Abstract
Background
The first-generation FreeStyle Navigator® Continuous Glucose Monitoring System (FreeStyle Navigator CGM) requires a 10 h warm-up period to avoid inaccurate glucose readings caused by sensor insertion trauma and wound-healing processes. The performance of a second-generation FreeStyle Navigator CGM that begins reporting glucose 1 h after sensor insertion is described.
Methods
Second-generation FreeStyle Navigator CGM performance was evaluated in an in-clinic study using the YSI Model 2300 STATPlus Glucose Analyzer as reference with 47 subjects with type 1 diabetes. The reference readings were taken at 15 min intervals, and the study was designed to emphasize the first 10 h of use.
Results
The second-generation FreeStyle Navigator CGM exhibited continuous glucose error grid analysis ratings of 93.7% “clinically accurate,” 3.6% “benign errors,” and 2.8% “clinical errors” and a mean and median absolute relative difference of 14.5% and 10.7%, respectively. The second-generation algorithm detected signal instability in the first 10 h of use and suspended the reporting of 14.1% of first day continuous glucose readings. The clinical accuracy of the second-generation FreeStyle Navigator CGM was similar for the first 10 h versus subsequent hours, with 92.6% and 94.2% “clinically accurate” readings, respectively.
Conclusion
The warm-up period for the second-generation FreeStyle Navigator CGM was reduced from 10 to 1 h, with minimal interruption of glucose reporting and without sacrificing clinical performance.
Keywords: accuracy, calibration, clinical performance, continuous glucose monitor
Introduction
The FreeStyle Navigator® Continuous Glucose Monitoring System (FreeStyle Navigator CGM) for measuring glucose concentration in interstitial fluid became commercially available in 2008. This first-generation product incorpo-rated a 10 h warm-up period before glucose values were reported.1
The reporting delay was the result of a phenomenon we have termed “postinsertion variability” (PIV), which is caused by the trauma of sensor insertion and the associated wound-healing process. It is characterized by depression of the sensor signal that would result in the reporting of inaccurately low glucose values. The signal interference associated with PIV is variable. Although 80–90% of FreeStyle Navigator CGM sensor insertions display little or no evidence of signal depression, in 5–10% of insertions, the effect can be severe.
The time course of PIV followed the wound-healing process, which is generally complete 3–10 h after insertion. To avoid reporting inaccurate glucose values, continuous glucose display was delayed for 10 h in the first-generation product.
The second-generation of FreeStyle Navigator CGM software incorporates an algorithm to detect PIV and prohibit display of glucose only during periods of significant signal depression. This new calibration algorithm, named TRUstart™, calls for five calibrations with self-monitoring of blood glucose (SMBG) measurements during the 5-day sensor lifetime to occur 1, 2, 10, 24, and 72 h after sensor insertion, with glucose values reported after the first calibration. If the sensor is inserted at a time when calibration is inconvenient (e.g., immediately before bedtime), the first-generation schedule of four calibrations occurring 10, 12, 24, and 72 h after insertion is an available option.
The TRUstart algorithm has additional features to make calibration more convenient. In the first-generation product, calibration was prohibited when the rate of glucose change was greater than ±2 mg/dl/min. This was because calibration is less accurate during times of rapid change, as a result of the time lag between SMBG glucose used for calibration and sensor-measured interstitial glucose.1
TRUstart corrects for the effect of interstitial glucose lag, and the window for calibration has been opened to rates up to ±3.5 mg/dl/min. Also, the acceptable glucose range for calibration was increased from 60–300 to 60–400 mg/dl, because data collected after the initial product was introduced have demonstrated sufficiently accurate calibration in the range of 300–400 mg/dl.
This article describes the TRUstart algorithm and its theoretical underpinnings and demonstrates in a clinical trial the performance of the second-generation FreeStyle Navigator CGM.
Methods
TRUstart Calibration Algorithm
FreeStyle Navigator CGM calibration is front-loaded with four of the five calibrations required in the first 24 hours, when there exists the highest probability of an unstable signal. After 24 hours, the probability of significant signal instability is negligible, and only one calibration is requested during the remaining 4 days of sensor life.
