Abstract
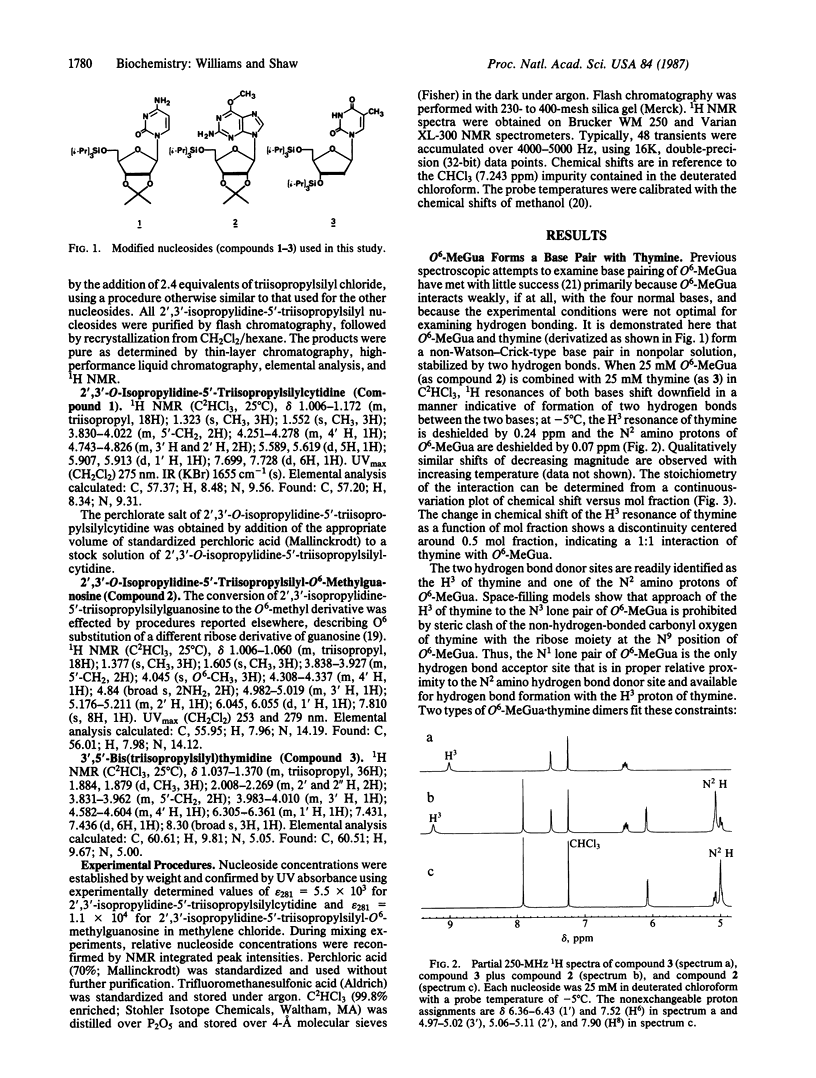
The base-pairing interactions of promutagenic O6-methylguanine (O6-MeGua) with cytosine and thymine in deuterated chloroform were investigated by 1H NMR spectroscopy. Nucleosides were derivatized at hydroxyl positions with triisopropylsilyl groups to obtain solubility in nonaqueous solvents and to prevent the ribose hydroxyls from forming hydrogen bonds. We were able to observe hydrogen-bonding interactions between nucleic acid bases in a solvent of low dielectric constant, a condition that approximates the hydrophobic interior of the DNA helix. O6-MeGua was observed to form a hydrogen-bonded mispair with thymine. Whereas O6-MeGua did not form hydrogen bonds with cytosine (via usual, wobble, or unusual tautomeric structures), it did form a 1:1 hydrogen-bonded complex with protonated cytosine. The pairing of unprotonated cytosine in chloroform is thus consistent with the known preference of O6-MeGua for thymine over cytosine in polymerase reactions. In contrast, the pairing of protonated cytosine is consistent with the greater stability of oligonucleotide duplexes containing cytosine.O6-MeGua as compared with thymine.O6-MeGua base pairs [Gaffney, B. L., Markey, L. A. & Jones, R. A. (1984) Biochemistry 23, 5686-5691]. Our observation that cytosine must be protonated in order to pair with O6-MeGua suggests that the cytosine.O6-MeGua base pair in DNA is stabilized by protonation of cytosine. Through this mechanism, methylation at the O6 position of guanine in double-stranded DNA could promote cross-strand deamination of cytosine (or 5-methylcytosine) to produce uracil (or thymine).
