Abstract
Bolus insulin calculators (BCs) became available in insulin pumps in 2002 and are being integrated into glucose meters and portable device applets for use with multiple daily injections. A retrospective analysis of continuous subcutaneous insulin infusion data from the Actual Pump Practices (APP) study is used in this article to generate formulas for more precise BC settings.
A well-designed BC determines accurate bolus doses for carbohydrate intake and for correcting elevated glucose levels. It should also provide the logic necessary to track residual bolus insulin and reduce bolus recommendations to minimize insulin stacking. To provide appropriate bolus doses, a BC requires accurate settings for the carbohydrate factor or insulin:carbohydrate ratio, glucose correction factor, duration of insulin action, and correction target. We provide guidelines to select BC settings from the user’s current total daily dose (TDD) of insulin and to determine more appropriate BC settings from an improved TDD based on the mean glucose level.
Keywords: basal rate, bolus calculator, carb factor, correction factor, insulin dose algorithm, insulin pump, TDD
Introduction
Bolus calculators (BCs) were introduced to insulin pumps in 2002 as a convenient way to automate bolus insulin dose calculations that better match varied lifestyle. A well-designed BC provides the logic and features to improve the accuracy of carbohydrate (carb) and correction doses and track bolus insulin on board (BOB or IOB or active insulin) to lessen insulin stacking. A BC offers a comprehensive insulin and glucose history that facilitates pattern management, and has some self-correcting capabilities for setting errors when proper BOB logic is used. BCs are becoming available for use with multiple daily injection (MDI) therapy as mobile device applets in cell phones, and in glucose meters where glucose values can be automatically entered into bolus calculations.
In an Actual Pump Practices (APP) study, we published data and formulas to derive average optimal BC settings for the carbohydrate factor (CarbF), correction factor (CorrF), and basal doses from the total daily dose (TDD) of insulin.1 Anonymous data from 396 Deltec Cozmo® insulin pumps (Smiths Medical MD, Inc., Saint Paul, MN) used throughout the United States and downloaded during a routine software upgrade in 2007 were analyzed. After dividing these pumps into tertiles by mean glucose level, formulas were derived from participants in the lower glucose tertile (LowGT) for determination of average daily basal doses, CarbF and CorrF. This tertile had a mean glucose of 144 mg/dl [8.0 mmol, ranging from 109 to 163 mg/liter (6.1 to 9.1 mmol)]. The BC was used to calculate carb boluses by 92.7% of these pumps and correction boluses by 96.5%.
In this article, we review how to determine BC settings from formulas based on an individual’s TDD and include additional steps to evaluate BC settings and derive an improved TDD (iTDD) when needed, from which settings may be further optimized.
Guidelines for Success with a Bolus Calculator
In the LowGT, we analyzed why pump CarbF (and CorrF) settings differed from actual CarbFs (calculated as the mean grams of carb in each meal divided by the carb bolus actually taken over an average of 244 meals per pump). We found that many pumps used numbers such as 5, 10, 15, or 20 g/U for their CarbF settings. These settings did not correspond with actual bolus doses given for carbs, indicating that pump users or the BC itself were compensating for faulty BC settings.
Further investigation found that the pump’s BC reduced bolus recommendations by an average of 1.40 U/day for BOB and by another 0.27 U/day for hypoglycemia. In contrast, users reduced their BC’s recommended doses by only 0.09 U/day. The BC increased bolus doses for hyperglycemia by 4.2 U/day, while users increased the BC’s recommended doses by 0.56 U/day. This confirmed that most corrections for previous dosing errors were made by the BC. The pump BC used in the APP study appears to compensated for errors in its own settings. Safe BC logic would include counting both carb and correction boluses as BOB, and subtracting BOB from carb and correction doses when a glucose test is performed. Appropriate individual BC settings, safe BC logic, and more glucose testing to measure BOB will improve bolus-dosing success. Additionally, the selection of an accurate duration of insulin action (DIA) time allows the BC to estimate residual BOB. Selection of a single correction target allows more exact tuning of other BC settings so that the desired glucose level may be reached 4 to 5 hours later. The following steps provide detailed recommendations to allow a BC to be set appropriately.