An SMBG measurement using the integrated FreeStyle blood glucose (BG) meter is requested 1 h after FreeStyle Navigator CGM detects sensor insertion. A sensitivity value is calculated for converting sensor current to glucose (sensitivity = current/BG level). Sequential continuous glucose monitoring (CGM) sensor currents are highly correlated in time, but the correlation largely disappears after 1 h.2 One hour after the first calibration, another SMBG test is requested, and a second sensitivity is calculated. The two sensitivities are temporally independent and compared for agreement. If the relative difference between the two values is within a proscribed range, they are deemed acceptable and averaged. Agreement ensures that an outlier CGM current or SMBG test will not be used for calibration, and averaging two independent calibrations reduces calibration error. Subsequent requested calibrations are likewise tested for agreement and averaged.
The system uses information from the CGM sensor and SMBG measurements to infer the presence of PIV during the first 10 h of sensor operation. For example, if the glucose descends into the hypoglycemic range, the system requests an SMBG test, because the glucose value could be inaccurately low due to a depressed signal. The BG tests that are performed without a request are also used to check for PIV. If there is no evidence of a depressed signal during the first 10 h, the algorithm for detecting PIV is suspended.
If signal depression is detected or if requested SMBG tests are not performed, CGM values are not displayed until signal recovery is indicated by an SMBG test. After suspension of continuous glucose reporting, SMBG tests are periodically requested to check for signal recovery, and tests performed without a request can also be used for this purpose. When there is evidence of signal recovery, the CGM readings are restored. After signal depression is detected, SMBG checks of sensitivity are performed up to 24 h after sensor insertion.
To obtain accurate calibration during times of glucose change, a first-order linear ordinary differential equation is used to describe the difference between blood and interstitial glucose.3 Using this model, the sensor current for sensitivity calculation is corrected for an average time lag of 10 min. The model requires an estimate of the rate of interstitial glucose change, which is calculated from the 1 min measurements ±7 min from the time of the BG calibration test.
Clinical Study Design
Forty-seven adult subjects with type 1 diabetes at two clinical sites (Diablo Clinical Research in Walnut Creek, CA, and Rainier Clinical Research in Renton, WA) tested the accuracy of the FreeStyle Navigator CGM versus the YSI Blood Glucose Analyzer Model 2300 (YSI; YSI Life Sciences, Yellow Springs, OH).
Subjects wore one sensor on the upper arm and one on the abdomen. Venous samples were obtained through intravenous placement of an angiocatheter, and YSI measurements were performed at 15 min intervals during two clinic sessions. For each subject, the first 14 h session was initiated with sensor insertion, and the second 12 h session was during one of the four following days. Subjects performed SMBG tests required for sensor calibration, additional tests requested by the study protocol, and tests to manage their normal treatment plan.
An insulin challenge was administered to each subject to obtain hypoglycemic measurements. FreeStyle Navigator BG meter values and system alerts were displayed, but CGM values were masked to subjects and research staff. The SMBG values and raw 1 min current and temperature readings were stored for later processing with the TRUstart and first-generation algorithms.
All subjects provided informed consent, and the study was registered with http://www.clinicaltrials.gov (registration number NCT01076218).
Analysis Methods
Sensor lag time versus reference was determined by a method based on a Poincaré plot.4 For the evaluation of sensitivity trends, normalized sensitivity for a sensor was calculated as a ratio of the sensitivity calculated from any SMBG reading to the median of all sensitivity values for that sensor; there was an average of 90 SMBG tests per sensor.
The continuous glucose error grid analysis (CG-EGA)5 was used to assess the clinical accuracy of all information provided by CGM sensors. Quantitative differences from reference glucose were assessed by mean absolute relative difference (ARD), median ARD, and percent within International Standards Organization (ISO) 15197 accuracy limits (within ±15 mg/dl for glucose ≤75 mg/dl and within ±20% for glucose >75 mg/dl).6
Low alarms were assessed by evaluating the percentage of detection of hypoglycemia (<70 mg/dl) within 30 min of the start of the hypoglycemic condition as determined by the reference glucose measurement. The percentage of false alarms (reference glucose > alarm setting when alarm activates) was also determined, and the range of alarm settings evaluated was 70–85 mg/dl. False alarms for glucose >85 mg/dl were also evaluated at each setting; when glucose is ≤85 mg/dl and descending, alarms are not necessarily false, because it is prudent to consider raising glucose to a safer level under these conditions.7
High alarms were evaluated differently than low alarms. Since the FreeStyle Navigator CGM is more accurate in the hyperglycemic range,1 the detection of a high glucose value was reported only for the alarm set at that value. The entire available range of high alarm settings was evaluated (140–300 mg/dl), because treatment of hyperglycemia depends on individual treatment goals. Because glucose monitor values within ±20% of reference are generally viewed as clinically accurate,6,8 detection of glucose levels 20% higher than the alarm setting and false alarms 20% lower than the alarm setting were also reported.9 The time window for hyperglycemic detection was also 30 min from the time reference glucose rose above the alarm setting.
