Abstract
Background
A strong relationship between glycemic variability and oxidative stress in poorly regulated type 2 diabetes (T2DM) on oral medication has been reported. However, this relationship was not seen in type 1 diabetes. The purpose of this study is to reexamine the relation between glycemic variability and oxidative stress in a cohort of T2DM patients on oral medication.
Methods
Twenty-four patients with T2DM on oral glucose lowering treatment underwent 48 hours of continuous glucose monitoring (CGMS® System GoldTM, Medtronic MiniMed) and simultaneous collection of two consecutive 24-hour urine samples for determination of 15(S)-8-iso-prostaglandin F2α (PGF2α) using high-performance liquid chromatography tandem mass spectrometry. Standard deviation (SD) and mean amplitude of glycemic excursions (MAGE) were calculated as markers of glycemic variability.
Results
Included in the study were 66.7% males with a mean age (range) of 59 (36–76) years and a mean (SD) HbA1c of 6.9% (0.7). Median [interquartile range (IQR)] urinary 15(S)-8-iso-PGF2α excretion was 176.1 (113.6–235.8) pg/mg creatinine. Median (IQR) SD was 31 (23–40) mg/dl and MAGE 85 (56–106) mg/dl. Spearman correlation did not show a significant relation for SD (ρ = 0.15, p = .49) or MAGE (ρ = 0.23, p = .29) with 15(S)-8-iso-PGF2α excretion. Multivariate regression analysis adjusted for age, sex, HbA1c, and exercise did not alter this observation.
Conclusions
We did not find a relevant relationship between glucose variability and 15(S)-8-iso-PGF2α excretions in T2DM patients well-regulated with oral medication that would support an interaction between hyperglycemia and glucose variability with respect to the formation of reactive oxygen species.
Keywords: 8-isoprostanes, glucose variability, MAGE, oxidative stress, type 2 diabetes
Introduction
The main cause of morbidity and mortality in both type 1 and type 2 diabetes (T1DM, T2DM) is the development of micro- and macrovascular complications. Several studies have shown a direct relation between glucose exposure, as measured by hemoglobin A1c (HbA1c) level, and complications of diabetes.1,2 The molecular mechanisms that explain this relation between hyper-glycemia and diabetic complications have not been fully elucidated. A suggested common pathway by which hyperglycemia leads to these complications is the formation of reactive oxygen species (ROS) by glucose overload in the mitochondria. This could lead to vascular damage through several molecular mechanisms.3 However, HbA1c only explains around 25% of the variation in risk of developing complications,4 suggesting the contribution of other factors. It has been suggested that glycemic variability contributes to diabetes complications via formation of ROS.5
Monnier and colleagues were the first to study the role of glucose variability in oxidative stress formation in T2DM.6 They measured 24-hour urinary excretion rates of free 8-iso-prostaglandin F2α (PGF2α), which is considered a good marker of oxidative stress.7,8 Continuous glucose monitoring (CGM) was performed for determination of glycemic variability. They found a strong correlation between 24-hour PGF2α excretion rates and glucose variability in T2DM patients on diet and/or oral antihyper-glycemic drugs. Subsequently, Wentholt and colleagues9 investigated the role of glucose variability in activation of oxidative stress in T1DM patients using the same oxidative stress marker and the same method to determine glucose variability. Despite more pronounced glycemic variability, no relationship was found between oxidative stress and glucose variability. They found oxidative stress to be increased in T1DM.
In T1DM patients, other pathways than glucose variability might be involved in activation of oxidative stress, perhaps explaining these conflicting findings. Moreover, different techniques were used to assess the PGF2α excretion rate. The immunoassay is less specific than the tandem mass spectrometry technique used by Wenthol et al., as already acknowledged by Monnier et al.10.11 Interestingly, a study in 2010 by Monnier confirmed the findings of Wentholt regarding the absence of a relation between glucose variability and oxidative stress in T1DM patients. Even more, T1DM patients and healthy controls did not differ in 8-iso-PGF2α excretion.12 Also their results suggest that the relation between glucose variability and ROS formation in T2DM patients depends on the HbA1c level, a relation only seen at higher HbA1c levels.
The aim of this study is to evaluate the role of acute glucose fluctuations in the activation of oxidative stress in T2DM patients using oral glucose-lowering agents, using tandem mass spectrometry for the assessment of PGF2α excretion rates.
