Abstract
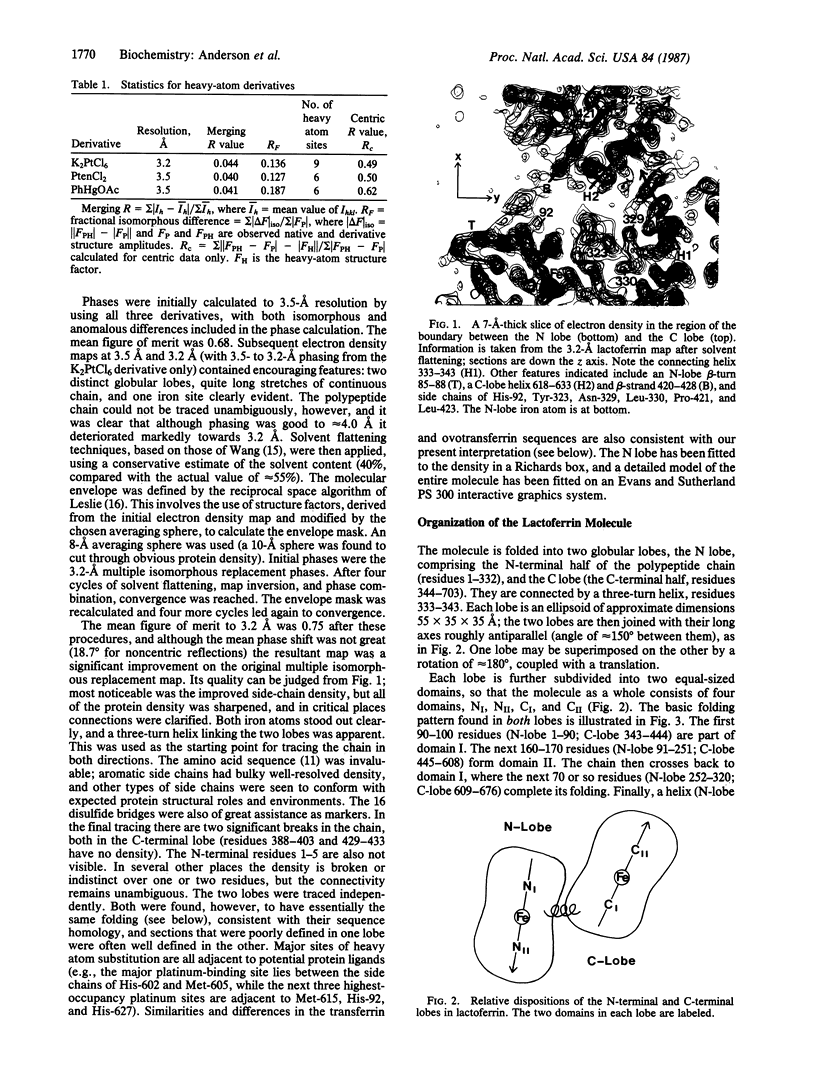
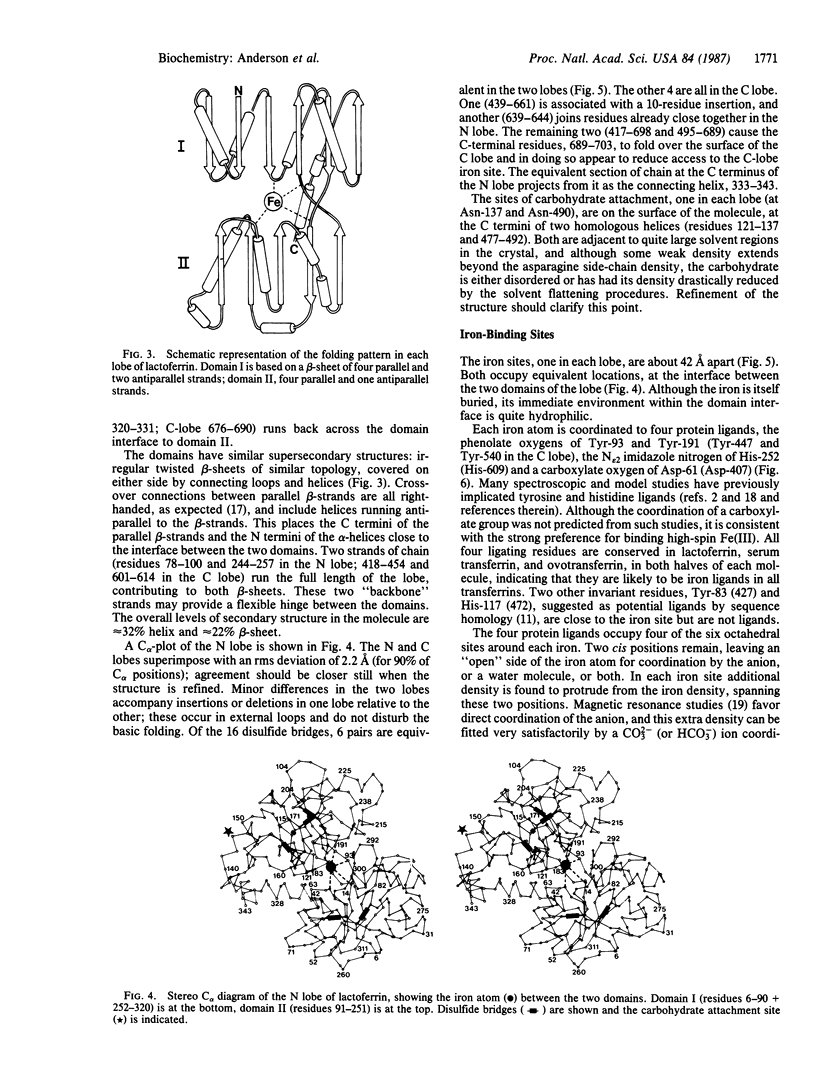
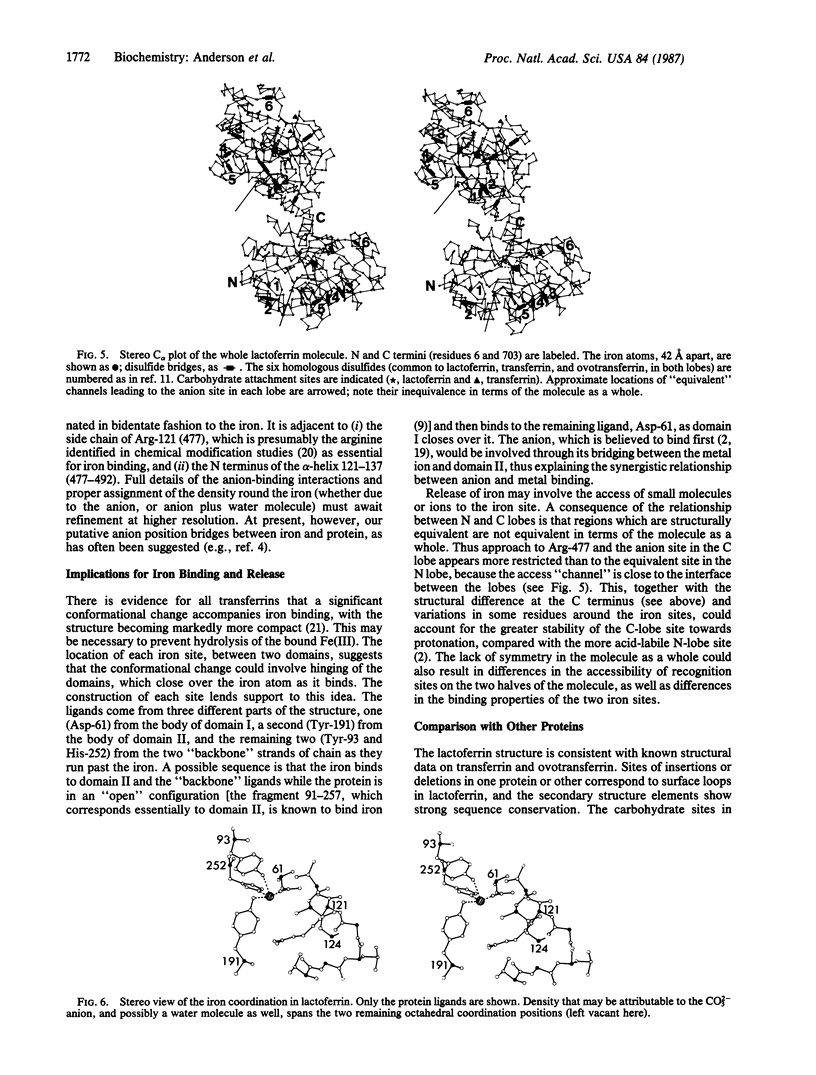
The three-dimensional structure of human milk lactoferrin, a member of the transferrin family, has been determined crystallographically at 3.2-A resolution. The molecule has two-fold internal homology. The N- and C-terminal halves form two separate globular lobes, connected by a short alpha-helix, and carry one iron-binding site each. Each lobe has the same folding, based on two domains of similar supersecondary structure, with the iron site at the domain interface. Each iron atom is coordinated by four protein ligands: two tyrosines, one histidine, and one aspartate. A probable CO3(2-) (or HCO3-) ion is suggested by the electron density, bound to iron and adjacent to an arginine side chain and a helix N terminus. The protein folding and location of the binding sites show marked similarities with those of other binding proteins, notably the sulfate-binding protein from Salmonella typhimurium.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aisen P., Listowsky I. Iron transport and storage proteins. Annu Rev Biochem. 1980;49:357–393. doi: 10.1146/annurev.bi.49.070180.002041. [DOI] [PubMed] [Google Scholar]
- Baker E. N., Rumball S. V. Crystallographic data for human lactoferrin. J Mol Biol. 1977 Apr;111(2):207–210. doi: 10.1016/s0022-2836(77)80124-1. [DOI] [PubMed] [Google Scholar]
- Baker E. N. Structure of actinidin: details of the polypeptide chain conformation and active site from an electron density map at 2-8 A resolution. J Mol Biol. 1977 Sep 25;115(3):263–277. doi: 10.1016/0022-2836(77)90154-1. [DOI] [PubMed] [Google Scholar]
- Baldwin D. A., Jenny E. R., Aisen P. The effect of human serum transferrin and milk lactoferrin on hydroxyl radical formation from superoxide and hydrogen peroxide. J Biol Chem. 1984 Nov 10;259(21):13391–13394. [PubMed] [Google Scholar]