Because CGM values are correlated in time and cannot be considered statistically independent, p values were calculated on a per-sensor basis to eliminate the temporal dependence. A paired t test was employed (JMP version 5.1, SAS Institute, Inc.), and the Bonferroni correction was used to determine the level of significance for multiple analyses of the same data set (p < .006).
Results
Study subject characteristics are listed in Table 1 . There was no significant influence of age (p= .345), body mass index (p= .092), years since diagnosis (p= .277), or sex (p= .816) on system accuracy, and the data were homogeneous between clinical sites. There was also no significant influence of insertion site (p= .253), but there was a significant influence of glucose range (p< .0001), with accuracy improving as glucose concentration increases. The average lag time was 9.6 min.
Table 1.
Subject Characteristics
| Characteristic | Average (standard deviation) |
|---|---|
| Age (years) | 39.3 (11.7) |
| Weight (lbs) | 173.8 (38.9) |
| Height (in.) | 67.2 (4.4) |
| Body mass index (kg/m2) | 26.9 (4.5) |
| Years since type 1 diagnosis | 21.1 (10.3) |
| Daily total insulin dosage (U) | 49.9 (30.3) |
| Reference glucose (mg/dl) | 169.4 (64.0) Diablo Research Clinic 162.0 (66.8) Rainier Clinical Research |
| Sex | 44.7% male 55.3% female |
| Ethnicity | 91.5% Caucasian 8.5% Noncaucasian |
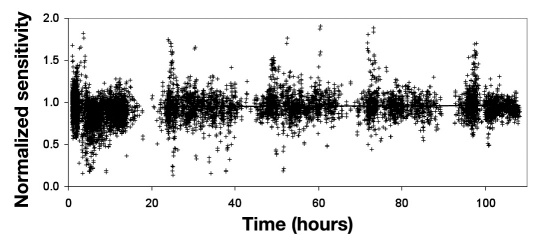
The relationship of normalized sensitivity with implant time (Figure 1 ) displays some depressed sensitivity values during the early hours. During the first 10 h, 11.3% of the normalized sensitivity values were more than 30% low compared to 3.0% during subsequent times. After the first day, the sensitivity was stable, with an average total increase of 1.8% during the following four days by linear regression analysis (p= .020). In the first day, the CGM values were blanked 14.1% of the time.
Figure 1.
Normalized sensitivity as a function of time (n = 9125). Values of normalized sensitivity include BG tests that do not meet the screening limits for calibration. The line is a statistically significant linear regression for days 2–5 (p = .020) and represents a total increase of 1.8% from day 2 to the end of sensor life.
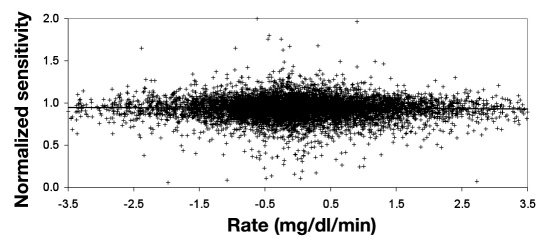
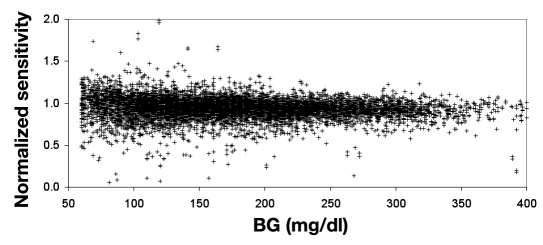
In Figures 2 and 3 , the normalized sensitivities were evaluated for BG values that meet the calibration screening requirements (60–400 mg/dl and rate of change of ±3.5 mg/dl/min). The normalized sensitivity as a function of rate (Figure 2 ) demonstrates little influence of rate on calibration accuracy. An average calibration bias of ±0.8% for rates of ±3.5 mg/dl/min was calculated by linear regression analysis, but the regression was not statistically significant (p= .060). The sensitivity check makes it unlikely that the outlier values in this plot would be used for calibration. The variability in normalized sensitivity decreased as glucose increased (Figure 3 ), and calibration accuracy would not be degraded in the 300–400 mg/dl glucose range.
