Abstract
Although current systems for continuous glucose monitoring (CGM) are the result of progressive technological improvement, and although a beneficial effect on glucose control has been demonstrated, few patients are using them. Something similar has happened to telemedicine (TM); in spite of the long-term experience, which began in the early 1980s, no TM system has been widely adopted, and presential visits are still almost the only way diabetologists and patients communicate. The hypothesis developed in this article is that neither CGM nor TM will ever be routinely implemented separately, and their consideration as essential elements for standard diabetes care will one day come from their integration as parts of a telemedical monitoring platform. This platform, which should include artificial intelligence for giving decision support to patients and physicians, will represent the core of a more complex global agent for diabetes care, which will provide control algorithms and risk analysis among other essential functions.
Keywords: artificial intelligence, continuous glucose monitoring, decision support, telemedicine
Introduction
The introduction of developments resulting from new technology and their impact on the management of diabetes has been adopted less rapidly than in other specialties, such as cardiology or neurology. Apart from the suspicion that we diabetologists are especially technophobic1 one can speculate that technology has yet to offer us a clear positive balance between pros and cons. An example would be the interpretation of electrocardiogram versus continuous glucose monitoring (CGM): while cardiologists receive a diagnosis of what is wrong (e.g., left ventricular hypertrophy), we receive a statistical analysis (e.g., 73% of fasting glucose values above objective). From this information, we know that we have a problem, but no help is given in finding a solution.
According to recent advances in communication technology, offering patients the use of a personal wireless system that integrates and synchronizes information from different devices and that communicates remotely with the medical center is almost a reality. Before introducing such a system that could be condemned to the “clinical assay world” instead of being used by a majority of patients in real life, it would be of interest to learn from past, not entirely successful results of its different components.
Clinical Evidence on Efficacy and Reasons for Continuous Glucose Monitoring Underuse
The results of the different studies on real-time continuous glucose monitoring (RT-CGM) effects on glycemic control in type 1 diabetes are controversial, but overall, we can affirm that RT-CGM has a beneficial effect on glucose control.2–7 But why did the first studies obtain negative results and the latest ones show a significant benefit? The answer could be that sensors have improved and are more user-friendly, but the main reason seems to be that researchers have learned what kind of patient should be included to obtain good results. In the beginning, RT-CGM appeared to be the solution for patients with very poor glycemic control despite being treated intensively, but we know now that patients who improve with RT-CGM are often those who are suboptimally controlled, feel very motivated, perform many blood glucose readings per day, and have a high level of diabetes education. One common conclusion is that patients should use CGM more than 70% of the time in order to obtain a clear benefit. However, there may be another interpretation; only the particular group of patients who are able to wear a CGM system more than 70% of the time can take full advantage from using this kind of system.
The current situation is that when, at last, after some initial failures,3,7 we have enough evidence regarding the impact of CGM on type 1 diabetes optimization,4 and even in those countries in which the cost of CGM is partially or totally reimbursed by local health care systems, the percentage of patients using CGM on a regular basis is rather low. So why do patients, who are otherwise accustomed to wearing chronically semi-invasive devices, not want to use CGM on a regular basis? Apart from some general reasons such as discomfort, high cost, and low reliability,8 the main cause is probably related to the primary reason for undergoing CGM, which is to allow patients to make better decisions. This aim, however, is something that seems to have been forgotten by manufacturers. At this point, the crude data displayed and the considerable amount of information generated is often useless if not confusing: discrepancies, glucose jumps after calibration, and, in other cases, correct information arrives too late to be of use for insulin or diet modification. Thus it seems crucial to include tools for data processing and interpretation that give patients predicted future glucose values and guarantee, as far as possible, that the system is working properly. At the same time, real-time information about the degree of uncertainty—something like a variable obtained from information such as the sensor’s useful lifetime, time from the last calibration, noise from the crude signal, or median absolute relative difference of the previous recordings—can encourage patients to rely on CGM data without significantly increasing the number of blood glucose analyses.
Clinical Evidence on Efficacy and Reasons for Telemedicine Underuse
A number of interesting telemedicine (TM) applications have been developed for diabetes, a condition that, a priori, could benefit greatly from remote supervision and teleconsulting, among other properties of TM. Some systematic reviews and meta-analyses have been published that examine the impact of TM on diabetes care,9,10 all of which include a large number of publications—over 500. However, few of those publications were finally evaluable. These results did not derive from excessively strict inclusion criteria but from the difficult task of evaluating the impact of TM following standard scientific methodology. The main conclusion drawn from the few evaluable studies seems to be that TM has but a minor benefit, if any, for glycemic control.
