Abstract
Background
The combination of telemedicine systems integrating mobile technologies with the use of continuous glucose monitors improves patients’ glycemic control but demands a higher interaction with information technology tools that must be assessed. In this article, we analyze patients’ behavior from the use-of-the-system point of view, identifying how continuous monitoring may change the interaction of patients with the mobile telemedicine system.
Methods
Patients’ behavior were evaluated in a clinical experiment consisting of a 2-month crossover randomized study with 10 type 1 diabetes patients. During the entire experiment, patients used the DIABTel telemedicine system, and during the intervention phase, they wore a continuous glucose monitor. Throughout the experiment, all user actions were automatically registered. This article analyzes the occurrence of events and the behavior patterns in blood glucose (BG) self-monitoring and insulin adjustments. A subjective evaluation was also performed based on the answers of the patients to a questionnaire delivered at the end of the study.
Results
The number of sessions established with the mobile Smart Assistant was considerably higher during the intervention period than in the control period (29.0 versus 18.8, p < .05), and it was also higher than the number of Web sessions (29.0 versus 22.2, p < .01). The number of daily boluses was higher during the intervention period than in the control period (5.27 versus 4.40, p < .01). The number of daily BG measurements was also higher during the intervention period (4.68 versus 4.05, p < .05) and, in percentage, patients increased the BG measurements not associated to meals while decreasing the percentage of preprandial measurements. The subjective evaluation shows that patients would recommend the use of DIABTel in routine care.
Conclusions
The use of a continuous glucose monitor changes the way patients manage their diabetes, as observed in the increased number of daily insulin bolus, the increased number of daily BG measurements, and the differences in the distribution of BG measurements throughout the day. Continuous monitoring also increases the interaction of patients with the information system and modifies their patterns of use. We can conclude that mobile technologies are especially useful in scenarios of tight monitoring in diabetes, and they are well accepted by patients.
Keywords: continuous data management, diabetes, evaluation, mobile telemedicine, multiaccess architecture, Smart Assistant
Introduction
Due to its multifactorial and systemic character, diabetes mellitus is considered a paradigm of chronic disorders, which has led to an extensive application of information technologies in diabetes care.1 Thus diabetes has been a major focus of biomedical engineering efforts. These efforts are promoted by the recommendations given by the World Health Organization in a 2002 report,2 which presents a number of actions to improve the treatment of chronic patients through a change in the paradigm of patient’s care. This change must be followed by an extended and better use of information and communication technologies (ICT) in chronic patients’ care.
Telemedicine has shown a positive influence on diabetes care, providing innovative solutions for the diagnosis, monitoring, and treatment of diabetes patients,3 but there are some problems that delay the widespread implement-ation of telemedicine systems and place telemedicine between fading and future.4 Earlier experiences aimed to facilitate remote monitoring of patients from home by the transmission of computerized blood glucose (BG) profiles to the hospital.5–7 Technology evolution has enabled the development of advanced systems based on more powerful and easy-to-use terminals and applications, including Web-based systems, in particular.8,9 Some telemedicine systems for diabetes patients use a distributed approxi-mation, where patients can download data that are sent to the hospital and can be accessed by doctors, enhancing physician–patient interaction.8,10,11
The rapid growth and development of mobile computing and mobile Internet is changing the way people access common services and is revolutionizing telemedicine and shared care services, providing independence, mobility, and ubiquity to the patients, extending the features of conventional telemedicine systems. The mobility and freedom provided by mobile technologies allows improving patients’ care in an ambulatory scenario.12
Some of the latest telemedicine systems in diabetes include manual sending of BG levels by handheld devices, which motivates self-care management of diabetes.13,14 Others are focused on manual transmission of BG results and real-time expert feedback by mobile phone,15 providing an added value that can only be reached by introducing mobile technologies. The natural next step is to include automatic data downloading from medical devices and to transfer the downloaded data to physicians, maintaining expert feedback.16,17
A modern mobile telemedicine system for diabetes care in ambulatory scenarios should facilitate the patient’s efforts to collect monitoring data and provide communication capabilities with a patient’s personal devices. A mobile-phone-based health management platform should automatically capture biological and physiological information. These health-related data must be analyzed, treated, and stored. The timely “personal health information” can be processed locally in the device, to be transferred immediately to a network-connected server for further processing and/or to be displayed for human analysis, and stored for later retrieval and analysis.18
The growing availability of continuous data coming from glucose sensors and subcutaneous insulin infusion pumps allows patients and physicians to make better therapeutic decisions but supposes a huge quantity of data that are difficult to manage using mobile terminals. Therefore, it is crucial to define new ICT architectures for efficient management of data storage, data transmission, and data visualization in the context of continuous monitoring.19
In previous studies, we observed that combining our telemedicine system with continuous monitoring improves patients’ glycemic control and modifies self-management of insulin treatment.20,21 In this article, we analyze patients’ behavior from the use-of-the-system perspective throughout a 2-month clinical experiment, identifying how continuous monitoring changes the interaction of patients with the mobile telemedicine system.