Figure 2.
Normalized sensitivity for BG tests that meet screening limits for calibration as a function of rate (n = 8584). The line is a nonstatistically significant linear regression (p = .060) and represents a calibration bias of ±0.8% for rates of ±3.5 mg/dl/min. The sensitivity check makes it very unlikely that the outlier values in this plot would be used for calibration.
Figure 3.
Normalized sensitivity for BG tests that meet screening limits for calibration as a function of glucose (n = 8584). The variability in calibration decreases with increasing glucose. The sensitivity check makes it very unlikely that the outlier values in this plot would be used for calibration.
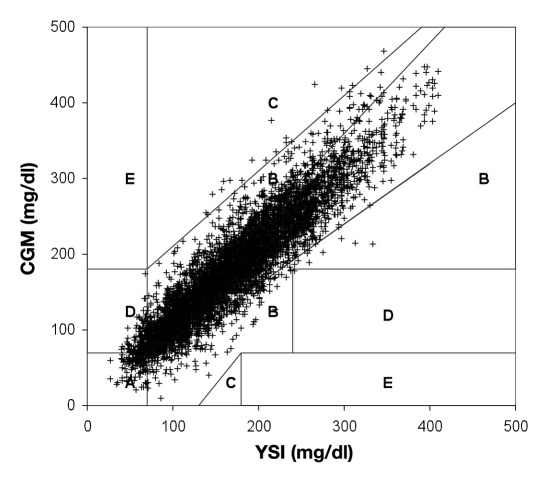
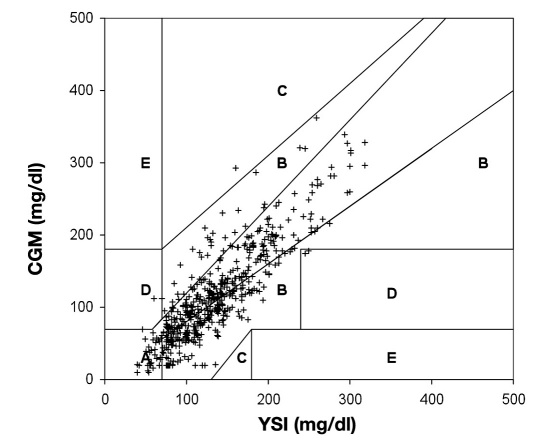
The Point error grid analysis (EGA), which is the part of the CG-EGA that evaluates individual glucose values, indicated 80.3% in zone A, 18.0% in zone B, and 1.7% in zones C–E (Table 2 and Figure 4 ), whereas the Rate EGA, which is the part of the CG-EGA that evaluates the rate of change in glucose, indicated 79.1% in zone A, 16.1% in zone B, and 4.8% in zones C–E. Zone A values are considered clinically accurate, zone B are benign errors, and zones C–E are clinical errors. The combined grids that assess all information provided by CGM rated all data 93.7% “clinically accurate,” 3.6% “benign errors,” and 2.8% “clinical errors.”
Table 2.
Performance Characteristics of the FreeStyle Navigator CGM with the TRUstart Algorithma
| Point EGA | ||||
| Zone A | 80.3 (6306) | |||
| Zone B | 18.0 (1410) | |||
| Zone C | 0.2 (16) | |||
| Zone D | 1.5 (120) | |||
| Zone E | 0 (0) | |||
| Rate EGA | ||||
| Zone A | 79.1 (6212) | |||
| Zone B | 16.1 (1266) | |||
| Zone C | 2.1 (162) | |||
| Zone D | 2.0 (158) | |||
| Zone E | 0.7 (54) | |||
| G-EGA (combined grids to give overall CGM clinical accuracy) | ||||
| Hypoglycemia (≤70 mg/dl) | Euglycemia (71–180 mg/dl) | Hyperglycemia (>180 mg/dl) | All data | |
| Clinically accurate | 53.6 (162) | 95.6 (4393) | 94.7 (2800) | 93.7 (7355) |
| Benign errors | 7.0 (21) | 3.7 (168) | 3.1 (91) | 3.6 (280) |
| Clinical errors | 39.4 (119) | 0.7 (32) | 2.2 (66) | 2.8 (217) |
| Statistical measures of concordance of CGM glucose with reference glucose values | ||||
| Mean ARD | 21.6 (302) | 15.5 (4593) | 10.5 (2957) | 14.5 (7852) |
| Median ARD | 17.5 (302) | 11.8 (4593) | 8.3 (2957) | 10.7 (7852) |
| ISO accuracy | 44.0 (133) | 72.3 (3319) | 87.4 (2584) | 76.9 (6036) |
Table entries are % (n). Zone A represents clinically accurate; zone B represents benign or no treatment errors; zone C represents overcorrection errors; zone D represents a potentially dangerous failure to treat; and zone E represents erroneous treatment.