First experiences in TM applied to diabetes management go back to the 1980s. One of the first was the DIACRONO project, carried out by the Bioengineering and Telemedicine Group in Madrid.11 They designed a microcomputer that allowed data interchange between doctor and patient. The next system developed by this group was called DIABTel12 and included a function of glucose data download from the glucometer, with subsequent versions that bring us to the present. Other TM experiences took further steps toward the model most of us recognize; the following ones are good examples: the TIDDM project,13 using Internet and including a smart analysis for insulin dose counseling; the Computer-Assisted Meal-Related Insulin Therapy project,14 one of the first to demonstrate a significant change in hemoglobin A1c; the Multiaccess Services for Diabetes Management project, allowing “multi-access” from many different devices; the Informatics for Diabetes Education and Telemedicine study,15 which included more than 2000 patients in two cohorts from socially underserved areas of New York, obtaining a reduction in hemoglobin A1c; and finally the Intelligent Control Assistant for Diabetes project,16 integrating, for the first time, CGM into a smart TM platform with positive results in terms of hemoglobin A1c and glucose variability.6
In general, new advances produced in communications technology have been incorporated progressively into different prototypes (Internet, email, video conferencing, mobile communications) but always with the restriction derived from the lack of interoperability. This fact is, without a doubt, one of the main obstacles to full TM implementation. Biomedical engineering and pharma-ceutical companies still design and produce information technology devices and software tools that are not interoperable, thus seriously limiting the application of technological advances to diabetes management. As an example of this absurd situation, we should remember at this point that the most relevant publication on closed loop is based on manually administered insulin.17 It is plausible that the time interval chosen for algorithm decision on insulin administration, in this case 15 minutes, is due to the lack of communication between devices, and a higher bolus frequency may well have obtained even better results. In summary, lack of interoperability together with the need for smart tools for data analysis, an issue discussed in the next section, are probably the principal factors that make TM use by diabetologists almost negligible.
Smart Telemedicine for Supporting Continuous Glucose Monitoring
With the introduction of glucose monitoring, we are now managing a huge amount of data. Patients and doctors have to analyze these data and extract the information most relevant to identifying glucose patterns, repetitive errors, and risky situations. It would be extremely helpful for us to have tools that we can count on to facilitate interpretation, a kind of “predigestion,” preprocessing of the data. Artificial intelligence should be applied here in order to obtain not only a statistical analysis, but also a diagnosis of what is wrong and a therapeutic proposal that can help patients and doctors to make better decisions. The application of artificial intelligence to decision support corresponds to TM’s “missing element,” as defined by Klonoff and True.18 In fact, several advisory systems for therapy planning based on blood glucose values have been described since 1990, among them, patient simulators,19 probabilistic network,20 case-based reasoning,13 bolus calculators,21 automatic monitoring data process,22–25 and prediction.26
The Next Scenario: The Global Agent for Diabetes Care
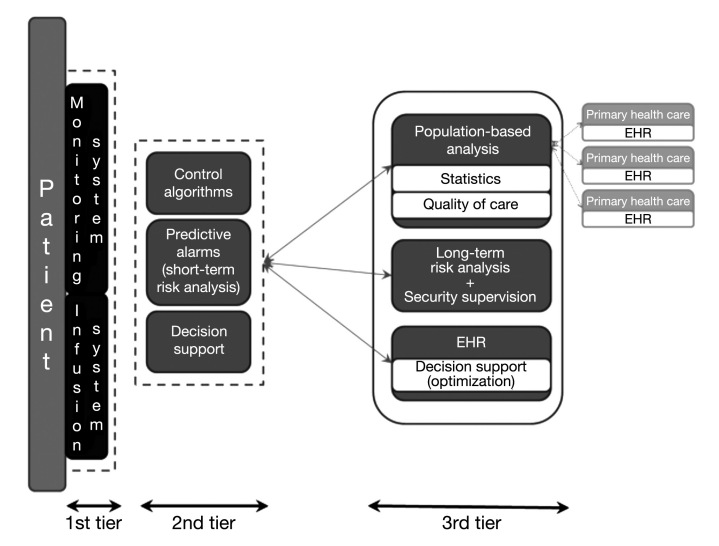
Advances in current technology allow us to foresee the creation of a complex framework to assist patients and health caregivers, comprising the different agents that have been developed into a personal loop for the patient and a remote loop for communication with the medical team, a concept that was defined years ago.27,28 This “global agent for diabetes care” would be based on wireless communication of first-tier devices (biological variables monitors, hormone infusers) with a smart assistant equipped with dual-mode low energy Bluetooth,29 which communicates remotely with the diabetes center. Its structure would be defined as a three-layer approach (Figure 1):
Figure 1.