- Bullen J. J., Rogers H. J., Leigh L. Iron-binding proteins in milk and resistance to Escherichia coli infection in infants. Br Med J. 1972 Jan 8;1(5792):69–75. doi: 10.1136/bmj.1.5792.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox T. M., Mazurier J., Spik G., Montreuil J., Peters T. J. Iron binding proteins and influx of iron across the duodenal brush border. Evidence for specific lactotransferrin receptors in the human intestine. Biochim Biophys Acta. 1979 Nov 15;588(1):120–128. doi: 10.1016/0304-4165(79)90377-5. [DOI] [PubMed] [Google Scholar]
- Gilliland G. L., Quiocho F. A. Structure of the L-arabinose-binding protein from Escherichia coli at 2.4 A resolution. J Mol Biol. 1981 Mar 5;146(3):341–362. doi: 10.1016/0022-2836(81)90392-2. [DOI] [PubMed] [Google Scholar]
- Gorinsky B., Horsburgh C., Lindley P. F., Moss D. S., Parkar M., Watson J. L. Evidence for the bilobal nature of diferric rabbit plasma transferrin. Nature. 1979 Sep 13;281(5727):157–158. doi: 10.1038/281157a0. [DOI] [PubMed] [Google Scholar]
- Legrand D., Mazurier J., Metz-Boutigue M. H., Jolles J., Jolles P., Montreuil J., Spik G. Characterization and localization of an iron-binding 18-kDa glycopeptide isolated from the N-terminal half of human lactotransferrin. Biochim Biophys Acta. 1984 May 31;787(1):90–96. doi: 10.1016/0167-4838(84)90111-0. [DOI] [PubMed] [Google Scholar]
- MONTREUIL J., TONNELAT J., MULLET S. [Preparation and properties of lactosiderophilin (lactotransferrin) of human milk]. Biochim Biophys Acta. 1960 Dec 18;45:413–421. doi: 10.1016/0006-3002(60)91478-5. [DOI] [PubMed] [Google Scholar]
- Metz-Boutigue M. H., Jollès J., Mazurier J., Schoentgen F., Legrand D., Spik G., Montreuil J., Jollès P. Human lactotransferrin: amino acid sequence and structural comparisons with other transferrins. Eur J Biochem. 1984 Dec 17;145(3):659–676. doi: 10.1111/j.1432-1033.1984.tb08607.x. [DOI] [PubMed] [Google Scholar]