Figure 4.
Point EGA of the FreeStyle Navigator CGM clinical data. Zone A represents clinically accurate (80.3%); zone B represents benign or no treatment errors (18.0%); zone C represents overcorrection errors (0.2%); zone D represents a potentially dangerous failure to treat (1.5%); and zone E represents erroneous treatment (0.0%). A slight modification of the Point-EGA was made for the purposes of this figure. Normally the ranges of the grid are adjusted for rate. To fit all points onto one grid, the adjustments to the grid were applied to the data points instead. For example, when the rate is 1–2 mg/dl/min, appropriate zones of the grid are adjusted by -10 mg/dl; for the purposes of this figure, the CGM glucose was adjusted by +10 mg/dl instead. This modification was only used for this figure; the CG-EGA data presented were performed as the analysis was described in the original publication.
The hypoglycemic range was significantly less accurate than the euglycemic and hyperglycemic ranges, with 56.2%, 95.6%, and 94.7% of values deemed “clinically accurate,” respectively.
For the entire glucose range, the mean and median ARD were 14.5% and 10.7%, respectively, and the ISO accuracy was 76.9%. The decrease in ARD and increase in ISO accuracy with increasing glucose indicated improved concordance with higher reference glucose values (Table 2 ).
During the time when CGM results were suppressed, the glucose readings exhibited an expected low bias (Figure 5 ), which resulted in less accurate glucose values: Point EGA zone A 57.5%, zone B 42.0%, and zones C–E 0.6%; mean and median ARD 25.3% and 21.3%, respectively; and ISO accuracy 47.8%. The overall clinical accuracy by CG-EGA, however, was not as severely affected: 91.7% “clinically accurate,” 6.3% “benign errors,” and 2.0% “clinical errors.”
Figure 5.
Point EGA of the FreeStyle CGM data during the period when readings were suppressed. The modification of the Point EGA described in Figure 4 was also applied to this figure.
There were no significant differences in clinical accuracy when the first 10 h were compared to subsequent hours with “clinically accurate” values 92.6% and 94.2% (p= .015), respectively (Table 3 ). (p values < .006 are statistically significant in this analysis.) When the data were stratified by glucose range, there was one statistically significant difference in the euglycemic range, but the magnitude of the difference was inconsequential, 95.2% versus 96.1% “clinically accurate” (p= .004). There were also no differences in the mean and median ARD when the first 10 h were compared to subsequent hours [mean ARD 13.4% versus 14.9% (p= .267), median ARD 10.0% versus 10.9% (p= .443), respectively].
Table 3.