Proposed design of the global agent for diabetes care.
First tier—The patient wears systems for biological variables monitoring (glucose, heart rate, physical activity) and infusion systems (insulin, glucagon), preferably as a disposable patch.
Second tier—Made up of the smart assistant, with three main functions: decision support; short-term risk analysis (glucose results and detection of anomalous function); and, finally, control algorithms.
Third tier—Corresponds to the remote loop and provides long-term risk analysis, decision support tools, and data integration into the electronic health record (EHR), and finally, the system should allow data aggregation for population-based analysis for quality of care assessment. To date, CGM and pump software has been designed for one-only user without taking into account that being able to aggregate data from different patients would mean clinical researchers could reach clearer conclusions and clinicians would have new markers for evaluating quality of care. In my opinion, this platform should communicate with primary health care centers in order to share some selected data from the EHR and even be useful in continuing education.
Once again, interoperability is an essential condition for the development of this scenario, which has been almost fully achieved—albeit in separate parts—by currently available technology, but which still requires the wholehearted input of all concerned.
Acknowledgments
This research was supported by a Spanish Grant of Ministry of Science and Innovation, Instituto de Salud Carlos III and FEDER (“Apriori” FIS PS09/01255).
Abbreviations
- CGM
continuous glucose monitoring
- EHR
electronic health record
- RT-CGM
real-time continuous glucose monitoring
- TM
telemedicine
References:
- 1.Albisser AM. Technophobia, prescription checking and the future of diabetes management. Diabetologia. 2009;52(6):1013–1018. doi: 10.1007/s00125-009-1341-8. [DOI] [PubMed] [Google Scholar]
- 2.Deiss D, Bolinder J, Riveline JP, Battelino T, Bosi E, Tubiana-Rufi N, Kerr D, Phillip M. Improved glycemic control in poorly controlled patients with type 1 diabetes using real-time continuous glucose monitoring. Diabetes Care. 2006;29(12):2730–2732. doi: 10.2337/dc06-1134. [DOI] [PubMed] [Google Scholar]
- 3.Hirsch IB, Abelseth J, Bode BW, Fischer JS, Kaufman FR, Mastrototaro J, Parkin CG, Wolpert HA, Buckingham BA. Sensor-augmented insulin pump therapy: results of the first randomized treat-to-target study. Diabetes Technol Ther. 2008;10(5):377–383. doi: 10.1089/dia.2008.0068. [DOI] [PubMed] [Google Scholar]
- 4.Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group. Tamborlane WV, Beck RW, Bode BW, Buckingham B, Chase HP, Clemons R, Fiallo-Scharer R, Fox LA, Gilliam LK, Hirsch IB, Huang ES, Kollman C, Kowalski AJ, Laffel L, Lawrence JM, Lee J, Mauras N, O’Grady M, Ruedy KJ, Tansey M, Tsalikian E, Weinzimer S, Wilson DM, Wolpert H, Wysocki T, Xing D. Continuous glucose monitoring and intensive treatment of type 1 diabetes. N Engl J Med. 2008;359(14):1464–1476. doi: 10.1056/NEJMoa0805017. [DOI] [PubMed] [Google Scholar]
- 5.Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group. Beck RW, Buckingham B, Miller K, Wolpert H, Xing D, Block JM, Chase HP, Hirsch I, Kollman C, Laffel L, Lawrence JM, Milaszewski K, Ruedy KJ, Tamborlane WV. Factors predictive of use and of benefit from continuous glucose monitoring in type 1 diabetes. Diabetes Care. 2009;32(11):1947–1953. doi: 10.2337/dc09-0889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rigla M, Hernando ME, Gómez EJ, Brugués E, García-Sáez G, Capel I, Pons B, de Leiva A. Real-time continuous glucose monitoring together with telemedical assistance improves glycemic control and glucose stability in pump-treated patients. Diabetes Technol Ther. 2008;10(3):194–199. doi: 10.1089/dia.2007.0273. [DOI] [PubMed] [Google Scholar]