Methods
Patients
Twenty-eight patients with T2DM were randomly recruited from the outpatient clinic of the Academic Medical Center in Amsterdam, The Netherlands, between April 2008 and February 2009. Inclusion criteria were a diagnosis of T2DM for more than 6 months and treatment with oral glucose-lowering agents or diet. Patients were excluded in case of use of steroid or nonsteroidal anti-inflammatory drugs, insulin treatment, an acute illness during the 3-month period prior to the investigation, or an estimated glomerular filtration rate (GFR) of less than 60 ml/min/1.73m2 according to the Cockcroft-Gault formula13 because of a potential influence on oxidative stress production. Also the use of heparin or oral anti-coagulants (except for aspirin) was not allowed as this could cause bleeding during sensor insertion. The study was approved by the local ethics committee and patients gave written informed consent after written and oral explanation of the study.
Study Design
On day 1, after giving written informed consent, the continuous glucose monitor (CGMS® System GoldTM, Medtronic MiniMed, Northridge, CA) was inserted subcutaneously in the abdominal wall. Patients were provided with home blood glucose meters (OneTouch® Ultra®, LifeScan, Inc., Milpitas, CA) and were instructed to perform the required sensor calibration procedure four times daily according to manufacturers instructions. Urine was collected for two consecutive 24-hour periods. Patients were asked to store the urine jars in the refrigerator, to avoid ex vivo formation of isoprostanes. Systolic and diastolic blood pressure was measured and venous blood samples were drawn for the following laboratory measurements: fasting plasma glucose (FPG), HbA1c, total cholesterol, high-density lipoprotein (HDL) cholesterol, low-density lipoprotein (LDL) cholesterol, triglycerides, and creatinine. Relevant patient characteristics were recorded [e.g., medical history, current medication use, body mass index (BMI), smoking habits]. The reported amount of physical exercise was graded as sedentary, moderately active, active, and fit. On day 4, patients returned to the clinical trial room with the two urine collection jars. From each 24-hour period (day 2 and day 3 of the study) a 7-ml urine sample was stored in a freezer at -80 °C until analysis of all urine samples in one run. For the analyses, the 15(S)-8-iso-PGF2α excretion rates over 48 hours were used calculated by averaging the first- and second-day samples. Lastly, the continuous glucose monitor was removed and data was downloaded and stored electronically. Only glucose data obtained during the 48 hours of urine sampling was used for further analysis.
Laboratory Measurements
Hemoglobin A1c was measured using a high-performance liquid chromatography (HPLC) assay (Variant II, Bio-Rad Laboratories, Inc., Montreal, Quebec, Canada). Activation of oxidative stress was estimated by determining the urinary isoprostane excretion [15(S)-8-iso-PGF2α], using HPLC tandem mass spectrometry (HPLC-MS/MS). Urine samples were collected and stored without additives as 7-ml aliquots at -80 °C. Initial creatinine concentrations were established by colorimetric Jaffé assay. Two milliliters of sample were mixed with 2H4-labelled 15(S)-8-iso-PGF2α as internal standard and applied to an 8-iso-PGF2α immunoaffinity column (Cayman Chemical Company, Ann Arbor, MI). After washing, the extract was eluted, evaporated to dryness (60 °C, N2) and reconstituted in 200-μl 0.05 mol/liter formic acid-ethanol (75:25, v/v); 50 μl was injected on the HPLC-MS/MS system. Chromatographic separation was achieved on a modular HPLC system (Surveyor®, Thermo Finnigan, San Jose, CA) consisting of a cooled autosampler (t = 12 °C) and a low-flow quaternary MS pump and analytical HPLC column: Alltima C8, 2.1 × 150 mm, 5 μm (Alltech, Lexington, KY). Samples were eluted with a flow rate of 200 μl/min and a programmed linear gradient between A (0.01% HCOOH in H2O, v/v) and B (CH3CN): from t = 0 min 45% A, 55% B toward t = 3 min 30% A, 70% B toward t = 3.1 min100% A until t = 6 min. MS/MS analyses were performed on a TSQ Quantum AM (Thermo Finnigan, San Jose, CA) operated in the negative ion electrospray ionization mode. The surface-induced dissociation was set at 2 V; spray voltage was 3500 V, and the capillary temperature was 400 °C. In the MS/MS experiments argon was used as collision gas at a pressure of 0.2 Pa; collision energy was 26 eV for the optimized transitions: m/z 353.24 → m/z 193.10 and m/z 357.24 → m/z 197.10. The interassay (n = 5) and intraassay (average of 5 days, n = 3) variability allowed for determination at physiological concentrations with a coefficient of variation of <7%.