Performance Characteristics with the TRUstart Algorithm in the First 10 h and Successive Time and Comparison of the TRUstart Algorithm to the First-Generation Algorithma
| TRUstart first 10 h | TRUstart after 10 h | P valuesb | ||||||||||
| CG-EGA | Hypoc | Euc | Hyperc | All Data | Hypo | Eu | Hyper | All Data | Hypo | Eu | Hyper | All Data |
| Clinically accurate | 54.6 (54) | 94.6 (1425) | 93.3 (874) | 92.6 (2353) | 53.2 (108) | 96.2 (2968) | 95.4 (1926) | 94.2 (5002) | 0.222 | 0.004 | 0.050 | 0.016 |
| Benign errors | 9.1 (9) | 4.4 (66) | 3.3 (31) | 4.2 (106) | 5.9 (12) | 3.3 (102) | 3.0 (60) | 3.3 (174) | 0.187 | 0.016 | 0.310 | 0.058 |
| Clinical errors | 36.4 (36) | 1.0 (15) | 3.4 (32) | 3.3 (83) | 40.9 (83) | 0.6 (17) | 1.7 (34) | 2.5 (134) | 0.431 | 0.067 | 0.070 | 0.058 |
| Mean ARDd | 19.2 (99) | 13.9 (1506) | 10.7 (937) | 13.4 (2542) | 22.8 (203) | 16.3 (3087) | 10.4 (2020) | 14.9 (5310) | 0.248 | 0.437 | 0.027 | 0.267 |
| Median ARDd | 14.8 (99) | 10.7 (1506) | 8.3 (937) | 10.0 (2542) | 18.3 (203) | 12.4 (3087) | 8.4 (2020) | 10.9 (5310) | 0.936 | 0.761 | 0.044 | 0.443 |
| TRUstart calibrated after 10 h | First-generation algorithm after 10 h | P values | ||||||||||
| CG-EGA | Hypo | Eu | Hyper | All Data | Hypo | Eu | Hyper | All Data | Hypo | Eu | Hyper | All Data |
| Clinically accurate | 54.4 (105) | 96.4 (2895) | 95.7 (1889) | 94.6 (4889) | 46.0 (87) | 96.7 (2843) | 96.8 (1798) | 94.8 (4719) | 0.632 | 0.187 | 0.448 | 0.527 |
| Benign errors | 4.7 (9) | 3.1 (92) | 2.6 (51) | 2.9 (152) | 3.7 (7) | 2.8 (82) | 1.8 (34) | 2.5 (123) | 0.190 | 0.364 | 0.130 | 0.064 |
| Clinical errors | 40.9 (79) | 0.6 (17) | 1.7 (33) | 2.5 (129) | 50.3 (95) | 0.5 (14) | 1.4 (25) | 2.7 (134) | 0.368 | 0.323 | 0.740 | 0.527 |
| Mean ARD | 22.5 (193) | 16.4 (3004) | 10.4 (1973) | 14.9 (5170) | 25.4 (189) | 16.4 (2939) | 9.1 (1848) | 14.8 (4976) | 0.409 | 0.715 | 0.016 | 0.725 |
| Median ARD | 18.2 (193) | 12.4 (3004) | 8.3 (1973) | 10.9 (5170) | 22.7 (189) | 12.8 (2939) | 7.3 (1848) | 10.5 (4976) | 0.269 | 0.761 | 0.015 | 0.294 |
Table entries are % (n).
P values were determined by paired t test, and the Bonferroni correction was used to determine the significance level for multiple analyses of the same data set (p< .006).
Hypo, hypoglycemia (≤70 mg/dl); Eu, euglycemia (71–180 mg/dl); Hyper, hyperglycemia (>180 mg/dl)
In the hypoglycemic region, the absolute difference rather than the ARD is reported.
When TRUstart was not calibrated until after 10 h and compared to the first-generation software version (Table 3 ), the clinical accuracy was nearly the same, with 94.6% and 94.8% “clinically accurate” (p= .527). There was no significant difference in mean ARD, with values of 14.9% versus 14.8% (p= .725), or in median ARD, with values of 10.9% versus 10.5% (p= .294), for TRUstart versus the first-generation algorithm, respectively. There were also no significant differences in performance when the data were stratified by glucose range.
The performance analysis of the low alarms shows the trade-off of hypoglycemia detection with false alarms as the alarm setting increases (Table 4 ). In the alarm range of 70–85 mg/dl, the detection increased from 60.2% to 86.1%, whereas alarms occurring with the BG >85 mg/dl increased from 19.1% to 23.2%. High alarm performance was similar throughout the range of 140–300 mg/dl. At the alarm threshold, detection ranged from 83.7% to 91.8%, and false alarms were 35.9–70.1%, but when ±20% is allowed for clinical accuracy, detection of BG 20% higher than the alarm setting varied from 97.4% to 100%, and false alarms 20% lower than the alarm setting were between 3.3% and 7.5%. There was a paucity of data with BG levels >300 mg/dl for performing the alarm analysis at 300 mg/dl (Table 5 ).
Table 4.