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chen F. M. Base protonation facilitates B-Z interconversions of poly(dG-dC) X poly(dG-dC). Biochemistry. 1984 Dec 4;23(25):6159–6165. doi: 10.1021/bi00320a041. [DOI] [PubMed] [Google Scholar]
- Eadie J. S., Conrad M., Toorchen D., Topal M. D. Mechanism of mutagenesis by O6-methylguanine. Nature. 1984 Mar 8;308(5955):201–203. doi: 10.1038/308201a0. [DOI] [PubMed] [Google Scholar]
- Gaffney B. L., Marky L. A., Jones R. A. Synthesis and characterization of a set of four dodecadeoxyribonucleoside undecaphosphates containing O6-methylguanine opposite adenine, cytosine, guanine, and thymine. Biochemistry. 1984 Nov 20;23(24):5686–5691. doi: 10.1021/bi00319a004. [DOI] [PubMed] [Google Scholar]
- Gerchman L. L., Ludlum D. B. The properties of O 6 -methylguanine in templates for RNA polymerase. Biochim Biophys Acta. 1973 May 10;308(2):310–316. doi: 10.1016/0005-2787(73)90160-3. [DOI] [PubMed] [Google Scholar]
- Goth R., Rajewsky M. F. Persistence of O6-ethylguanine in rat-brain DNA: correlation with nervous system-specific carcinogenesis by ethylnitrosourea. Proc Natl Acad Sci U S A. 1974 Mar;71(3):639–643. doi: 10.1073/pnas.71.3.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray D. M., Cui T., Ratliff R. L. Circular dichroism measurements show that C.C+ base pairs can coexist with A.T base pairs between antiparallel strands of an oligodeoxynucleotide double-helix. Nucleic Acids Res. 1984 Oct 11;12(19):7565–7580. doi: 10.1093/nar/12.19.7565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwahashi H., Sugeta H., Kyogoku Y. Detection of separated amino proton resonance signals of adenine derivatives of low temperature and its application to estimation of population of the adenine-uracil dimers in solution. Biochemistry. 1982 Feb 16;21(4):631–638. doi: 10.1021/bi00533a005. [DOI] [PubMed] [Google Scholar]
- Katz L., Penman S. Association by hydrogen bonding of free nucleosides in non-aqueous solution. J Mol Biol. 1966 Jan;15(1):220–231. doi: 10.1016/s0022-2836(66)80222-x. [DOI] [PubMed] [Google Scholar]
- Kyogoku Y., Lord R. C., Rich A. Hydrogen bonding specificity of nucleic acid purines and pyrimidines in solution. Science. 1966 Oct 28;154(3748):518–520. [PubMed] [Google Scholar]
- LAWLEY P. D., BROOKES P. FURTHER STUDIES ON THE ALKYLATION OF NUCLEIC ACIDS AND THEIR CONSTITUENT NUCLEOTIDES. Biochem J. 1963 Oct;89:127–138. doi: 10.1042/bj0890127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt M. Computer simulation of DNA double-helix dynamics. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 1):251–262. doi: 10.1101/sqb.1983.047.01.030. [DOI] [PubMed] [Google Scholar]
- Loveless A. Possible relevance of O-6 alkylation of deoxyguanosine to the mutagenicity and carcinogenicity of nitrosamines and nitrosamides. Nature. 1969 Jul 12;223(5202):206–207. doi: 10.1038/223206a0. [DOI] [PubMed] [Google Scholar]
- Mehta J. R., Ludlum D. B. Synthesis and properties of O6-methyldeoxyguanylic acid and its copolymers with deoxycytidylic acid. Biochim Biophys Acta. 1978 Dec 21;521(2):770–778. doi: 10.1016/0005-2787(78)90316-7. [DOI] [PubMed] [Google Scholar]
- Newmark R. A., Cantor C. R. Nuclear magnetic resonance study of the interactions of guanosine and cytidine in dimethyl sulfoxide. J Am Chem Soc. 1968 Aug 28;90(18):5010–5017. doi: 10.1021/ja01020a041. [DOI] [PubMed] [Google Scholar]
- Patel D. J., Shapiro L., Kozlowski S. A., Gaffney B. L., Jones R. A. Structural studies of the O6meG.C interaction in the d(C-G-C-G-A-A-T-T-C-O6meG-C-G) duplex. Biochemistry. 1986 Mar 11;25(5):1027–1036. doi: 10.1021/bi00353a012. [DOI] [PubMed] [Google Scholar]
- Patel D. J., Shapiro L., Kozlowski S. A., Gaffney B. L., Jones R. A. Structural studies of the O6meG.T interaction in the d(C-G-T-G-A-A-T-T-C-O6meG-C-G) duplex. Biochemistry. 1986 Mar 11;25(5):1036–1042. doi: 10.1021/bi00353a013. [DOI] [PubMed] [Google Scholar]
- Pulleyblank D. E., Haniford D. B., Morgan A. R. A structural basis for S1 nuclease sensitivity of double-stranded DNA. Cell. 1985 Aug;42(1):271–280. doi: 10.1016/s0092-8674(85)80122-7. [DOI] [PubMed] [Google Scholar]
- Singh U. C., Weiner S. J., Kollman P. Molecular dynamics simulations of d(C-G-C-G-A) X d(T-C-G-C-G) with and without "hydrated" counterions. Proc Natl Acad Sci U S A. 1985 Feb;82(3):755–759. doi: 10.1073/pnas.82.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snow E. T., Foote R. S., Mitra S. Base-pairing properties of O6-methylguanine in template DNA during in vitro DNA replication. J Biol Chem. 1984 Jul 10;259(13):8095–8100. [PubMed] [Google Scholar]
- Sussman J. L., Holbrook S. R., Warrant R. W., Church G. M., Kim S. H. Crystal structure of yeast phenylalanine transfer RNA. I. Crystallographic refinement. J Mol Biol. 1978 Aug 25;123(4):607–630. doi: 10.1016/0022-2836(78)90209-7. [DOI] [PubMed] [Google Scholar]
- Toorchen D., Topal M. D. Mechanisms of chemical mutagenesis and carcinogenesis: effects on DNA replication of methylation at the O6-guanine position of dGTP. Carcinogenesis. 1983 Dec;4(12):1591–1597. doi: 10.1093/carcin/4.12.1591. [DOI] [PubMed] [Google Scholar]
- Watanabe M., Sugeta H., Iwashashi H., Kyogoku Y., Kainosho M. Detection of proton-acceptor sites of hydrogen bonding in adenine X uracil base pairs by the use of 15N magnetic resonance. Eur J Biochem. 1981 Jul;117(3):553–558. doi: 10.1111/j.1432-1033.1981.tb06372.x. [DOI] [PubMed] [Google Scholar]