Assessment of Glycemic Variability
In literature, there is no universally accepted ‘gold’ standard to measure variability in glucose values.14 We calculated the SD of the glucose measurements and the mean amplitude of glycemic excursions (MAGE) as described by Service and colleagues15 because SD is the best mathematically validated measure and both are commonly used in literature. The MAGE over 48 hours is the mean of the absolute differences between peak and nadir values over 48 hours. The peaks and nadirs are defined as glucose values preceded and followed by an increase and decrease or a decrease and increase, respectively. Only increases or decreases larger than 1 SD of the mean glucose are taken into account. If a decrease of more than 1 SD was the first excursion, only peak-to-nadir excursions (>1 SD) were included in the calculation of the MAGE and vice versa.
Assessment of Postprandial Glucose Excursions
We determined the incremental areas above preprandial glucose values (breakfast, lunch, dinner) over a 4-hour period following the beginning of each meal using the trapezoidal rule.16 The six incremental areas of each patient during the 48 hours of continuous glucose monitoring were summed and averaged to calculate the mean postprandial incremental area under the curve (AUCpp).6
Statistical Analysis
Means and standard deviations of patient characteristics, urinary excretion of 15(S)-8-iso-PGF2α, and glucose varia-bility measures were assessed using standard statistics. Levels of urinary 15(S)-8-iso-PGF2α in the samples from both days were compared using a Wilcoxon signed-rank test. Spearman correlation was calculated in order to evaluate the relation between glycemic variability measures, HbA1c, FPG, postprandial glucose excursions, and the excretion of urinary 15(S)-8-iso-PGF2α. The effect of glucose variability on 15(S)-8-iso-PGF2α excretion was also assessed in a multivariate regression model adjusting for variables that have been reported to be involved in oxidative stress activation, i.e., sex, age, smoking, and HbA1c,17 and for variables that showed a relation with urinary 15(S)-8-iso-PGF2α excretion in Spearman correlation analysis. Analyses were performed using SPSS version 16.0.2.
Results
From the 28 recruited patients, 4 patients were excluded from data analysis; 2 patients showed an estimated GFR of less than 60 ml/min/1.73m2, in 1 patient the glucose sensor failed to record any data, and 1 patient did not perform the urine collection adequately.
From the remaining 24 patients, 16 were male, mean (range) age was 58.9 years (36–76) and mean (SD) HbA1c was 6.9% (0.7). Median [interquartile range (IQR)] urinary 15(S)-8-iso-PGF2α excretion was 176.1 (113.6–235.8) pg/mg creatinine. There was no significant difference between the first- and second-day urine samples (Wilcoxon signed-rank test, p = .95). Median (IQR) SD and MAGE were 31 mg/dl (23–40) and 85 (56–106), respectively. Patient characteristics are listed in Table 1 .
Table 1.
Baseline Characteristics
| Characteristicsa | Patients (n = 24) |
|---|---|
| Age (year) | 58.9 (36–76) |
| Men/women (n) | 16/8 |
| Diabetes duration (year) | 7.2 (4.2) |
| Diabetes treatment [n (%)] | |
| Metformin | 23 (96) |
| Sulfonylurea | 15 (63) |
| Rosiglitazone | 2 (8) |
| Other treatments [n (%)] | |
| ACE inhibitor | 9 (38) |
| Statin | 19 (79) |
| Aspirin | 7 (29) |
| Cigarette smoking [n (%)] | 2 (8) |
| BMI, kg/m2 | 30.5 (5.5) |
| Systolic blood pressure (mm Hg) | 135 (17) |
| Diastolic blood pressure (mm Hg) | 82 (10) |
| Plasma creatinine (µmol/liter) | 76.3 (13.1) |
| Total cholesterol (mmol/liter) | 4.18 (0.80) |
| HDL cholesterol (mmol/liter) | 1.10 (0.20) |
| LDL cholesterol (mmol/liter) | 2.31 (0.71) |
| Triglycerides (mmol/liter) | 1.71 (0.72) |
| HbA1c (%) | 6.9 (0.7) |
| FPG (mg/dl) | 144 (32) |
| Mean sensor glucose (mg/dl) | 146 (27) |
| AUCppb (mg/dl/h) | 129 (54) |
| Markers of glucose variability [median (IQR)] | |
| SD (mg/dl) | 31 (23–40) |
| MAGE (mg/dl) | 85 (56–106) |
| Urinary 15(S)-8-iso-PGF2α, pg/mg creatinine [median (IQR)] | 176.1 (113.6–235.8) |
Data are means (SD) or means (range), unless stated otherwise in parentheses. To convert mean glucose, AUCpp, MAGE, and SD from mg/dl to mmol/liter, multiply by 0.0555.