1. Determine the Current TDD
An individual’s TDD is the primary determinant of their mean glucose level. Accurate basal doses and CarbF and CorrF settings can be closely estimated from formulas based on an accurate TDD, and will provide more appropriate bolus doses than settings based on easy-to-use numbers. An average TDD is available on a history screen in most insulin pumps but must be reconstructed for those who use an insulin pen, syringe, or written records.
Those using MDI can determine their TDD by adding up averages of typical injection doses in Table 1 . In the rapid insulin column, averages of carb plus correction boluses taken at each time of day over the last 2 weeks are entered. Averages of the long-acting insulin doses are entered into the long-acting insulin column. These are added together to find the current TDD.
Table 1.
Find the Current TDD on Injections
| 1. Enter below averages of the insulin doses taken at each time of day over the last 2 weeks. Rapid insulin includes usual meal doses PLUS an average of the extra correction doses you take for highs at the time. | 2. Total these doses to find the current average TDD. | ||
|---|---|---|---|
| Insulin | Rapid | Long | |
| Breakfast | __________ U | _________ U | |
| Lunch | __________ U | _________ U | |
| Dinner | __________ U | _________ U | |
| Bedtime | __________ U | _________ U | |
| Total = | __________ U + _________ U = ________ U/day Current TDD | ||
With regular use, a BC dramatically improves tracking of actual bolus doses. After a week or two, the actual bolus portion of the BC can be compared to the initial estimate. New basal, CarbF, and CorrF settings can be calculated if glucose control is poor, based on the lower or higher TDD found when significant differences exist between estimated and actual bolus doses.
2. Determine Basal Doses
Although basal doses are not entered in a BC, determining an appropriate basal dose is a prerequisite for optimal BC performance. If basal doses are excessive, CarbF and CorrF numbers must rise (become “weaker”) to create smaller bolus doses. With a weakened CarbF, carb boluses cannot prevent hyperglycemia following large carb meals, and the weaker CorrF number will not optimally lower high readings. Likewise, basal doses that are too low force the CarbF and CorrF numbers to decrease (become “stronger”) than ideal, creating a risk of hypoglycemia following large carb meals and high readings. Appropriate basal delivery enables the CarbF and CorrF to work over a wide range of carb intakes and glucose values.
Note that bedtime-only neutral protamine Hagedorn (NPH) in MDI does not provide adequate basal coverage to determine CarbFs and CorrFs that will work reliably if daily carb intake and glucose levels vary. Three daily NPH doses are preferred for adequate basal replacement.2
Basal doses in the APP study averaged 48% of the TDD in each glucose tertile, similar to other pump3 and MDI4 studies. An optimal basal percentage will be higher than 48% for someone who consumes a low carb diet and lower for someone on a high carb diet. Once an individual’s average TDD is accurately determined, basal doses can be closely approximated as
| (1) |
3. Determine the Carb Factor
Accuracy of the CarbF is important because small changes in this number create large changes in postmeal glucose outcomes. For example, a change from 1 U per 10 g to 1 U per 9 g causes all subsequent carb boluses to increase by 11%, sufficient to lower postmeal glucose levels for a 160 lb (76.4 kg) individual by 34 to 57 mg/dl (1.9–3.2 mmol) for each subsequent carb intake of 60 to 100 g. Calculation for 60g intake is as follows: 160 lb × 0.24 U/lb = 38.4 U (average TDD per day); CorrF = 1960 mg/dl ÷ 38.4 U = 51 mg/dl/U; 60 g ÷ 9 g/U – 60 g ÷ 10 g/U = 0.67 U; 0.67 U × 51 mg/dl/U = an additional fall of 34 mg/dl for each 60 g intake.
An individual’s CarbF is directly related to their insulin sensitivity, and measures how many grams of carb one unit of insulin covers. From our data, the CarbF formula in the best control tertile can be represented as
| (2) |
4. Determine the Correction Factor
An individual’s CorrF is inversely related to their TDD and measures how far an individual’s elevated glucose concentration will fall per unit of insulin. Even with optimal control, correction doses still make up about 9% of the TDD as they compensate for deficits in basal rates or carb boluses.