Detection of Hypoglycemia (Glucose < 70 mg/dl) with FreeStyle Navigator CGM Low Alarms at Various Settingsa
| Alarm setting (mg/dl) | 70 | 75 | 80 | 85 |
|---|---|---|---|---|
| Detection of <70 mg/dl | 60.2 (65/108) | 68.5 (74/108) | 78.7 (85/108) | 86.1 (93/108) |
| False alarms with reference glucose > alarm setting | 36.3 (32/94) | 30.0 (33/110) | 23.6 (33/140) | 23.2 (39/168) |
| False alarms with reference glucose >85 mg/dl | 19.1 (18/94) | 19.1 (21/110) | 18.6 (26/140) | 23.2 (39/168) |
Detection is within 30 min of the start of hypoglycemia. Table entries are % (n/N).
Table 5.
Detection of High Glucose Levels between 140 and 300 mg/dl with FreeStyle Navigator CGM High Alarmsa
| Alarm setting (mg/dl) | 140 | 180 | 240 | 300 |
|---|---|---|---|---|
| Detection of glucose at alarm setting | 91.8 (213/232) | 88.7 (196/221) | 83.7 (82/98) | 86.4 (22/26) |
| Detection of glucose 20% higher than alarm setting | 98.7 (225/298) | 99.3 (143/144) | 97.4 (38/39) | 100.0 (8/8) |
| False alarm with reference glucose < alarm setting | 36.8 (91/247) | 35.9 (89/248) | 58.7 (88/150) | 70.1 (47/67) |
| False alarm with reference glucose < 20% lower than alarm setting | 4.5 (11/247) | 4.0 (10/248) | 3.3 (5/150) | 7.5 (5/67) |
Detection is within 30 min of the start of the high glucose event. Table entries are % (n/N).
Discussion
There has been little sacrifice in accuracy of display data in the first 10 h (Table 3 ). The TRUstart algorithm is deliberately conservative in that it is more likely to suppress CGM glucose when signal depression is insignificant than it is to allow the reporting of inaccurate CGM values (Figure 5 ). Although the suppression of CGM is relatively moderate, averaging 14.1% in the first day of use, it is variable from one sensor insertion to the next. Although the first-generation algorithm had a 10 h wait, the TRUstart algorithm has a degree of unpredictability. To minimize exposure to unreliable CGM values, users must tolerate some level of inconvenience with either algorithm.
Extending the allowable glucose range and rates for calibration eliminates some failures to calibrate after a calibration attempt. Again, these measures to enhance convenience were achieved with no deterioration of accuracy; the new algorithm exhibited the same clinical accuracy as the first-generation algorithm with its more restricted calibration conditions (Table 3 ).
The FreeStyle Navigator CGM performance characterization was chosen to give a complete picture of clinical utility. The ARD and ISO accuracy are simple statistical measures of concordance with the reference method. These measure only the accuracy of individual glucose values and do not capture the temporal nature of the measurements, which is an essential feature of CGM.
Understanding the effect of lag is important for inter-preting CGM-reported glycemic dynamics. The 9.6 min average lag measured in this study is consistent with previous lag measurements.1 Although a small fraction of the lag is due to the sensor, approximately 2 min, the larger portion of the lag time is due to the physiological difference between interstitial and venous glucose concentrations.5,10
The CG-EGA represents the most complete analysis of CGM and assesses the most crucial aspect of performance: clinical accuracy. The alarm analysis, however, is an essential additional element for CGM characterization. The CG-EGA rates FreeStyle Navigator CGM relatively poorly in the hypoglycemic range, with 53.6% “clinically accurate” values and 32.7% “clinical errors.” This evaluation is highly influenced by a +19.1 mg/dl bias to YSI in the hypoglycemic range that seriously interferes with the detection of hypoglycemia according to CG-EGA.
The alarm analysis, however, showed that an alarm setting of 85 mg/dl provided 86.1% detection of hypoglycemia within ±30 min, and only 23.2% of alarms occurred when attention to low BG is unnecessary (BG >85 mg/dl). The effect of the bias can be negated by the strategic use of the low alarms, and the resulting performance is virtually unobtainable using the traditional methods of noticing symptoms and performing SMBG tests to detect hypoglycemia.11 If a system exhibits high glucose variability and also has a significant negative bias, the clinical accuracy can be highly rated by CG-EGA (Figure 5); the high variability, however, would give poor alarm performance. Therefore, both CG-EGA and alarm analysis are necessary for an unambiguous assessment of CGM clinical performance.