Methods
Clinical Experiment
The clinical experiment consisted of a 2-month crossover randomized study, each phase lasting 4 weeks, with an interim wash-out period of 6 weeks.20 Ten type 1 patients [5 women, 41.2 (range, 21–62) years old, duration of diabetes 14.9 (range, 3–52) years] were included, and they used the DIABTel system,22–24 which supported the interactions between doctors and patients.
In both experimental phases, patients used a personal assistant, called Smart Assistant (SA),21 running on a personal digital assistant (PDA), which provides patients with flexible tools for data visualization, management of his/her monitoring data, and downloading of data from an insulin pump (D-tron plus, Disetronic, Burgdorf, Switzerland), via Infrared Data Association or BluetoothTM. The SA application is able to manage a local database, allowing the patient to work isolated from the telemedicine system. Self-monitoring of BG data were measured with the Roche Accutrend® glucose meter (Boehringer Mannheim, Mannheim, Germany) and were transmitted through a conventional phone line using the Roche Acculink® modem (Roche Diagnostics, Basel, Switzerland). After transmission, BG data were immediately available on the Web. During both experimental phases, patients used the SA whenever they had to communicate with the insulin pump. On patient’s demand, the SA application communicates with the DIABTel server and automatically updates all the new information in both directions. In the 24 h period following data reception, doctors analyzed patients’ data and advised treatment changes as necessary. Patients received instruction to synchronize the SA at least once a week without any specific guideline about the Web or SA functionalities they should use.
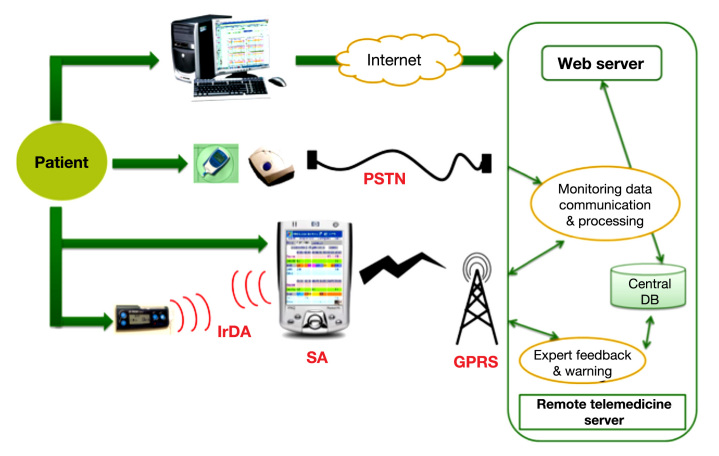
During the control phase, patients used the DIABTel system. During the intervention phase, patients also wore a continuous glucose monitoring (CGM) device (Guardian® RT, Continuous Glucose Monitoring System, Medtronic MiniMed, Northdridge, CA) 3 days per week. Patients had real-time information of their BG levels; however, the CGM data were retrospectively analyzed. While wearing the CGM device, patients were not expressly asked to perform a predefined number of finger-prick tests apart from the two a day required for calibration. Figure 1 shows an overview of the devices and applications the patients used during the clinical experimental phases.