AUCpp is the 4-hour postprandial incremental area under the curve.
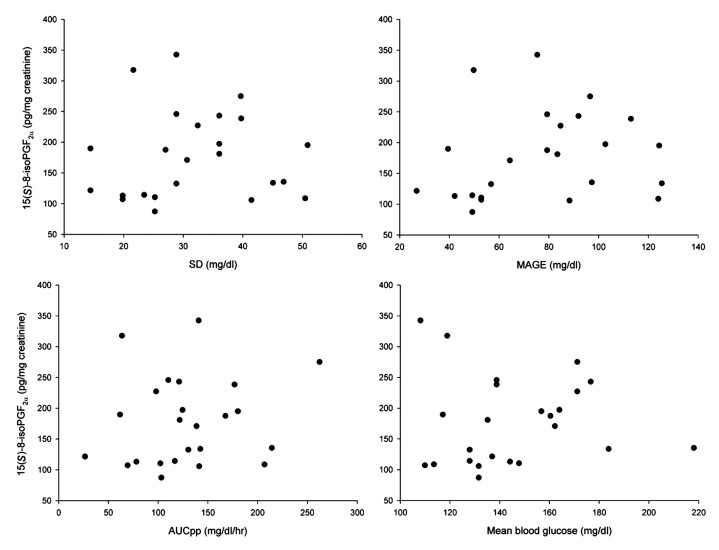
Spearman correlation did not reveal a significant relation for any glucose variability parameter with 15(S)-8-iso-PGF2α excretion [SD, ρ = 0.15, p = .49 (Figure 1 ); MAGE, ρ = 0.23, p = .29]. Multivariate regression analysis was performed to adjust for sex, age, smoking, and HbA1c,17 and for the variable that showed a significant correlation with 15(S)-8-iso-PGF2α, i.e., the amount of physical exercise. This did not alter the results from Spearman correlation described above (SD, r2 = 0.003, p = .77; MAGE, r2= <0.001, p= .98).
Figure 1.
Correlations between glycemic markers and oxidative stress. X-axis: glycemic markers over 48 hours of glucose measurements expressed as SD, MAGE, AUCpp, and mean blood glucose. Y-axis: oxidative stress, expressed as 15(S)-8-iso prostaglandin F2α in pg/mg creatinine, over 48 hours of urine collection.
No significant correlation was found between 15(S)-8-iso-PGF2a excretion and HbA1c (ρ = 0.27, p = .20)or AUCpp (ρ = 0.14, p = .51), FPG (ρ = 0.27, p = .20) and the mean of sensor glucose measurements (ρ = 0.23, p = .29), Table 2 . The plots do not reveal a pattern that would suggest a threshold phenomenon. Also, no significant correlations were found for sex, age, BMI, smoking, systolic or diastolic blood pressure, or lipid concentrations (Table 2 ). A multivariate regression model with age, sex, smoking, exercise, and HbA1c as covariates did show significant inverse relations between 15(S)-8-iso-PGF2a excretion rates and age (r = -0.49, p = .01) and the amount of physical exercise (r = -0.61, p = .004).
Table 2.