In our data, the mean for individual CarbFs times average TDDs was 1960 mg/dl (109 mmol) in the best control tertile. The CorrF can be determined as
| (3) |
The number 1960 is an average of individual actual CorrFs multiplied by their respective TDDs in the LowGT. Because correction boluses must increase when glucose readings are routinely elevated and larger basal/carb bolus deficits exist, a lower number such as 1500 may work better for someone with a high average glucose, while a higher CorrF number such as 2200 may work better when average glucoses are close to normal.
Table 2 converts an individual TDD into approximate daily basal doses, CarbF, and CorrF using Equations (1) , (2) , and (3) . More precise calculations are available online using our automated pump settings tool at www.opensourcediabetes.org.
Table 2.
Estimated Basal, CarbF, and CorrF Basal from TDD and Weighta
| TDD/day | Basalb (U/day) | Basal (U/h) | CorrFc (mg/dl/U) (mmol/U) | CarbFd (g/U) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 100 lb. 45.4 kg | 110 lb. 49.9 kg | 120 lb. 54.4 kg | 130 lb. 60.0 kg | 140 lb. 63.5 kg | 150 lb. 68.0 kg | 160 lb. 72.6 kg | 170 lb. 77.1 kg | 180 lb. 81.6 kg | ||||
| 16 | 7.7 | 0.32 | 122 (6.8) | 16.3 | 17.9 | 19.5 | 21.1 | 22.8 | ||||
| 20 | 9.6 | 0.40 | 98.0 (5.4) | 13.0 | 14.3 | 15.6 | 16.9 | 18.2 | 19.5 | 20.8 | ||
| 24 | 11.5 | 0.48 | 81.7 (4.5) | 10.8 | 11.9 | 13.0 | 14.1 | 15.2 | 16.3 | 17.3 | 19.5 | 21.7 |
| 28 | 13.4 | 0.56 | 70.0 (3.9) | 9.3 | 10.2 | 11.1 | 12.1 | 13.0 | 13.9 | 14.9 | 16.7 | 18.6 |
| 32 | 15.4 | 0.64 | 61.3 (3.4) | 8.1 | 8.9 | 9.8 | 10.6 | 11.4 | 12.2 | 13.0 | 14.6 | 16.3 |
| 36 | 17.3 | 0.72 | 54.4 (3.0) | 7.2 | 7.9 | 8.7 | 9.4 | 10.1 | 10.8 | 11.6 | 13.0 | 14.4 |
| 40 | 19.2 | 0.80 | 49.0 (2.7) | 6.5 | 7.2 | 7.8 | 8.5 | 9.1 | 9.8 | 10.4 | 11.7 | 13.0 |
| 45 | 21.6 | 0.90 | 43.6 (2.4) | 5.8 | 6.4 | 6.9 | 7.5 | 8.1 | 8.7 | 9.2 | 10.4 | 11.6 |
| 50 | 24.0 | 1.00 | 39.2 (2.2) | 5.2 | 5.7 | 6.2 | 6.8 | 7.3 | 7.8 | 8.3 | 9.4 | 10.4 |
| 55 | 26.4 | 1.10 | 35.6 (2.0) | 4.7 | 5.2 | 5.7 | 6.1 | 6.6 | 7.1 | 7.6 | 8.5 | 9.5 |
| 60 | 28.8 | 1.20 | 32.7 (1.8) | 4.3 | 4.8 | 5.2 | 5.6 | 6.1 | 6.5 | 6.9 | 7.8 | 8.7 |
| 65 | 31.2 | 1.30 | 30.2 (1.7) | 4.0 | 4.4 | 4.8 | 5.2 | 5.6 | 6.0 | 6.4 | 7.2 | 8.0 |
| 70 | 33.6 | 1.40 | 28.0 (1.6) | 3.7 | 4.1 | 4.5 | 4.8 | 5.2 | 5.6 | 5.9 | 6.7 | 7.4 |
| 80 | 38.4 | 1.60 | 24.5 (1.4) | 3.3 | 3.6 | 3.9 | 4.2 | 4.6 | 4.9 | 5.2 | 5.9 | 6.5 |
| 90 | 43.2 | 1.80 | 21.8 (1.2) | 2.9 | 3.2 | 3.5 | 3.8 | 4.0 | 4.3 | 4.6 | 5.2 | 5.8 |
| 100 | 48.0 | 2.00 | 19.6 (1.1) | 2.6 | 2.9 | 3.1 | 3.4 | 3.6 | 3.9 | 4.2 | 4.7 | 5.2 |
For exact calculations, use the Pump Setting Tool at www.opensourcediabetes.org
Basal = TDD × 0.48
CorrF = 1960 ÷ TDD
CarbF = 10.8 × relative insulin sensitivity = [2.6 × Weight(lb)] ÷ TDD
5. Select an Accurate Duration of Insulin Action