Figure 1.
Overall architectural overview of the system. PSTN, public switched telephone network; IrDA, Infrared Data Association; DB, database; GPRS, general packet radio service.
System Evaluation
The system was evaluated from an objective point of view with the automatic tracking of a user’s interactions with the PDA or the Web to obtain information about the use of the system. Additionally, a subjective evaluation was made based on a questionnaire fulfilled online by patients at the end of the experiment, where they reflected their impressions about the system and especially about the SA application. This questionnaire was specifically created for the experiment, and among others, it asks whether the PDA and the SA are easy to use, whether patients think that the SA is secure and keeps their intimacy, whether it helps patients with their daily diabetes self-management, whether it reduces the number and level of incidences and whether it helps solving or avoiding them, and whether they would recommend the use of the system to other patients.
The questionnaire includes a section about the utility of the CGM device, where patients were asked if CGM makes them more confident with their decisions regarding diabetes, if it helps preventing problematic situations, and if it provides them with more freedom.
Objective evaluation was made studying the user-interaction events that were automatically registered in DIABTel’s database during the experiment. For each event, data related to the patient’s identification, the type of action, and the date and time of the generation of the event is registered. This information allows the study of patients’ behavior patterns of use of the system at each experimental phase.
The events registered are start session, finish session, database synchronization, data downloaded from the insulin pump, read serial number, new treatment notification, logbook visualization, insert glucose data, insert diet data, insert additional data, modify additional data, graphics visualization, pump therapy visualization, and diet therapy visualization. These actions can be performed by the patient using the SA or using other applications of the system like the Web application, accepting data downloading from the insulin pump, reading the serial number, or database synchronization that can only be carried out with the SA.
For each experimental phase, we computed the number of sessions established in the SA application and the functionalities of the system used by the patients. To asses the impact of CGM in patients’ behavior, we analyzed the changes in the number of boluses carried out by the patients and also the number of capillary BG measurements and the time of these measurements. We aimed to discover if the number of changes in the insulin regime, and measurements are higher during the intervention period than in the control period.
We finished the analysis by comparing the number of sessions registered at the SA application versus the number of Web sessions for each period. Our intention was to check if patients used the SA functionalities that are also available in the Web application, with which patients could be more familiar.
Results
Objective Evaluation of the Smart Assistant
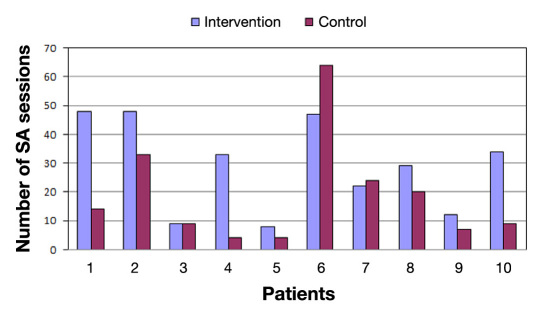
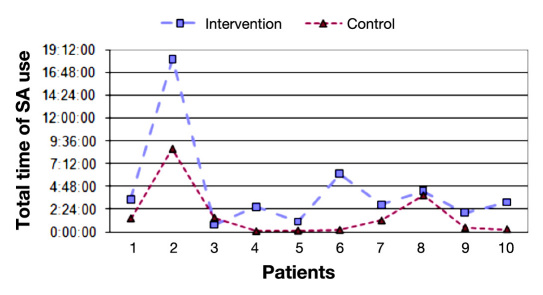
Figure 2 shows the number of sessions in the SA for the intervention and control periods. We can observe that the number of sessions during the intervention period is considerably higher than in the control period (29.0 versus 18.8, p <.05). Figure 3 shows the total time of SA usage for each experimental phase. The total time spent by patients with the system was greater during the intervention phase (04:27:11 versus 01:47:07, p <.01). If we analyze the average duration of each session, there is no statistically significant difference between the two periods.