- 7.Chase HP, Kim LM, Owen SL, MacKenzie TA, Klingensmith GJ, Murtfeldt R, Garg SK. Continuous subcutaneous glucose monitoring in children with type 1 diabetes. Pediatrics. 2001;107(2):222–226. doi: 10.1542/peds.107.2.222. [DOI] [PubMed] [Google Scholar]
- 8.Mazze RS, Strock E, Borgman S, Wesley D, Stout P, Racchini J. Evaluating the accuracy, reliability, and clinical applicability of continuous glucose monitoring (CGM): is CGM ready for real time? Diabetes Technol Ther. 2009;11(1):11–18. doi: 10.1089/dia.2008.0041. [DOI] [PubMed] [Google Scholar]
- 9.Farmer A, Gibson OJ, Tarassenko L, Neil A. A systematic review of telemedicine interventions to support blood glucose self-monitoring in diabetes. Diabet Med. 2005;22(10):1372–1378. doi: 10.1111/j.1464-5491.2005.01627.x. [DOI] [PubMed] [Google Scholar]
- 10.Verhoeven F, Van Gemert-Pijnen L, Dijkstra K, Nijland N, Seydel E, Steehouder M. The contribution of teleconsultation and videoconferencing to diabetes care: a systematic literature review. J Med Internet Res. 2007;9(5):e37. doi: 10.2196/jmir.9.5.e37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gómez-Aguilera EJ, del Pozo F, Serrano RM. DIACRONO: a new portable microcomputer system for diabetes management. Proc. 9th Ann. Int. IEEE Engineering in Medicine and Biology Society; Nov. 13–16, 1987; pp. 1231–1232. [Google Scholar]
- 12.Gómez EJ, del Pozo F, Hernando ME. Telemedicine for diabetes care: the DIABTel approach towards diabetes telecare. Med Inform (Lond) 1996;21(4):283–295. doi: 10.3109/14639239608999290. [DOI] [PubMed] [Google Scholar]
- 13.Bellazzi R, Larizza C, Montani S, Riva A, Stefanelli M, d’Annunzio G, Lorini R, Gomez EJ, Hernando E, Brugues E, Cermeno J, Corcoy R, de Leiva A, Cobelli C, Nucci G, Del Prato S, Maran A, Kilkki E, Tuominen J. A telemedicine support for diabetes management: the T-IDDM project. Comput Methods Programs Biomed. 2002;69(2):147–161. doi: 10.1016/s0169-2607(02)00038-x. [DOI] [PubMed] [Google Scholar]
- 14.Schrezenmeir J, Dirting K, Papazov P. Controlled multicenter study on the effect of computer assistance in intensive insulin therapy of type 1 diabetics. Comput Methods Programs Biomed. 2002;69(2):97–114. doi: 10.1016/s0169-2607(02)00034-2. [DOI] [PubMed] [Google Scholar]
- 15.Shea S, Weinstock RS, Teresi JA, Palmas W, Starren J, Cimino JJ, Lai AM, Field L, Morin PC, Goland R, Izquierdo RE, Ebner S, Silver S, Petkova E, Kong J, Eimicke JP, IDEATel Consortium A randomized trial comparing telemedicine case management with usual care in older, ethnically diverse, medically underserved patients with diabetes mellitus: 5 year results of the IDEATel study. J Am Med Inform Assoc. 2009;16(4):446–456. doi: 10.1197/jamia.M3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gómez EJ, Hernando Pérez ME, Vering T, Rigla Cros M, Bott O, García-Sáez G, Pretschner P, Brugués E, Schnell O, Patte C, Bergmann J, Dudde R, de Leiva A. The INCA System: a further step towards a telemedical artificial pancreas. IEEE Trans Inf Technol Biomed. 2008;12(4):470–479. doi: 10.1109/TITB.2007.902162. [DOI] [PubMed] [Google Scholar]
- 17.Hovorka R, Allen JM, Elleri D, Chassin LJ, Harris J, Xing D, Kollman C, Hovorka T, Larsen AF, Nodale M, De Palma A, Wilinska ME, Acerini CL, Dunger DB. Manual closed-loop insulin delivery in children and adolescents with type 1 diabetes: a phase 2 randomised crossover trial. Lancet. 2010;375(9716):743–751. doi: 10.1016/S0140-6736(09)61998-X. [DOI] [PubMed] [Google Scholar]