Spearman Correlation Coefficients (ρ) between Urinary Excretion Rates of 15(S)-8-iso-PGF2α, Clinical Characteristics and Glycemic Markers
| Sex | Age | BMI | Exercise | HbA1c | FPG | MBG | SD | MAGE | AUCpp | |
|---|---|---|---|---|---|---|---|---|---|---|
| FPG | 0.09 | 0.33 | -0.12 | -0.15 | 0.48a | |||||
| MBG | -0.08 | 0.36 | -0.22 | -0.20 | 0.63b | 0.85b | ||||
| SD | -0.29 | -0.04 | -0.45a | -0.04 | 0.43a | 0.34 | 0.45a | |||
| MAGE | -0.27 | -0.13 | -0.41a | 0.01 | 0.44a | 0.38 | 0.44a | 0.95b | ||
| AUCpp | -0.41a | -0.18 | -0.28 | 0.03 | 0.39 | 0.29 | 0.35 | 0.85b | 0.86b | |
| 8-iso-PGF2α | -0.09 | -0.27 | 0.23 | -0.50a | 0.27 | 0.27 | 0.23 | 0.15 | 0.23 | 0.14 |
p < .05
p < .01
Discussion
We did not find a relevant association between markers of glycemic variability and oxidative stress, estimated by 15(S)-8-iso-PGF2α excretion rates, in patients with T2DM who were well-regulated with oral glucose lowering agents only. At first, these results seem in contrast with earlier data, showing a firm correlation between glycemic variability and oxidative stress in T2DM patients using oral hypoglycemic agents,6 despite use of the same continuous glucose monitor and mathematical methods to assess glucose variability.
A possible explanation for the seemingly opposing findings in T2DM patients might be the difference in glucose regulation between the study populations. The mean glucose and HbA1c of the original Monnier population was markedly higher than in our population (mean glucose 190 and 146 mg/dl, HbA1c 9.6 and 6.9%, respectively) though the mean MAGE was roughly comparable (75 and 85 mg/dl, respectively). In a paper published by the same group,12 there seemed to be a modifying effect of HbA1c on the relation between glucose variability and ROS formation in patients with T2DM on oral medication. No effect of MAGE on the formation of 15(S)-8-iso-PGF2α was seen in the group with an HbA1c below the median of 8.2%, while people with higher MAGE values had more oxidative stress in the subgroup with an HbA1c above the median. Thus, the absence of a relation between glucose variability and oxidative stress in our population with a mean HbA1c of 6.9% is in agreement with their findings. It might be possible that the fluctuations observed in patients with a high HbA1c level reach a higher maximum glucose value and in that way cross a threshold for oxidative stress. In line with this is the observation that the AUCpp in the Monnier population was much larger than in our well-regulated population (407 and 129 mg/dl/h respectively) suggesting that the variability in the Monnier population depended more on large postprandial excursions, which are known to enhance oxidative stress.18
Another difference between our results and those reported by Monnier et al. is a difference in technique for quantification of oxidative stress. Monnier et al. used an enzyme immunoassay (EIA) to quantify urinary excretion of 15(S)-8-iso-PGF2α, whereas we used HPLC-MS/MS, the same method used by Wentholt et al.Measurement of F2-isoprostanes by way of mass spectro-metry has been reported to be more specific and sensitive than measurement by immunoassay.10,11 Studies have shown that MS is not hampered by cross reactivity of structurally (un)related components of 8-iso-PGF2α in that way selectively determining 15(S)-8-iso-PGF2α,whereas several substances in biological fluids that are not products of lipid peroxidation interfere with the immunoassay that includes its enantiomer ent-15(S)-8-iso-PGF2α in the quantification of oxidative stress.8,11 This is also why in literature 8-iso-PGF2α levels are often reported to be higher when assessed by EIA compared to MS.
The excretion rates of 15(S)-8-iso-PGF2α in our T2DM population [median (IQR) 176.1 (113.6–235.8) pg/mg creatinine] are of the same magnitude as the excretion rates in the T1DM patient group reported by Wentholt and colleagues [median (IQR) 161 (140–217) pg/mg creatinine] that were significantly higher than in their healthy control group (median [IQR] 118 (101–146) pg/mg creatinine). Also, when we match 9 of our patients with 9 of the controls used in the Wentholt study according to age and sex, the PGF excretion rate in our well-regulated T2DM patients is significantly larger [median (IQR) controls 108.2 (90.3–144.0) and patients 243.3 (147.8–287.5), p = .006, Mann-Whitney U Test]. The excretion rates of the patient group reported by Monnier and colleagues are nearly threefold higher [mean (SD) 482 (206) pg/mg creatinine] than those of our patient group. This disparity is likely to be caused by the different methods used to assess 8-iso-PGF2α excretion rates as explained earlier, together with the higher mean glucose and HbA1c of the Monnier population, as a higher mean glucose is likely to cause more oxidative stress.3,19
Our findings are supported by the only intervention trial assessing the effect of lowering glucose variability on oxidative stress in T2DM patients.20 This crossover trial comparing prandial vs basal insulin did not find any influence of lowering glucose variability on oxidative stress, assessed by the 24-hour excretion rates of 8-iso-PGF2α, measured by HPLC-MS/MS. Additionally, no correlation between glucose variability (MAGE) and oxidative stress was found in this insulin-treated population. Again, this is in line with the recent findings of Monnier, who reported no relation between glucose variability and urinary excretion of 15(S)-8-iso-PGF2α in insulin-treated T2DM patients.