An accurate DIA time allows a close estimation of residual BOB from prior boluses to lessen the risk of insulin stacking. This is important because 65% of pump boluses are given within 4.5 hours of a prior bolus,5 well within the time during which today’s rapid insulins remain active.6,–9 In at least 10.8% of boluses, the BOB exceeds the correction bolus needed to cover the current glucose level.5
For a BC to accurately track BOB with aspart, lispro, or glulisine, the DIA must be set to at least 4 to 6 hours. When a short DIA such as 3 hours is chosen, the BC calculates that no residual bolus insulin activity remains after 3 hours. In a study by Mudaliar and colleagues of 20 nondiabetic subjects, 40% of aspart’s (0.2 U/kg) glucose-lowering effect remained at 3 hours after an injection.6 A short DIA hides residual bolus insulin activity, causes unrecognized insulin stacking, and may lead to errors in other settings as the user attempts to compensate for this hidden insulin stacking.
Some BCs approximate residual bolus insulin activity linearly (i.e., 20% per hour for a 5-hour DIA), while others use a curvilinear formula that more closely approximates insulin’s delayed onset of action and its gradual tailing off in activity. DIA times between 4.0 to 5.5 hours work well for BCs that use a linear approach, while DIA times of 4.5 to 6.0 hours are more appropriate for curvilinear systems. The standard deviation for interindividual variations in DIA can be roughly approximated as ±45 minutes (coefficient of variation for late t50% = 17% × 264 min = 44.8 min as standard deviation).10 Any new insulins or methods that speed up insulin activity may require shorter DIA time settings.
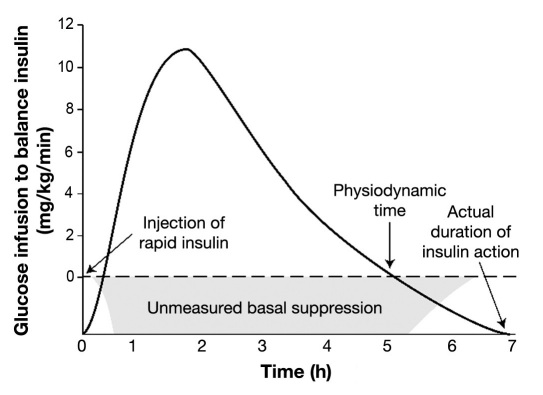
One study found that the DIA appeared to depend on the size of the insulin dose,8 but this apparent dose/time dependency may be an artifact of the glucose infusion rate (GIR) methodology used. In this and Mudaliar’s GIR study, the DIA or insulin pharmacodynamics was measured as the time over which glucose infusion was needed to offset an insulin bolus given to healthy individuals. In this scenario, the bolus also suppresses the nondiabetic subject’s basal insulin delivery. The glucose infusion (DIA) ends once the individual’s basal delivery restarts, well before the bolus activity actually stops as shown in Figure 1 (times on x axis are examples, not research-based). A smaller insulin dose (0.1 U/kg) cannot suppress basal insulin as long as a larger dose (0.2 or 0.3 U/kg), making smaller doses appear to have a shorter DIA. We believe DIAs determined using larger doses measure true DIA more accurately in insulin dependent individuals who receive ongoing basal replace-ment. More research is needed to confirm actual DIA times in type 1 diabetes where basal delivery is not suppressed.