- 18.Klonoff DC, True MW. The missing element of telemedicine for diabetes: decision support software. J Diabetes Sci Technol. 2009;3(5):996–1001. doi: 10.1177/193229680900300501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Salzsieder E, Albrecht G, Fischer U, Freyse EJ. Kinetic modeling of the glucoregulatory system to improve insulin therapy. IEEE Trans Biomed Eng. 1985;32(10):846–855. doi: 10.1109/TBME.1985.325500. [DOI] [PubMed] [Google Scholar]
- 20.Hernando ME, Gómez EJ, Corcoy R, del Pozo F. Evaluation of DIABNET, a decision support system for therapy planning in gestational diabetes. Comput Methods Programs Biomed. 2000;62(3):235–248. doi: 10.1016/s0169-2607(00)00070-5. [DOI] [PubMed] [Google Scholar]
- 21.Palerm CC, Zisser H, Bevier WC, Jovanovic L, Doyle FJ 3rd. Prandial insulin dosing using run-to-run control: application of clinical data and medical expertise to define a suitable performance metric. Diabetes Care. 2007;30(5):1131–1136. doi: 10.2337/dc06-2115. [DOI] [PubMed] [Google Scholar]
- 22.García-Sáez G, Alonso JM, Molero J, Rigla M, Martínez-Sarriegui I, Leiva A, Gómez EJ, Hernando EM. Mealtime blood glucose classifier based on fuzzy logic for DIABTel telemedicine system. Artif Intell Med. 2009;5651:295–304. [Google Scholar]
- 23.Salzsieder E, Augstein P, Vogt L, Kohnert KD, Heinke P, Freyse E, Azim Ahmed A, Metwali Z, Salman I, Attef O. Telemedicine-based KADIS combined with CGMS has high potential for improving outpatient diabetes care. J Diabetes Sci Technol. 2007;1(4):511–521. doi: 10.1177/193229680700100409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Augstein P, Vogt L, Kohnert KD, Freyse EJ, Heinke P, Salzsieder E. Outpatient assesment of Karslburg Diabetes Management System-based decision support. Diabetes Care. 2007;30(7):1704–1708. doi: 10.2337/dc06-2167. [DOI] [PubMed] [Google Scholar]
- 25.Holbrook A, Thabane L, Keshavjee K, Dolovich L, Bernstein B, Chan D, Troyan S, Foster G, Gerstein H, COMPETE II Investigators Individualized electronic decision support and reminders to improve diabetes care in the community: COMPETE II randomized trial. CMAJ. 2009;181(1-2):37–44. doi: 10.1503/cmaj.081272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pérez-Gandía C, Facchinetti A, Sparacino G, Cobelli C, Gómez EJ, Rigla M, de Leiva A, Hernando ME. Artificial neural network algorithm for online glucose prediction from continuous glucose monitoring. Diabetes Technol Ther. 2010;12(1):81–88. doi: 10.1089/dia.2009.0076. [DOI] [PubMed] [Google Scholar]
- 27.Bellazzi R, Arcelloni M, Bensa G, Blankenfeld H, Brugués E, Carson E, Cobelli C, Cramp D, D’Annunzio G, De Cata P, De Leiva A, Deutsch T, Fratino P, Gazzaruso C, Garcìa A, Gergely T, Gómez E, Harvey F, Ferrari P, Hernando E, Boulos MK, Larizza C, Ludekke H, Maran A, Nucci G, Pennati C, Ramat S, Roudsari A, Rigla M, Stefanelli M. Design, methods, and evaluation directions of a multi-access service for the management of diabetes mellitus patients. Diabetes Technol Ther. 2003;5(4):621–629. doi: 10.1089/152091503322250640. [DOI] [PubMed] [Google Scholar]
- 28.Bellazzi R, Siviero C, Stefanelli M, De Nicolao G. Adaptative controllers for intellingent monitoring. Artif Intell Med. 1995;7(6):515–540. doi: 10.1016/0933-3657(95)00025-x. [DOI] [PubMed] [Google Scholar]
- 29.Omre AH. Bluetooth low energy: wireless connectivity for medical monitoring. J Diabetes Sci Technol. 2010;4(2):457–463. doi: 10.1177/193229681000400227. [DOI] [PMC free article] [PubMed] [Google Scholar]