrochloride) and poly(ethylene imine), and of negatively-charged polymers including poly(styrene sulfonate) and chondroitin sulfate induced activation of platelets during the encapsulation process. In contrast, no obvious activation was observed in platelets encapsulated with (glycol-chitosan/Poly-L-Glu)2, (glycol-chitosan/Poly-L-Glu)3, and (glycol-chitosan/Poly-L-Glu)4. It is important not to activate the platelets during the encapsulation process, because most platelet contents including growth factors and cytokines will be released upon activation and will lose their biofunctions within a very short time period.16
FIG. 2.
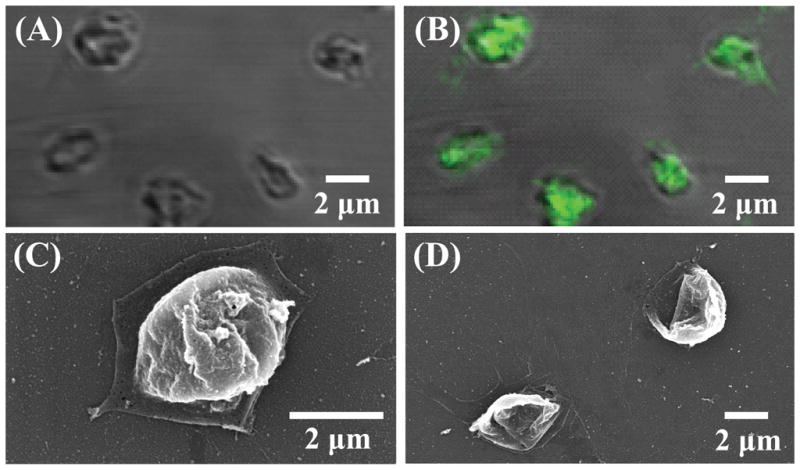
Images of (glycol-chitosan/Poly-L-Glu)2 encapsulated platelets. CLSM images under (A) transmittance mode and (B) fluorescence mode. SEM image (C) before and (D) after platelet dissolution.
FIG. 3.
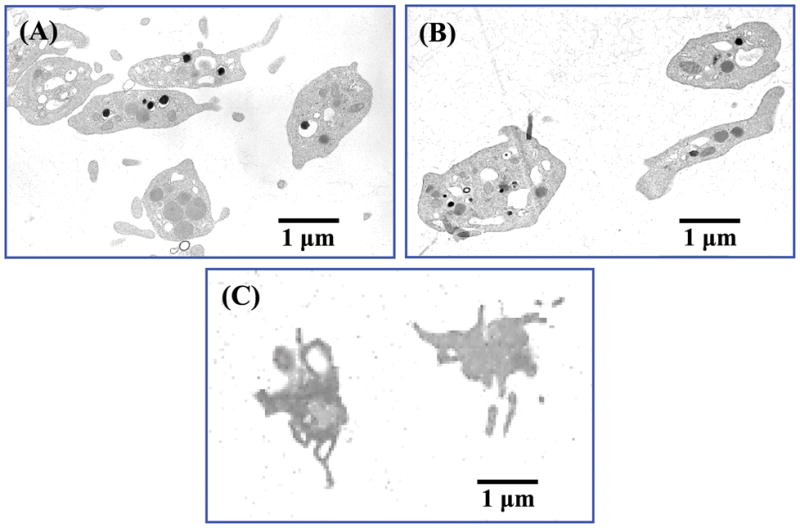
TEM images of platelets (A) before and (B) after nanoencapsulating with (glycol-chitosan/Poly-L-Glu)2, and (C) after activation using thrombin.
IV. CONCLUSIONS
In summary, biocompatible glycol-chitosan and Poly-L-Glu were successfully deposited onto platelets using LBL technology, and the internal structures of platelets were preserved during cell encapsulation. Future experiments will focus on immune protection, biocompatibility, and controlled release of growth factors and cytokines from platelets. By tuning the properties of polyelectrolyte multilayer coating on cells, one may be able to protect cells from immunological responses, to obtain sustained and controlled release of biological molecules (e.g. growth factors, cytokines), and to target cell therapies to tumors. This work and similar studies by others may open interesting perspectives of encapsulating living biological cells using LBL technology for a variety of biomedical applications.
Acknowledgments
Financial support from the NSF (OISE-0737735), NSF EPSCoR RII, AO Foundation, Osteosynthesis and Trauma Care Foundation, and WV NASA EPSCoR is acknowledged. Project S-07-43L was supported by the AO Research Fund of the AO Foundation. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the funding agencies. Microscope experiments and image analysis were performed in part in the West Virginia University Imaging Facility, which is supported in part by the Mary Babb Randolph Cancer Center and NIH grant P20 RR016440. The authors also appreciate the use of the electron microscope at the Microscopic Imaging Facilities at the National Institute for Occupational Safety and Health (NIOSH), Morgantown, WV. The authors thank Diane Schwegler-Berry at NIOSH and Karen Martin at WVU for imaging, Bingbing Jiang for the critical point drying treatment of platelets, and Suzanne Smith for proofreading.
Footnotes
No commercial associations, current and within the past five years, that might pose a potential, perceived or real conflict of interest, were reported by the authors of this paper.
Disclaimer: The findings and conclusions in this report are those of the author(s) and do not necessarily represent the views of the funding agencies.