Figure 1.
Physiodynamics in nondiabetic subjects not equivalent to DIA.
6. Use a Single Correction Target
Current BCs do not offer settings for both a single correction target (CT) where an elevated glucose will end up 4 to 5 hours after a correction bolus, as well as a glucose target range (GTR) of acceptable glucose values through the day. When a GTR is selected as a CT, no correction bolus is recommended for values within that range. The wider a GTR, the less precise correction boluses become.
When the glucose lies outside the GTR, one BC corrects glucose levels to the high and low ends of the range, while other BCs correct to the mean. For example, if a person’s glucose is 181 mg/dl (10.1 mmol) and their range is 80 to 180 mg/dl (4.4 to 10 mmol), the first BC corrects to 180 mg/dl (10 mmol) while others correct to the mean value of 130 mg/dl (7.2 mmol). If a wide GTR is selected for the first BC, users often compensate by selecting lower (“stronger”) CorrF numbers to receive correction boluses large enough to lower the glucose below 180 mg/dl (10 mmol). But if they use the same CorrF number to bring down a very high reading, a correction bolus may become excessive.
With current BCs, a single CT (or a narrow GTR) gives more precise correction doses than use of a wide GTR. Always select a CT for the glucose desired about 5 hours later, such as starting a bedtime CT before the evening meal.
7. Determine the iTDD
The frequency and severity of hypo- and hyperglycemia show how well an individual’s current TDD is working. Among people with type 1 diabetes, 37% experience a severe hypoglycemia event11 each year with prevalences found in two large studies of 1.3 events11 and 1.15 events12 per patient year. Frequent hypoglycemia indicates the TDD should be lowered, while ongoing hyperglycemia indicates it needs to be raised. Always ask individuals how often they may have had hypoglycemia and consumed carbs, but did not document the hypoglycemia by doing a glucose test at the time. Ask whether more than one meter is being used that contains essential data.
Hyperglycemia is far more common than hypoglycemia. In our APP study, 79% of type 1 pump users had an average meter glucose level above 154 mg/dl (8.6 mmol), equivalent roughly to hemoglobin A1cs of 7% or higher.13 Increased TDDs are more commonly needed than lower ones once frequent hypoglycemia is eliminated.
Some BC users may adjust only a single BC setting or their basal doses to fix all glucose problems. This can lead to a basal/bolus imbalance and dose errors that worsen control. Periodically check an individual’s current doses against the optimal basal and bolus percentages found in the LowGT in Table 3 . Individual BC settings can be compared to the optimal settings found in Table 2 or by using our online pump settings tool at www.opensourcediabetes.org
Table 3.
Optimal Insulin Use: Mean Values for Optimal Doses in Best Control Tertile
| Insulin Source | % of TDD | Interquartile Range (%) |
|---|---|---|
| Basal | 47.8% | 39.6% to 54.9% |
| Carb Boluses | 43.1% | 35.6% to 51.2% |
| Corr Boluses | 9.0% | 6.2% to 11.3% |
If an individual’s settings differ from these average optimal values, consider whether an adjustment is needed.
With frequent hypoglycemia, lower the TDD immediately by 5% or more to improve glucose stability. To correct the more common problem of hyperglycemia, two methods can be used to find an improved TDD (iTDD) from which new basal rates, CarbF, and CorrF can be derived to improve glucose levels.
Method 1
If the mean glucose is elevated (without frequent hypo-glycemia), correction boluses make up a greater than desired portion of the TDD as they compensate for deficits in basal or carb doses. Control can be quickly improved by using this TDD with its excess correction boluses to select more appropriate basal doses, CarbF, and CorrF using Equations (1) , (2) , and (3) . Over a few iterations, an iTDD can be determined as excess correction boluses are redistributed into more appropriate basal doses and BC settings. With a mean of 4.5 glucose tests per day in these 396 pump users, the majority of pump users are testing sufficiently often to use this method.