Figure 2.
Number of SA sessions per period.
Figure 3.
Total time of SA use per period.
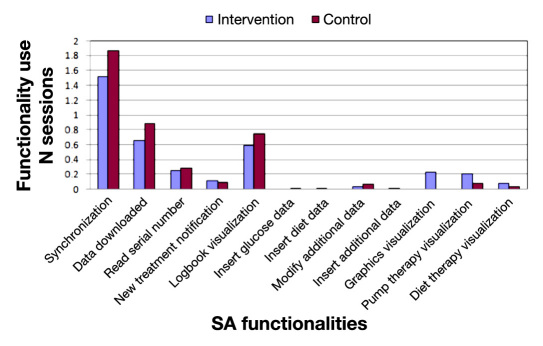
In Figure 4 , shows the use of the functionalities of the SA weighted by the number of sessions. We detected that, during the control phase, patients used the functionalities related to “logbook visualization,” “data downloaded from the insulin pump,” or “read serial number” events more times per session. The functionalities “insulin pump therapy visualization” and “diet therapy visualization” were employed more often during the intervention phase. The “insulin pump therapy visualization” was used significantly more during the intervention phase (p <.05) than in control phase, and “graphics visualization” was only generated during the intervention phase.
Figure 4.
Use of the SA functionalities per period weighted by the number of sessions.
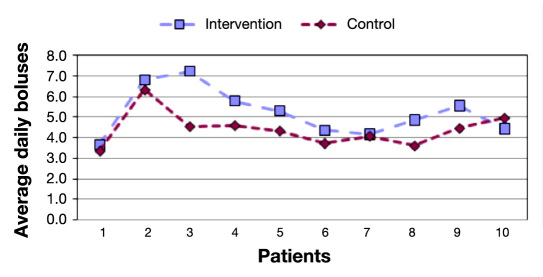
The average number of daily BG measurements and the number of daily boluses can be seen in Figures 5 and 6 . During the intervention phase, the number of boluses has higher than in the control period (5.27 versus 4.40, p <.01), and the same happens for the number of BG measurements (4.68 versus 4.05, p <.05).
Figure 5.
Average number of daily boluses per period.
Figure 6.
Average number of daily BG measurements per period.
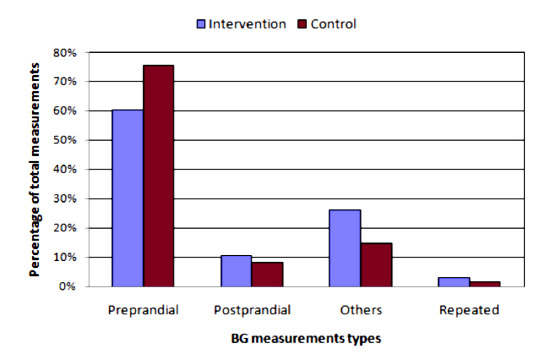
Figure 7 shows the distribution of BG measurements in moments of the day. The number of measurements not associated to meals was higher during the intervention phase than in the control phase.
Figure 7.
Distribution of BG measurements.
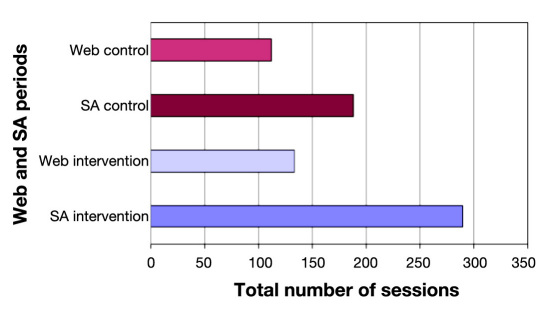
Figure 8 shows a comparison between the number of sessions established by the patients in the Web application and the SA for each period. The number of SA sessions during the intervention phase was higher than the number of Web sessions (29.0 versus 22.2, p <.01). There was no statistical significance when comparing the number of sessions between Web and SA during the control phase.