- Pflugrath J. W., Quiocho F. A. Sulphate sequestered in the sulphate-binding protein of Salmonella typhimurium is bound solely by hydrogen bonds. Nature. 1985 Mar 21;314(6008):257–260. doi: 10.1038/314257a0. [DOI] [PubMed] [Google Scholar]
- Richardson J. S. Handedness of crossover connections in beta sheets. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2619–2623. doi: 10.1073/pnas.73.8.2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers T. B., Børresen T., Feeney R. E. Chemical modification of the arginines in transferrins. Biochemistry. 1978 Mar 21;17(6):1105–1109. doi: 10.1021/bi00599a026. [DOI] [PubMed] [Google Scholar]
- Rosseneu-Motreff M. Y., Soetewey F., Lamote R., Peeters H. Size and shape determination of apotransferrin and transferrin monomers. Biopolymers. 1971 Jun;10(6):1039–1048. doi: 10.1002/bip.360100610. [DOI] [PubMed] [Google Scholar]
- Saper M. A., Quiocho F. A. Leucine, isoleucine, valine-binding protein from Escherichia coli. Structure at 3.0-A resolution and location of the binding site. J Biol Chem. 1983 Sep 25;258(18):11057–11062. [PubMed] [Google Scholar]
- Schlabach M. R., Bates G. W. The synergistic binding of anions and Fe3+ by transferrin. Implications for the interlocking sites hypothesis. J Biol Chem. 1975 Mar 25;250(6):2182–2188. [PubMed] [Google Scholar]
- Vyas N. K., Vyas M. N., Quiocho F. A. The 3 A resolution structure of a D-galactose-binding protein for transport and chemotaxis in Escherichia coli. Proc Natl Acad Sci U S A. 1983 Apr;80(7):1792–1796. doi: 10.1073/pnas.80.7.1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B. C. Resolution of phase ambiguity in macromolecular crystallography. Methods Enzymol. 1985;115:90–112. doi: 10.1016/0076-6879(85)15009-3. [DOI] [PubMed] [Google Scholar]
- Zweier J. L., Wooten J. B., Cohen J. S. Studies of anion binding by transferrin using carbon-13 nuclear magnetic resonance spectroscopy. Biochemistry. 1981 Jun 9;20(12):3505–3510. doi: 10.1021/bi00515a031. [DOI] [PubMed] [Google Scholar]