Method 2
Another way to determine an iTDD is to add sufficient correction doses per day to an individual’s current TDD to lower their average meter glucose (averaged over 14 days or more of representative readings) down to a desired average glucose level. For example, when the average glucose level is higher than the LowGT’s mean of 144 mg/dl (8.0 mmol), an iTDD can be calculated using this formula:
| (4) |
The constants 2.5, 1960 mg/dl (109 mmol), and 144 mg/dl (8.0 mmol) can all be modified for more aggressive or more conservative insulin and glucose adjustments. For example, 2.5 is a reasonable estimate for the number of times that correction doses will be needed per day to lower the current average glucose down to a patient’s desired average glucose on their meter. This value can be replaced with less frequent corrections such as 1.5 or 2 times a day for a slower lowering of glucose levels, or by 3 or 3.5 times a day for more aggressive corrections. The value 1960 mg/dl (109 mmol) can be modified as discussed earlier to address basal or carb bolus deficits. The mean glucose of 144 mg/dl (8.0 mmol) can be decreased for pregnancy or increased for hypoglycemia unawareness. This average of individual glucose readings will be a higher number than that selected for the CT value.
An iTDD from methods 1 or 2 can be used to determine more appropriate settings, using Equations (1) , (2) , and (3) . On the path toward an iTDD, pattern management and basal/bolus testing would be simultaneously used to improve glucose control. Keep in mind that after the TDD has been increased to counteract hyperglycemia, it may later need to be reduced somewhat once more normal glucose levels reduce insulin resistance.
BC Limitations
For 60 to 90 minutes following a carb bolus, a blind spot prevents a BC from accurately estimating how the rising glucose from digesting carbs will be counterbalanced by the rise in insulin level from the carb bolus that was given. During this time, a BC cannot recommend an accurate bolus dose based on the glucose. Afterward, it can inform the user whether they have an insulin deficit or a carb deficit based on the balance between BOB and their current glucose. Additionally, a BC cannot accurately balance BOB against carb digestion in situations where food absorption is significantly delayed, such as after low glycemic index meals, with use of Symlin or Precose, with off-label use of glucagons-like peptide-1 agonists, or with gastroparesis.
Conclusions
These BC setting guidelines from 132 individuals in excellent control provide well-balanced basal/bolus doses to improve control, especially once an individual’s TDD has gradually improved to minimize frequent hypo-glycemia and lower elevated mean glucose levels. Testing of each individual’s basal doses and BC settings are needed to optimize individual basal doses,14,15 CarbF, CorrF, and DIA. Outpatient testing methods have been outlined for injections,16 pumps,17 and DIA.18
In most clinical situations, accurate BC settings and appropriate dose logic can provide tremendous improve-ments in blood glucose management. Once a glucose value is entered, a well-designed BC can add precision to bolus doses and minimize insulin stacking even when glucose values are relatively normal, following increased physical activity, when BC setting errors are present, and when a user intentionally increases a recommended correction dose to hasten a fall in glucose. It can also recommend carb intake whenever BOB exceeds the current carb and correction needs. The BC used in the APP study implemented these abilities, but other BCs available in pumps as of November 2010, do so only partially. The need for appropriate BC settings and logic will increase as meter and applet BC tools are introduced for MDI.
Most of the protection against hypoglycemia in our pump analysis arose through dose reductions made by the BC rather than from users overriding a BC’s bolus recommendation, and the same was true for correction of hyperglycemia. This suggests that accurate BC settings have more impact on glucose levels than bolus adjustments by users, especially when a BC is routinely used as it was in the APP study. Even when BC settings are accurate and appropriately used, situational dose modifications by users will always be needed for changes in activity, weight, stress, and other variables.
The BC setting guidelines presented here are appropriate for adults with type 1 diabetes or with insulin-requiring type 2 diabetes who use basal-bolus therapy with a pump or MDI. These BC formulas are similar to those found in another large pump study,19 but differ from those found in a study of patients using pumps and CGM.20–22 A clinical trial is needed to prospectively validate this approach to BC dose optimization.