Despite the vast amount of studies using F2-isoprostanes as a biomarker of oxidative stress, not all (patho)physiological factors contributing to the formation of F2-isoprostanes are known.17 Multivariate regression analysis showed the amount of physical exercise and age to be inversely related to oxidative stress activation in our study population. This has been reported in another study, although for both parameters, also positive relations and no relations have been reported (for review, see Basu and Helmersson17).
A limitation of this study is that from the broad spectrum of markers of oxidative stress, we only measured the urinary excretion of F2-isoprostanes. Measurement of another marker(s) could have complemented the present data. On the other hand, measurement of F2-isoprostanes is regarded the best option for addressing lipid peroxidation.7,8 A further limitation is the absence of people with higher HbA1c values on oral medication in our cohort. We simply could not find such patients, who apparently had already received treatment intensification according to current treatment guidelines.
Conclusions
We found no relevant relationship between glycemic variability, assessed by continuous glucose monitoring, and 15(S)-8-iso-PGF2α excretion, assessed by HPLC-MS/MS in a population of well-regulated T2DM patients. These results argue against a role of glycemic variability in the activation of oxidative stress in this group of patients, and together with findings from literature suggest that the relation between glucose variability and oxidative stress is only seen at higher HbA1c levels.
Acknowledgments
We thank H. van Lenthe, Laboratory Genetic Metabolic Diseases, Academic Medical Center, Amsterdam, The Netherlands, who performed all the laboratory analyses of the urine samples for determination of 15(S)-8-iso-PGF2α.
Abbreviations
- AUCpp
postprandial incremental area under the curve
- BMI
body mass index
- CGM
continuous glucose monitoring
- EIA
enzyme immunoassay
- FPG
fasting plasma glucose
- GFR
glomerular filtration rate
- HbA1c
hemoglobin A1c
- HDL
high-density lipoprotein
- HPLC
high-performance liquid chromatography
- IQR
interquartile range
- LDL
low-density lipoprotein
- MAGE
mean amplitude of glycemic excursions
- MS
mass spectrometry
- PGF2α
prostaglandin F2α
- ROS
reactive oxygen species
- SD
standard deviation
- T1DM
type 1 diabetes mellitus
- T2DM
type 2 diabetes mellitus
References:
- 1.The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329(14):977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 2.Stratton IM, Adler AI, Neil HA, Matthews DR, Manley SE, Cull CA, Hadden D, Turner RC, Holman RR. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321(7258):405–412. doi: 10.1136/bmj.321.7258.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414(6865):813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 4.The Diabetes Control and Complications Trial Research Group. The relationship of glycemic exposure (HbA1c) to the risk of development and progression of retinopathy in the diabetes control and complications trial. Diabetes. 1995;44(8):968–983. [PubMed] [Google Scholar]
- 5.Hirsch IB, Brownlee M. Should minimal blood glucose variability become the gold standard of glycemic control? J Diabetes Complications. 2005;19(3):178–181. doi: 10.1016/j.jdiacomp.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 6.Monnier L, Mas E, Ginet C, Michel F, Villon L, Cristol JP, Colette C. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA. 2006;295(14):1681–1687. doi: 10.1001/jama.295.14.1681. [DOI] [PubMed] [Google Scholar]