Figure 8.
Comparison of total number of sessions per period between SA and Web.
Subjective Evaluation of the Smart Assistant
Regarding the results obtained with the questionnaire, in the section related to the technical validation and utility of the SA (easy to use and to learn, utility in downloading data from the insulin pump and later visualization, respects my intimacy), the system obtained 150 points out of 162. The aspects related to global usability were pointed positively, obtaining 134 points out of 144. Among the aspects asked in this global usability section, we can highlight that 87.5% of the patients disagree with the statement that “the system has made my daily life harder.”
Related to the utility of the DIABTel system (impact in the communication with the doctor, number and level of incidences, the system helps me taking decisions, makes me feel safer in my diabetes self-management), the score was 133 points out of 141. All patients would recommend the use of the DIABTel system for diabetes care.
The answers regarding the utility of CGM show that 80% of the patients agree or partially agree with the statement that the use of a CGM makes them more confident about diabetes, 60% think that CGM helps them preventing problematic situations, and 80% believe that the CGM provides them with more freedom.
Discussion
Patients used the system more intensively when they wore the continuous glucose sensor, in terms of number of sessions and total time of system use.
During the control phase, the number of SA sessions was significantly lower, but patients performed more tasks per session. Our interpretation is that patients used the SA less frequently, so they concentrate more actions per session.
When analyzing patients’ behavior within a SA session, we observe that, during the intervention phase, patients access more often the graphic representation of data and the consultation of insulin therapy and diet. In our opinion, one of the reasons is that they have access to more information integrated in the SA, and this helps them to make their decisions.
Patients’ behavior change is reflected in the use of the functionalities of the system, the average number of daily insulin boluses, and the number of BG measurements, which were higher during the intervention phase than in the control phase. The availability of the CGM information drove patients to administer extra boluses frequently to control the glycemic levels better after having confirmed the CGM readings with extra BG measurements. For this reason, the percentage of BG measurements in times of the day not associated to meals was higher during the intervention phase.
The mobility and independence provided by the PDA application competes with the higher usability of the Web application. Objective results show that patients prefer mobility instead of usability, because they used the SA more intensively than the Web, even for those functionalities that are common to both applications. Additionally, patients explicitly stated that the SA helped them with their control of diabetes without making their daily life harder.
The mobility of the whole system could be incremented if BG data could be directly downloaded into the PDA instead of using the Acculink modem that requires a wired connection. However, the Acculink modem makes it possible to download data from anywhere a phone line is available, without the need of having a computer, so the SA can be an alternative to feedback for the patients regarding data transfer, providing them with more freedom than computer access. From a technical point of view, it is possible to directly download glucose meters to the presented SA using both a data cable or a wireless (Bluetooth) connection,21 but this requires a specific software development for each commercial device since there are no communication standards implemented by medical device manufacturers.
There are international initiatives, like Continua Health Alliance,25 to get a common standard for medical device communication in ambulatory scenarios by extending the family of standards International Organization of Standardization 11073/IEEE 1073 (X73)26 and encouraging manufacturers to adapt their products to the standard. To the best of the authors’ knowledge there is only one “diabetes product” certified by Continua, Roche Accu-Check®Smart Pix Device Reader, limited to Roche glucose meters and insulin pumps and using the USB interface for data transfer. Therefore, a lot of work remains to be done in the area of device integration into mobile scenarios for diabetes care.
Conclusions
The use of CGM changes the way patients manage their diabetes, as observed in the increased number of daily insulin bolus, the increased number of daily BG measurements, and the differences in the distribution of BG measurements along the day. Continuous monitoring also increases the interaction of patients with the information system and modifies their patterns of use.
We can conclude that mobile technologies are especially useful in scenarios of tight monitoring in diabetes and that they are well accepted by patients, who would recommend the use of DIABTel in routine care.