Acknowledgments
We are especially indebted to Paul Davidson, M.D., of Atlanta Diabetes Associates, Atlanta, GA, for his pioneering efforts to clarify the rules and logic behind insulin dose therapy. Also, a special thanks to the late Rhall Pope B.S., M.S., Ph.D., Vice President of Research and Development, and Mike Blomquist, Director of Software Systems, Smiths Medical MD, Inc. Their desire to improve glucose control for people with diabetes led to the development of an advanced insulin pump and bolus calculator to acomplish this.
Abbreviations
- APP
Actual Pump Practices
- BC
bolus calculator
- BOB
bolus insulin on board
- carb
carbohydrate
- CarbF
carbohydrate factor
- CorrF
glucose correction factor
- CT
correction target
- DIA
duration of insulin action
- GIR
glucose infusion rate
- GTR
glucose target range
- IOB
insulin on board
- iTDD
improved total daily dose
- LowGT
lower glucose tertile
- MDI
multiple daily injection
- NPH
neutral protamine Hagedorn
- TDD
total daily dose
References:
- 1.Walsh J, Roberts R, Bailey T. Guidelines for insulin dosing in continuous subcutaneous insulin infusion using new formulas from a retrospective study of individuals with optimal glucose levels. J Diabetes Sci Technol. 2010;4(5):1174–1181. doi: 10.1177/193229681000400516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Del Sindaco P, Ciofetta M, Lalli C, Perriello G, Pampanelli S, Torlone E, Brunetti P, Bolli GB. Use of the short-acting insulin analogue lispro in intensive treatment of type 1 diabetes mellitus: importance of appropriate replacement of basal insulin and time-interval injection-meal. Diabet Med. 1998;15(7):592–600. doi: 10.1002/(SICI)1096-9136(199807)15:7<592::AID-DIA625>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 3.Davidson PC, Hebblewhite HR, Bode BW, Steed RD, Welch NS, Greenlee MC, Richardson PL, Johnson J. Statistically based CSII parameters: correction factor, CF (1700 rule), carbohydrate-to-insulin ratio, CIR (2.8 rule), and basal-to-total ratio. Diabetes Technol Ther. 2003;3:237–242. [Google Scholar]
- 4.Garg SK, Rosenstock J, Ways K. Optimized basal-bolus insulin regimens in type 1 diabetes: insulin glulisine versus regular human insulin in combination with basal insulin glargine. Endocr Pract. 2005;11(1):11–17. doi: 10.4158/EP.11.1.11. [DOI] [PubMed] [Google Scholar]
- 5.Walsh J, Wroblewski D, Bailey TS. Disparate bolus on board recommendations in insulin pump therapy. AACE meeting; 2007; poster. [Google Scholar]
- 6.Mudaliar SR, Lindberg FA, Joyce M, Beerdsen P, Strange P, Lin A, Henry RR. Insulin aspart (B28 asp-insulin): a fast-acting analog of human insulabsorption kinetics and action profile compared with regular human insulin in healthy nondiabetic subjects. Diabetes Care. 1999;22(9):1501–1506. doi: 10.2337/diacare.22.9.1501. [DOI] [PubMed] [Google Scholar]
- 7.Heise T, Weyer C, Serwas A, Heinrichs S, Osinga J, Roach P, Woodworth J, Gudat U, Heinemann L. Time-action profiles of novel premixed preparations of insulin lispro and NPL insulin. Diabetes Care. 1998;21(5):800–803. doi: 10.2337/diacare.21.5.800. [DOI] [PubMed] [Google Scholar]
- 8.Rave K, Nosek L, de la Peña A, Seger M, Ernest CS 2nd, Heinemann L, Batycky RP, Muchmore DB. Dose response of inhaled dry-powder insulin and dose equivalence to subcutaneous insulin lispro. Diabetes Care. 2005;28(10):2400–2405. doi: 10.2337/diacare.28.10.2400. [DOI] [PubMed] [Google Scholar]