For glucose >70 mg/dl, the bias to YSI was 10.4 mg/dl, but it had a relatively small impact on clinical performance. The CG-EGA rates FreeStyle Navigator CGM accurate, with “clinically accurate” values of 95.6% and 94.7% for the euglycemic and hyperglycemic ranges, respectively. The high alarm analysis is consistent with average hyperglycemia detection >95% and false alarms averaging <5% when incorporating an allowable error in glucose values of ±20%. It is important to note that the high bias is partially the result of an artifact in the venous reference, which read an average of 10 mg/dl higher than the SMBG measurements used to guide diabetes therapy and calibrate CGM sensors.
Conclusion
The FreeStyle Navigator CGM TRUstart algorithm allows the reporting of glucose values 1 h, rather than 10 h, after sensor insertion, but CGM readings can be suppressed because of instability caused by insertion trauma. In this study, 14.1% of data in the first day was suppressed. The restrictions on calibration were also relaxed to facilitate ease of calibration. These improvements in convenience were achieved without deterioration of system performance.
Abbreviations
- ARD
absolute relative difference
- BG
blood glucose
- CGM
continuous glucose monitoring
- CG-EGA
continuous glucose error grid analysis
- EGA
error grid analysis
- ISO
International Standards Organization
- PIV
postinsertion variability
- SMBG
self-monitoring of blood glucose
References:
- 1.Weinstein RL, Schwartz SL, Bragz RL, Bugler JR, Peyser TA, McGarraugh GV. Accuracy of the 5-day FreeStyle Navigator continuous glucose monitoring system: comparison with frequent laboratory reference measurements. Diabetes Care. 2007;30(5):1125–1130. doi: 10.2337/dc06-1602. [DOI] [PubMed] [Google Scholar]
- 2.Kovatchev B, Clarke W. Peculiarities of the continuous glucose monitoring data stream and their impact on developing closed-loop control technology. J Diabetes Sci Technol. 2008;2(1):158–163. doi: 10.1901/jaba.2008.2-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rebrin K, Steil GM, van Antwerp WP, Mastrototaro JJ. Subcutaneous glucose predicts plasma glucose independent of insulimplications for continuous monitoring. Am J Physiol. 1999;277(3 Pt 1):E561–E571. doi: 10.1152/ajpendo.1999.277.3.E561. [DOI] [PubMed] [Google Scholar]
- 4.Kovatchev BP, Shields D, Breton M. Graphical and numerical evaluation of continuous glucose sensing time lag. Diabetes Technol Ther. 2009;11(3):139–143. doi: 10.1089/dia.2008.0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kovatchev BP, Gonder-Frederick LA, Cox DJ, Clarke WL. Evaluating the accuracy of continuous glucose-monitoring sensors: continuous glucose-error grid analysis illustrated by TheraSense Freestyle Navigator data. Diabetes Care. 2004;27(8):1922–1928. doi: 10.2337/diacare.27.8.1922. [DOI] [PubMed] [Google Scholar]
- 6.International Standards Organization. In vitro diagnostic test systems: requirements for blood-glucose monitoring systems for self-testing in managing diabetes mellitus. ISO 15197:2003(E) Geneva: International Standards Organization; 2003. [Google Scholar]
- 7.McGarraugh G. Alarm characterization for continuous glucose monitors used as adjuncts to self-monitoring of blood glucose. J Diabetes Sci Technol. 2010;4(1):41–48. doi: 10.1177/193229681000400106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clarke WL, Cox D, Gonder-Frederick LA, Carter W, Pohl SL. Evaluating clinical accuracy of systems for self-monitoring of blood glucose. Diabetes Care. 1987;10(5):622–628. doi: 10.2337/diacare.10.5.622. [DOI] [PubMed] [Google Scholar]
- 9.McGarraugh G. Alarm characterization for a continuous glucose monitor that replaces traditional blood glucose monitoring. J Diabetes Sci Technol. 2010;4(1):49–56. doi: 10.1177/193229681000400107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Keenan DB, Mastrototaro JJ, Voskanyan G, Steil GM. Delays in minimally invasive continuous monitoring devices: a review of current technology. J Diabetes Sci Technol. 2009;3(5):1207–1214. doi: 10.1177/193229680900300528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McGarraugh GV, Bergenstal RM. Detection of hypoglycemia with continuous interstitial and traditional blood glucose monitoring using the FreeStyle Navigator continuous glucose monitoring system. Diabetes Technol Ther. 2009;11(3):145–150. doi: 10.1089/dia.2008.0047. [DOI] [PubMed] [Google Scholar]