Abbreviations
- BG
blood glucose
- CGM
continuous glucose monitoring
- ICT
information and communication technologies
- PDA
personal digital assistant
- SA
Smart Assistant
References:
- 1.Andreassen S, Gómez EJ, Carson ER. Computers in diabetes 2000. Comput Methods Programs Biomed. 2002;69(2):93–95. doi: 10.1016/s0169-2607(00)00063-8. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization (WHO) Innovative care for chronic conditions: building blocks for action. Geneva: 2002. WHO Global Report. [Google Scholar]
- 3.Verhoeven F, Tanja-Dijkstra K, Nijland N, Eysenbach G, van Gemert-Pijnen L. Asynchronous and synchronous teleconsultation for diabetes care: a systematic literature review. J Diabetes Sci Technol. 2010;4(3):666–684. doi: 10.1177/193229681000400323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bellazzi R. Telemedicine and diabetes management: current challenges and future research directions. J Diabetes Sci Technol. 2008;2(1):98–104. doi: 10.1177/193229680800200114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marrero DG, Kronz KK, Golden MP, Wright JC, Orr DP, Fineberg NS. Clinical evaluation of a computer-assisted self-monitoring of blood glucose system. Diabetes Care. 1989;12(5):345–350. doi: 10.2337/diacare.12.5.345. [DOI] [PubMed] [Google Scholar]
- 6.Ahring KK, Ahring JP, Joyce C, Farid NR. Telephone modem access improves diabetes control in those with insulin-requiring diabetes. Diabetes Care. 1992;15(8):971–975. doi: 10.2337/diacare.15.8.971. [DOI] [PubMed] [Google Scholar]
- 7.Billiard A, Rohmer V, Roques MA, Joseph MG, Suraniti S, Giraud P, Limal JM, Fressinaud P, Marre M. Telematic transmission of computerized blood glucose profiles for IDDM patients. Diabetes Care. 1991;14(2):130–134. doi: 10.2337/diacare.14.2.130. [DOI] [PubMed] [Google Scholar]
- 8.Lanzola G, Capozzi D, D´Annunzio G, Ferrari P, Bellazzi R, Larizza C. Going mobile with a multiaccess service for the manage-ment of diabetic patients. J Diabetes Sci Technol. 2007;1(5):730–737. doi: 10.1177/193229680700100518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nigrin DJ, Kohane IS. Glucoweb: a case study of secure, remote biomonitoring and communication. Proc AMIA Symp. 2000:610–614. [PMC free article] [PubMed] [Google Scholar]
- 10.Gómez EJ, Hernando ME, García A, Del Pozo F, Cermeño J, Corcoy R, Brugués E, De Leiva A. Telemedicine as a tool for intensive management of diabetes: the DIABTel experience. Comput Methods Programs Biomed. 2002;69(2):163–177. doi: 10.1016/s0169-2607(02)00039-1. [DOI] [PubMed] [Google Scholar]
- 11.Biermann E, Dietrich W, Rihl J, Standl E. Are there time and cost savings by using telemanagement for patients on intensified insulin therapy? A randomised, controlled trial. Comput Methods Programs Biomed. 2002;69(2):137–146. doi: 10.1016/s0169-2607(02)00037-8. [DOI] [PubMed] [Google Scholar]
- 12.Perakis K, Konnis G, Pavlopoulos S. Telemedicine and third generation (3G) cellular networks. J Inform Technol Healthcare. 2007;5(3):141–150. [Google Scholar]
- 13.Franklin V, Waller A, Pagliari C, Greene S. “Sweet talk”: text messaging support for intensive insulin therapy for young people with diabetes. Diabetes Technol Ther. 2003;5(6):991–996. doi: 10.1089/152091503322641042. [DOI] [PubMed] [Google Scholar]