References
- 1.Lanza RP, Chick WL. Transplantation of encapsulated cells and tissues. Surgery. 1997;121(1):1. doi: 10.1016/s0039-6060(97)90175-6. [DOI] [PubMed] [Google Scholar]
- 2.Decher G. Fuzzy nanoassemblies: Toward layered polymeric multicomposites. Science. 1997;277(5330):1232. [Google Scholar]
- 3.Li B, Jiang B, Boyce B, Lindsey B. Multilayer polypeptide nanoscale coatings for the prevention of biomedical device associated infections. Biomaterials. 2009;30:2552. doi: 10.1016/j.biomaterials.2009.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li B, Jiang B, Dietz MJ, Smith ES, Clovis NB, Rao KMK. Evaluation of local MCP-1 and IL-12 nanocoatings for infection prevention in open fractures. J Orthop Res. 2010;28:48. doi: 10.1002/jor.20939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li H, Ogle H, Jiang B, Hagar M, Li B. Cefazolin embedded biodegradable polypeptide nanofilms promising for infection prevention: A preliminary study on cell responses. J Orthop Res. 2010 Feb; doi: 10.1002/jor.21115. Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jiang B, Li B. Tunable drug incorporation and release from polypeptide multilayer nanofilms. Int J Nanomedicine. 2009;4:37. doi: 10.2147/ijn.s4970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jiang B, Li B. Polypeptide nanotechnology coatings for preventing dental and orthopaedic device-associated infection. J Biomed Mater Res. 2009;88B(2):332. doi: 10.1002/jbm.b.31021. [DOI] [PubMed] [Google Scholar]
- 8.Cortez C, Tomaskovic-Crook E, Johnston APR, Radt B, Cody SH, Scott AM, Nice EC, Heath JK, Caruso F. Targeting and uptake of multilayered particles to colorectal cancer cells. Adv Mater. 2006;18:1998. [Google Scholar]
- 9.Heuberger R, Sukhorukov GB, Vörös J, Textor M, Möhwald H. Biofunctional polyeletrolyte multilayers and microcapsules: Control of non-specific and bio-specific protein adsorption. Adv Funct Mater. 2005;15:357. [Google Scholar]
- 10.Diaspro A, Silvano D, Krol S, Cavalleri O, Gliozzi A. Single living cell encapsulation in nano-organized polyelectrolyte shells. Langmuir. 2002;18:5047. [Google Scholar]
- 11.Krol S, Guerra S, Grupillo M, Diaspro A, Gliozzi A, Marchetti P. Multilayer nanoencapsulation. New approach for immune protection of human pancreatic islets. Nano Lett. 2006;6:1933. doi: 10.1021/nl061049r. [DOI] [PubMed] [Google Scholar]
- 12.Neu B, Voigt A, Mitlöhner R, Leporatti S, Gao CY, Donath E, Kiesewetter H, Möhwald H, Meiselman HJ, Bäumler H. Biological cells as templates for hollow microcapsules. J Microencapsul. 2001;18(3):385. doi: 10.1080/02652040010000398. [DOI] [PubMed] [Google Scholar]
- 13.Ai H, Fang M, Jones SA, Lvov YM. Electrostatic layer-by-layer nanoassembly on biological microtemplates: Platelets. Biomacromolecule. 2002;3:560. doi: 10.1021/bm015659r. [DOI] [PubMed] [Google Scholar]
- 14.Donath E, Moya S, Neu B, Sukhorukov GB, Georgieva R, Voigt A, Bäumler H, Kiesewetter H, Möhwald H. Hollow polymer shells from biological templates: fabrication and potential applications. Chemistry. 2002;8(23):5481. doi: 10.1002/1521-3765(20021202)8:23<5481::AID-CHEM5481>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 15.Onishi H, Machida Y. Biodegradation and distribution of water-soluble chitosan in mice. Biomaterials. 1999;20:175. doi: 10.1016/s0142-9612(98)00159-8. [DOI] [PubMed] [Google Scholar]
- 16.Li HS, Li B. Platelet-rich plasma: its biological properties and applications. (in press) [Google Scholar]
- 17.Uludag H, De Vos P, Tresco PA. Technology of mammalian cell encapsulation. Adv Drug Deliv Rev. 2000;42:29. doi: 10.1016/s0169-409x(00)00053-3. [DOI] [PubMed] [Google Scholar]
- 18.Kühtreiber WM, Lanza RP, Chick WL, editors. Cell Encapsulation Technology and Therapeutics. Boston: Birkhäuser; 1999. [Google Scholar]
- 19.Visted T, Bjerkvig R, Enger PO. Cell encapsulation technology as a therapeutic strategy for CNS malignancies. Neuro Oncol. 2001;3:201. doi: 10.1093/neuonc/3.3.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhong Y, Li B, Haynie DT. Fine tuning of physical properties of designed polypeptide multilayer films by control of pH. Biotechnol Prog. 2006;22(1):126. doi: 10.1021/bp050130h. [DOI] [PubMed] [Google Scholar]
- 21.Mehta S, Watson JT. Platelet rich concentrate: basic science and current clinical applications. J Orthop Trauma. 2008;22(6):432. doi: 10.1097/BOT.0b013e31817e793f. [DOI] [PubMed] [Google Scholar]
- 22.White JG. Platelet ultrastructure. In: Bloom AL, Forbes CD, Thomas DP, Tuddenham EGD, editors. Hemostasis and Thrombosis. 3. Edinburgh: Churchill Livingstone; 1994. [Google Scholar]
- 23.Parise LV, Smyth SS, Shet AS, Coller BS. Platelet morphology, biochemistry, and function. In: Lichtman MA, Beutler E, Kipps TJ, Seligsohn U, Kaushansky K, Prchal JT, editors. Williams Hematology. 7. McGraw-Hill Companies, Inc; Columbus, OH: 2006. [Google Scholar]