- 7.Morrow JD, Hill KE, Burk RF, Nammour TM, Badr KF, Roberts LJ 2nd. A series of prostaglandin F2-like compounds are produced in vivo in humans by a non-cyclooxygenase, free radical-catalyzed mechanism. Proc Natl Acad Sci. 1990;87(23):9383–9387. doi: 10.1073/pnas.87.23.9383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roberts LJ, Morrow JD. Measurement of F2-isoprostanes as an index of oxidative stress in vivo. Free Radic Biol Med. 2000;28(4):505–513. doi: 10.1016/s0891-5849(99)00264-6. [DOI] [PubMed] [Google Scholar]
- 9.Wentholt IM, Kulik W, Michels RP, Hoekstra JB, DeVries JH. Glucose fluctuations and activation of oxidative stress in patients with type 1 diabetes. Diabetologia. 2008;51(1):183–190. doi: 10.1007/s00125-007-0842-6. [DOI] [PubMed] [Google Scholar]
- 10.Proudfoot J, Barden A, Mori TA, Burke V, Croft KD, Beilin LJ, Puddey IB. Measurement of urinary F(2)-isoprostanes as markers of in vivo lipid peroxidation-A comparison of enzyme immunoassay with gas chromatography/mass spectrometry. Anal Biochem. 1999;272(2):209–215. doi: 10.1006/abio.1999.4187. [DOI] [PubMed] [Google Scholar]
- 11.Saenger AK, Laha TJ, Edenfield MJ, Sadrzadeh SM. Quantification of urinary 8-iso-PGF2alpha using liquid chromatography-tandem mass spectrometry and association with elevated troponin levels. Clin Biochem. 2007;40(16-17):1297–1304. doi: 10.1016/j.clinbiochem.2007.07.023. [DOI] [PubMed] [Google Scholar]
- 12.Monnier L, Colette C, Mas E, Michel F, Cristol JP, Boegner C, Owens DR. Regulation of oxidative stress by glycaemic control: evidence for an independent inhibitory effect of insulin therapy. Diabetologia. 2010;53(3):562–571. doi: 10.1007/s00125-009-1574-6. [DOI] [PubMed] [Google Scholar]
- 13.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16(1):31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 14.Siegelaar SE, Holleman F, Hoekstra JB, DeVries JH. Glucose variability; does it matter? Endocr Rev. 2010;31(2):171–182. doi: 10.1210/er.2009-0021. [DOI] [PubMed] [Google Scholar]
- 15.Service FJ, Molnar GD, Rosevear JW, Ackerman E, Gatewood LC, Taylor WF. Mean amplitude of glycemic excursions, a measure of diabetic instability. Diabetes. 1970;19(9):644–655. doi: 10.2337/diab.19.9.644. [DOI] [PubMed] [Google Scholar]
- 16.Rohlfing CL, Wiedmeyer HM, Little RR, England JD, Tennill A, Goldstein DE. Defining the relationship between plasma glucose and HbA(1c): analysis of glucose profiles and HbA(1c) in the Diabetes Control and Complications Trial. Diabetes Care. 2002;25(2):275–278. doi: 10.2337/diacare.25.2.275. [DOI] [PubMed] [Google Scholar]
- 17.Basu S, Helmersson J. Factors regulating isoprostane formation in vivo. Antioxid Redox Signal. 2005;7(1-2):221–235. doi: 10.1089/ars.2005.7.221. [DOI] [PubMed] [Google Scholar]
- 18.Ceriello A, Bortolotti N, Motz E, Crescentini A, Lizzio S, Russo A, Tonutti L, Taboga C. Meal-generated oxidative stress in type 2 diabetic patients. Diabetes Care. 1998;21(9):1529–1533. doi: 10.2337/diacare.21.9.1529. [DOI] [PubMed] [Google Scholar]
- 19.Davi G, Ciabattoni G, Consoli A, Mezzetti A, Falco A, Santarone S, Pennese E, Vitacolonna E, Bucciarelli T, Costantini F, Capani F, Patrono C. In vivo formation of 8-iso-prostaglandin f2〈 and platelet activation in diabetes mellitus: effects of improved metabolic control and vitamin E supplementation. Circulation. 1999;99(2):224–229. doi: 10.1161/01.cir.99.2.224. [DOI] [PubMed] [Google Scholar]
- 20.Siegelaar SE, Kulik W, van Lenthe H, Mukherjee R, Hoekstra JB, DeVries JH. A randomized controlled trial comparing the effect of basal insulin and inhaled mealtime insulin on glucose variability and oxidative stress. Diabetes Obes Metab. 2009;11(7):709–714. doi: 10.1111/j.1463-1326.2009.01037.x. [DOI] [PubMed] [Google Scholar]