- 14.Kim SI, Kim HS. Effectiveness of mobile and internet intervention in patients with obese type 2 diabetes. Int J Med Inform. 2008;77(6):399–404. doi: 10.1016/j.ijmedinf.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 15.Farmer AJ, Gibson OJ, Dudley C, Bryden K, Hayton PM, Tarassenko L, Neil A. A randomized controlled trial of the effect of real-time telemedicine support on glycemic control in young adults with type 1 diabetes (ISRCTN 46889446) Diabetes Care. 2005;28(11):2697–2702. doi: 10.2337/diacare.28.11.2697. [DOI] [PubMed] [Google Scholar]
- 16.Gammon D, Arsand E, Walseth OA, Andersson N, Jenssen M, Taylor T. Parent–child interaction using a mobile and wireless system for blood glucose monitoring. J Med Internet Res. 2005;7(5):e57. doi: 10.2196/jmir.7.5.e57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee HJ, Lee SH, Ha KS, Jang HC, Chung WY, Kim JY, Chang YS, Yoo DH. Ubiquitous healthcare service using Zigbee and mobile phone for elderly patients. Int J Med Inform. 2009;78(3):193–198. doi: 10.1016/j.ijmedinf.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 18.Hernando ME, García-Sáez G, Martínez-Sarriegui I, Rodríguez-Herrero A, Pérez-Gandía C, Rigla M, de Leiva A, Capel I, Pons B, Gómez EJ. Automatic data processing to achieve a safe telemedical artificial pancreas. J Diabetes Sci Technol. 2009;3(5):1039–1046. doi: 10.1177/193229680900300507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hernando ME, Pascual M, Salvador CH, García-Sáez G, Rodríguez-Herrero A, Martínez-Sarriegui I, Gómez EJ. Definition of information technology architectures for continuous data management and medical device integration in diabetes. J Diabetes Sci Technol. 2008;2(5):899–905. doi: 10.1177/193229680800200523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rigla M, Hernando ME, Gómez EJ, Brugués E, García-Sáez G, Capel I, Pons B, de Leiva A. Real-time continuous glucose monitoring together with telemedical assistance improves glycemic control and glucose stability in pump-treated patients. Diabetes Technol Ther. 2008;10(3):194–199. doi: 10.1089/dia.2007.0273. [DOI] [PubMed] [Google Scholar]
- 21.García-Sáez G, Hernando ME, Martínez-Sarriegui I, Rigla M, Torralba V, Brugués E, de Leiva A, Gómez EJ. Architecture of a wireless personal assistant for telemedical diabetes care. Int J Med Inform. 2009;78(6):391–403. doi: 10.1016/j.ijmedinf.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 22.Rigla M, Hernando ME, Gómez EJ, Brugués E, García-Sáez G, Torralba V, Prados A, Erdozain L, Vilaverde J, de Leiva A. A telemedicine system that includes a personal assistant improves glycemic control in pump-treated patients with type 1 diabetes. J Diabetes Sci Technol. 2007;1(4):505–510. doi: 10.1177/193229680700100408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gómez EJ, Hernando Pérez ME, Vering T, Rigla Cros M, Bott O, García-Sáez G, Pretschner P, Brugués E, Schnell O, Patte C, Bergmann J, Dudde R, de Leiva A. The INCA system: a further step towards a telemedical artificial pancreas. IEEE Trans Inf Technol Biomed. 2007;12(4):470–479. doi: 10.1109/TITB.2007.902162. [DOI] [PubMed] [Google Scholar]
- 24.Martínez-Sarriegui I, García Sáez G, Hernando ME, Rigla M, Brugués E, de Leiva A, Gómez EJ. Mobile telemedicine for diabetes care. In: Xiao Y, Chen H, editors. Mobile telemedicine: a computing and networking perspective. Boca Raton: Auerbach; 2008. [Google Scholar]
- 25.Continua Health Alliance. http://www.continuaalliance.org/index.html. Accessed July 12, 2010.
- 26.IEEE 1073-1996 IEEE standard for medical device communications-overviewandframework. http://ieeexplore.ieee.org/xpls/abs_all.jsp?arnumber=536423. Accessed July 12, 2010.