- 9.Heinemann L. Time-action profiles of insulin preparations. Mainz, Germany: Kirchheim & Co GmbH; 2004. [Google Scholar]
- 10.Heinemann L, Weyer C, Rauhaus M, Heinrichs S, Heise T. Variability of the metabolic effect of soluble insulin and the rapid-acting insulin analog insulin aspart. Diabetes Care. 1998;21(11):1910–1914. doi: 10.2337/diacare.21.11.1910. [DOI] [PubMed] [Google Scholar]
- 11.Pedersen-Bjergaard U, Pramming S, Heller SR, Wallace TM, Rasmussen AK, Jørgensen HV, Matthews DR, Hougaard P, Thorsteinsson B. Severe hypoglycaemia in 1076 patients with type 1 diabetes: influence of risk markers and selection. Diabetes Metab Res Rev. 2004;20(6):479–486. doi: 10.1002/dmrr.482. [DOI] [PubMed] [Google Scholar]
- 12.Donnelly LA, Morris AD, Frier BM, Ellis JD, Donnan PT, Durrant R, Band MM, Reekie G, Leese GP, DARTS/MEMO Collaboration Frequency and predictors of hypoglycaemia in type 1 and insulin-treated type 2 diabetes: a population-based study. Diabet Med. 2005;22(6):749–755. doi: 10.1111/j.1464-5491.2005.01501.x. [DOI] [PubMed] [Google Scholar]
- 13.Nathan DM, Kuenen J, Borg R, Zheng H, Schoenfeld D, Heine RJ, A1c-Derived Average Glucose Study Group Translating the hemoglobin A1C assay into estimated average glucose values. Diabetes Care. 2008;31(8):1473–1478. doi: 10.2337/dc08-0545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holterhus P, Odendahl R, Oesingmann S, Lepler R, Wagner V, Hiort O, Holl R, German/Austrian DPV Initiative. German Pediatric CSII Working Group Classification of distinct baseline insulin infusion patterns in children and adolescents with type 1 diabetes on continuous subcutaneous insulin infusion therapy. Diabetes Care. 2007;30(3):568–573. doi: 10.2337/dc06-2105. [DOI] [PubMed] [Google Scholar]
- 15.Scheiner G, Boyer BA. Characteristics of basal insulin requirements by age and gender in type-1 diabetes patients using insulin pump therapy. Diabetes Res Clin Pract. 2005;69(1):14–21. doi: 10.1016/j.diabres.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 16.Walsh J, Roberts R, Varma C, Bailey T. Using insulin. San Diego: Torrey Pines Press; 2003. [Google Scholar]
- 17.Walsh J, Roberts R. Pumping insulin. 4th ed. San Diego: Torrey Pines Press; 2006. [Google Scholar]
- 18.Walsh J, Roberts R. An accurate DIA prevents excessive insulin stacking. Available from: http://www.diabetesnet.com/diabetes_technology/dia.php. Accessed on October 1, 2010.
- 19.Davidson PC, Hebblewhite HR, Steed RD, Bode BW. Analysis of guidelines for basal-bolus dosing: basal insulin, correction factor, and carbohydrate-to-insulin ratio. Endocr Pract. 2008;14(9):1095–1101. doi: 10.4158/EP.14.9.1095. [DOI] [PubMed] [Google Scholar]
- 20.King A, Armstrong D. A prospective evaluation of insulin dosing recommendations in patients with type 1 diabetes at near normal glucose control: basal dosing. J Diabetes Sci Technol. 2007;1(1):36–41. doi: 10.1177/193229680700100106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.King A. How much insulin do I give? Reevaluation of insulin dosing estimation formulas using continuous glucose monitoring. Endocr Pract. 2010;16(3):428–432. doi: 10.4158/EP09308.OR. [DOI] [PubMed] [Google Scholar]
- 22.Walsh J, Roberts R. Letter to the editor. A study that glucose control can be achieved in 31 type 1 patients who follow a controlled diet and use an insulin pump that is programmed. J Diabetes Sci Technol. 2007;1(4):L3–L6. doi: 10.1177/193229680700100422. [DOI] [PMC free article] [PubMed] [Google Scholar]